International Journal of Physical Medicine & Rehabilitation
Open Access
ISSN: 2329-9096
ISSN: 2329-9096
Research Article - (2024)Volume 12, Issue 3
Background: After acute injury there are a variety both modifiable and unknown factors that contribute to the liver’s ability to recover. This group previously demonstrated that treatment with activated umbilical cord Mesenchymal Stem Cells (MSCs) increased the survival of mice with humanized livers who developed alcoholinduced liver injury.
Objective: This study’s primary objective was to evaluate the safety and efficacy of various doses of frozen-thawed activated MSCs compared to placebo in the treatment of acute alcohol-induced liver injury in humanized mouse livers. Secondary objectives included evaluating hepatic chemistries, biomarkers and pathology at various doses.
Methods: Sixty-two humanized mice that were fed high fat diets and alcohol binge drinking for 24 days were randomized to receive 1 million, 500,000, 250,000, 100,000 or 28,000 activated umbilical cord MSCs, or vehicle (plasmalyte) only injections via tail vein three times in the first week and weekly for two additional weeks. Mice were followed for survival at 4 weeks with surviving mice euthanized.
Results: At the highest administered dose, 1 million activated umbilical cord MSCs, there was a statistically significant survival increase compared to placebo (p=0.03). Histologic findings correlated with survival with 27 surviving animals demonstrating 1 to 2+ steatosis with no necrosis. Of the 35 animals that died, 23 demonstrated necrosis and 9 showed various degrees of steatosis. Using food consumption and body weight as measures of safety showed no statistical differences between study groups.
Conclusion: Treatment with high-dose frozen-thawed activated umbilical cord MSCs can result in improved survival and histology in mice with humanized livers and alcohol-induced liver injury. Additionally, this therapy does not appear to be associated with adverse effects.
Mesenchymal stem cells; Treatment; Pyroptosis; Necroptosis; Recovery; Bone marrow
ALD: Alcoholic Liver Disease; MSC: Mesenchymal Stem Cells; HSC: Human Hematopoietic Stem Cell; HFCD: High-Fat High-Cholesterol Diet; PBS: Phosphate-Buffered Saline; ALT: Alanine Aminotransferase; AST: Aspartate Aminotransferase; LPS: Lipopolysaccharide; hUCMSC: Human Umbilical Cord Mesenchymal Stem Cells
Alcoholic liver disease is increasing in the United States [1]. This increase is more pronounced in the COVID era [2,3]. Highlighting this trend is the increase in the number of patients that are placed on the liver transplant waitlist and the number that have undergone transplantation over recent years [4].
The liver is the main site of alcohol metabolism and a target of alcohol-induced injury. The spectrum of liver disease varies from the development of steatosis, steatohepatitis and fibrosis to acute alcoholic hepatitis and advanced liver disease including cirrhosis. Acute alcoholic hepatitis is an inflammatory disease of the liver associated with recent heavy binge drinking. It is characterized by steatosis, hepatocyte ballooning, Mallory-Denk bodies, and lobular inflammation with a prominent component of neutrophils [5]. Outcomes are variable with a 30-day mortality rate for severe cases, defined by the discriminant function, as high as 30%-50% [6]. Treatments are primarily supportive and different therapeutic techniques have variable efficacy. Criteria for transplantation vary between centers and the supply of organs is limited. The need for an effective treatment for acute alcoholinduced liver injury is imperative.
The remarkable regenerative properties of the liver can change the balance between recovery and failure. Human Umbilical Cord Mesenchymal Stem Cells (hUCMSCs) have the potential to promote regeneration while decreasing the inflammatory response to the liver injury. In previously published work, our group demonstrated improvement in survival in mice with humanized livers that underwent liver injury with binge alcohol drinking using repeated 1 million hUCMSC injections [5]. In addition, survival was significantly improved using activated umbilical cord MSCs compared to non-activated cells and both were significantly better than placebo. Additionally, our work has shown that hUCMSCs may exert this protective effect by protecting against liver necrosis, apoptosis, and reducing the inflammation in the liver to allow for hepatocyte regeneration.
The primary goal of this study is to better determine the optimal dose of activated hUCMSCs, and to evaluate whether MSCs can be frozen and then thawed and maintain similar efficacy as was observed in our previous study, which would be essential for their use in clinical practice. Further, we evaluate for toxicities at various dose levels and follow up hepatic chemistries and histologic findings to better understand the necroptotic pathway in acute liver injury.
Preparation of humanized mice
After obtaining IACUC approval, we utilized the FRG KO liverhumanized mice. This process was conducted as per the original publication [5].
Breeding of FRG mice: Fah-/-; Rag2-/-; Il2rgc-/- (FRG) were generated by crossbreeding of Fah-/- mice (RIKEN) and Rag2-/-; Il2rgc-/- (Jackson Lab).
Irradiation of newborn FRG pups: Irradiation was performed at 250 kV, 16 mA, 50 cm FSD using 2 mm filter. Dose exposure rate (cGy): 150 cGy on a field size of 10 cm by 10 cm. Mice were housed in a pathogen-free facility with microisolator cages and monitored to assure there was no acute illness.
Transplant procedures: Human Hematopoietic Stem Cells (HSC) were collected from fetal liver (Donor cells). Human HSCs were prepared in sterile media and injected into the irradiated recipients intrahepatically. One day after irradiation, 5 × 10^5 cells were injected per mouse.
Sterilized water feeding: Animals were fed autoclaved/irradiated food and maintained on acidified autoclaved water with or without SMZ (7.8 ml of SMZ per 250 ml of drinking water) on alternate weeks for the duration of their lives after animals were weaned at 3 weeks of age. Animals were monitored daily by the investigator following irradiation and HSC transplant for potential adverse events and body weight was monitored.
In vivo blood studies of humanized immune cells in FRG mice: To determine if humanized immune cells are kept in FRG mouse blood stream at the enough levels, blood was collected from the facial vein to detect the human stem cell xenografted mice.
FACS analysis using peripheral blood cells from FRG mouse reconstituted with human HSCs: Approximately 100 μl of blood per mouse was collected in 1.5 ml sterile microcentrifuge tubes containing 100 μl of 20 mM PBS-EDTA and place it on ice. Peripheral Blood Mononuclear Cells (PBMC’s) were resuspended the bottom portion in red blood cell lysis buffer (1 × ACK lysis buffer), incubate for 5 min at room temperature (25°C). The cells were centrifuged at 469 g twice and resuspended in 2% (vol/vol) FBS/PBS containing human CD45, mouse CD45 antibodies and 7-AAD mixture. We measured the human immune reconstitution (% of human CD45+ cells/total CD45+ cells) was examined using flow cytometry analyses.
Preparation of umbilical cord mesenchymal cells and activated cells
Human umbilical cord-msc culture: Human Umbilical Cord Mesenchymal Stromal Cells (hUCMSCs) under informed consent were isolated from the perivascular Wharton’s Jelly region of the human umbilical cord were provided by RoosterBio Inc (Frederick, MD; RoosterVial-hUC-XF manufactured and sold by RoosterBio, INC and supported by licensed technology from Tissue Regeneration Therapeutics Inc. (TRT) core technology and patent family: US: 8,790,923; US: 8,278,102; US: 7,547,546; US: 9,611,456; US: 9,611,456; US: 8,481,311; US: 9,611,456). The purchased hUCMSC vials were additionally fully characterized according to the International Society for Cell and Gene Therapy’s (ISCT) minimal criteria performed by RB. RoosterBio further performed additional tests for hUCMSC characterizations for the expression of surface markers by flow cytometry, trilineage mesoderm differentiation potential (adipocytes, osteocytes, and chondrocytes), Indoleamine 2,3-dioxygenase (IDO) activity, sterility, endotoxin, and mycoplasma test (Data not shown) [7]. The hUCMSCs were cultured and harvested following RB manufacturing protocols. One million five hundred thousand to two million cells hUCMSCs were plated in T225 vented flask (Corning or ThermoFIsher) in 25 ml of RB complete medium RoosterNourish-MSC-XF (RoosterBio Inc.) and cultured for 48 hours and incubated at 37°C with 5% CO2.
Activation of hUCMSCs: At 36-38 hours after initial culture of hUCMSCs, PrimeGen’s Triple activation consisting of human TNF-a (PeproTech,Inc), human INF-g (PeproTech,Inc) and human IL-17 (PeproTech,Inc) was added to each T225 Flask with hUCMSCs at final concentration of 2 ng/mL for each cytokine [8]. Each hUCMSC Flasks were allowed to culture with added activation media for an additional 10-12 hours in at 37°C with 5% CO2 incubator.
Collection of hUCMSCs: Cell harvesting from theT225 flasks was performed as follows: Rooster culture medium was removed and washed with 10 mL D-PBS-/-(Gibco) and removed, then added 10 mL of CTS-TrypLE (Gibco) and incubated at 37°C for 5-6 mins. 10 mL of Rooster media was then added to quench the trypsin activity. Then cell suspension was removed, and T225 Flask was additionally washed with 25 ml D-PBS-/- and placed in centrifuge tube. All the cell suspension mixture was centrifuged at 280 × g for 10 min at 4°C and supernatant removed.
Freezing of activated hUCMSCs: Pelleted Activated hUCMSCs was resuspended in 1 ml of CS10 freezing media (BioLife) at a concentration of 5,000,000 or 10,000,000 cells/mL and aliquoted into 1.8 ml freezing vials (Nunc). Aliquoted Cell vials were then frozen using Planer Kryo-550-16 Control Rate Freezer (Planer Limited). Then transferred to vapor phase LN2 tank for storage until use.
Shipping cells and syringe preparation of hUCMSCs: One frozen vial of Activated hUCMSCs was thawed using ThawStar Automated Cell Thawing System (BioLife Solutions). Resuspend thawed frozen cells slowly in 7 ml of Complete Rooster Nourish Media. Frozen-thawed cell suspension tube was centrifuged at 280 × g for 10 min at 4°C. Remove supernatant and pelleted Activated hUCMSCs was resuspended in 10 mL of Rooster Nourish Complete Media. Cell counts were taken using NuceloCounter NC-200 Cell Counter (Chemometec). Cells were aliquoted at a concentration of ˜1,300,000 cells/mL in Complete Rooster Nourish Media into a 1.8 mL vial for each dose (this will ensure that 1,000,000 hUC-MSCs will be injected into each subject). Then, individual activated hUC-MSCs dose vials were placed into and transported using PrimeGen’s proprietary Validated 4°C Shipping Transportation Box. When ready to use for treatment groups, each vial with activated hUCMSC suspension was centrifuged at 280 × g for 10 min at 4°C. Remove supernatant and pelleted Activated hUC-MSCs were resuspended at a concentration of 1,300,000 cells in 200 uL Plasma-Lyte. The hUC-MSC/Plasma-Lyte solution (200 uL) was loaded into one U-100 BD Ultra-Fine Short Insulin Syringes (Beckton, Dickinson, and Company) for a tail vein injection in mice immediately.
Induction of liver injury and treatment
Binge Drinking: Humanized mice were fed high fat diet and alcohol binge drinking for 24 days. 62 mice survived the humanization process as well as the binge drinking and were then randomized into the following 6 treatment groups according to sex:
Group 1: Injected 1 million activated MSCs
Group 2: Injected 500,000 activated MSCs
Group 3: Injected 250,000 activated MSCs
Group 4: Injected 100,000 activated MSCs
Group 5: Injected 28,000 activated MSCs
Group 6: Injected vehicle Plasma-Lyte only
Mice were injected 3 times a week for the first week and then weekly for the remaining 2 weeks. Mice were injected via the tail vein. Each group was assigned 5 male mice and and 5 female mice except for the control group assigned 4 females and 6 males. The additional 2 female mice were assigned one each to Group 1 and Group 4.
Half of the mice began their first injection on Day 1 following binge drinking and half on Day 2 following binge drinking. The reason for dividing the beginning injection over two days was due to the time required to draw blood and inject the animals and maintain the appropriate documentation. Following the first injection, each group continued injections 3, 7, 14 and 21 days after the first injection and were followed for up to 28 days following the start of the first injections.
Mice were examined during the follow up period for body stance, grooming, respiratory rates, weight and food and water consumption.
Blood was drawn for AST and ALT prior to each injection and at the time of death. Necropsy was performed for mice that died before endpoint. Mice with symptoms were monitored and once their body conditions deteriorated to the threshold of euthanasia end point, mice were euthanized based on USC IACUC guideline.
Pathology analysis and evaluation of liver and blood of humanized FRG mice
We collected blood from FRG-hu HSC/Hep mice to measure human leukocyte reconstitution at 4 weeks post infection and at euthanasia endpoint, 60 days after initiation of MSC treatment (day 167). This blood was collected to measure human transplant efficacy of fetal liver cells. Blood was collected at baseline and at death to measure AST and ALT. After isoflurane vapormediated anesthesia, blood was collected form facial veins. At euthanasia, after Ketamine/Xylazine anesthesia, cardiopuncture was performed to collect blood. After euthanasia, mouse organs were collected.
Representative sections of liver tissue were fixed with neutral buffered 10% formalin, processed and hematoxylin eosin stained slides were preparedfor histological evaluation.
Statistical design
Median and inter-quantile range were reported for non-normally distributed continuous variables. Kruskal-Wallis test was used to test median across treatment levels.
Time-to-event data were analyzed using Kaplan Meier curve and Wilcoxon rank test. For post-hoc comparison of each treatment and control group, Sidak adjusted p-value was reported with multiple comparison adjustments. Significance level was 0.05, 2-sided. All analyses were performed using SAS 9.4 (SAS Institute Inc., Cary, NC, USA).
Group comparisons
In total 62 mice were included in our study. Median age was 9 weeks with no difference noted between groups, p=0.72. There were 33 female mice and 29 male mice. There was no difference in sex noted between groups (p=0.99). Baseline liver function tests were measured with median AST 231.5 with no difference noted between groups (p=0.13). Baseline median ALT was 298.5 and there was a significant difference noted between groups with the lowest value noted in 100,000 cell group with median 228 and highest in the 500,000 cell group with median 384.5 (p=0.002) (Tables 1A and 1B).
| Total | Placebo | Tx 28,000 cells | Tx 100,000 cells | Tx 250,000 cells | Tx 500,000 cells | Tx 1 million cells | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N | Median (Q1-Q3) | N | Median (Q1-Q3) | N | Median (Q1-Q3) | N | Median (Q1-Q3) | N | Median (Q1-Q3) | N | Median (Q1-Q3) | N | Median (Q1-Q3) | p-value | |
| Age (weeks) | 62 | 9 (8-10) | 10 | 9 (8-10) | 10 | 9.5 (9-10) | 11 | 9 (9-10) | 10 | 8.5 (8-10) | 10 | 9 (8-9) | 11 | 9 (8-10) | 0.72 |
| AST at baseline | 62 | 231.5 (188-270) | 10 | 211 (183-383) | 10 | 244 (207-311) | 11 | 234 (194-289) | 10 | 218 (128-253) | 10 | 249 (245-336) | 11 | 169 (120-239) | 0.13 |
| ALT at baseline | 62 | 298.5 (228-378) | 10 | 372.5 (291-401) | 10 | 242 (160-345) | 11 | 228 (178-267) | 10 | 270.5 (182-338) | 10 | 384.5 (355-514) | 11 | 308 (264-353) | 0.002 |
Table 1A: Age and baseline AST and ALT for all 6 cohorts.
| Total | Placebo | Tx 28,000 | Tx 100,000 cells | Tx 250,000 cells | Tx 500,000 cells | Tx 1 million cells | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N | % | N | % | N | % | N | % | N | % | N | % | N | % | p-value | |
| Sex | 0.99 | ||||||||||||||
| F | 33 | 53.2 | 6 | 60 | 5 | 50 | 6 | 55 | 5 | 50 | 5 | 50 | 6 | 55 | |
| M | 29 | 46.8 | 4 | 40 | 5 | 50 | 5 | 46 | 5 | 50 | 5 | 50 | 5 | 46 | |
Table 1B: Sex for all 6 cohorts.
Survival
Overall male mice had an improved survival as compared to female mice, but as previously mentioned sex was equal amongst groups, (p=0.03) (Supplementary Figure 1). A survival benefit was noted for mice that received 1 million cells and was the only cohort found to have significantly better survival than the Placebo group (p=0.03) (Table 2) (Figure 1).
| Number of cells per injection compared to Placebo | Adjusted p-value |
|---|---|
| 28,000 | 0.81 |
| 1,00,000 | 0.27 |
| 2,50,000 | 0.18 |
| 5,00,000 | 0.46 |
| 1 million | 0.03 |
Table 2: Pairwise comparison of treatment groups as compared to placebo.
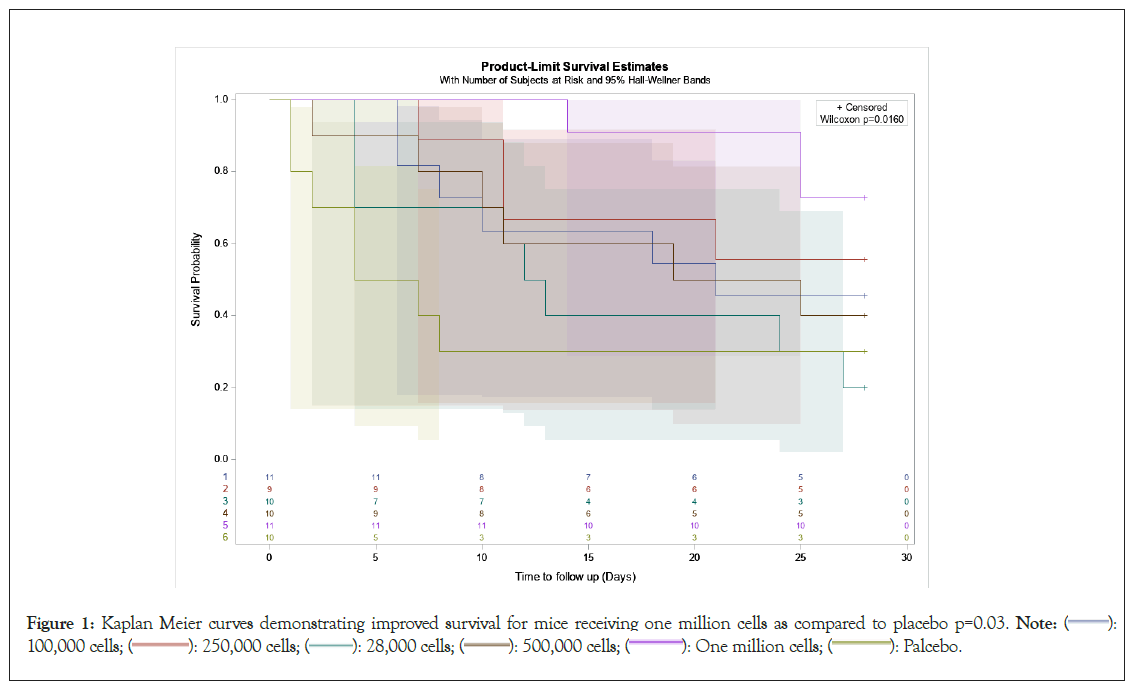
Figure 1: Kaplan Meier curves demonstrating improved survival for mice receiving one million cells as compared to placebo p=0.03. Note: ( ): 100,000 cells; (
): 100,000 cells; ( ): 250,000 cells; (
): 250,000 cells; ( ): 28,000 cells; (
): 28,000 cells; ( ): 500,000 cells; (
): 500,000 cells; (  ): One million cells; (
): One million cells; (  ): Palcebo.
): Palcebo.
Post treatment laboratory values
Median last AST and ALT for all mice was 221 and 280.5 respectively. No differences were noted amongst groups, AST (p=0.4) and ALT (p=0.14). Change from baseline of AST was +7 amongst all patients and no difference was noted amongst treatment groups (p=0.12). Median change of ALT-47 amongst all mice. The largest increase was seen in the placebo group of +153.5 as compared to the largest decrease noted in the 500,000 cell group of -156.5 (p=0.01) (Table 3).
| Total | Reference | Tx LV1 | Tx LV2 | Tx LV3 | Tx LV4 | Tx LV5 | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N | Median (Q1-Q3) | N | Median (Q1-Q3) | N | Median (Q1-Q3) | N | Median (Q1-Q3) | N | Median (Q1-Q3) | N | Median (Q1-Q3) | N | Median (Q1-Q3) | p-value | ||
| Last AST | 48 | 221 (165-275) | 4 | 347 (277.5-369.5) | 7 | 221 (149-255) | 9 | 185 (140-249) | 9 | 235 (210-305) | 8 | 197 (187-250) | 11 | 193 (163-272) | 0.4 | |
| Last ALT | 48 | 280.5 (206-336) | 4 | 379.5 (371.5-449.5) | 7 | 252 (203-351) | 9 | 297 (202-321) | 9 | 293 (190-410) | 8 | 272 (205.5-334.5) | 11 | 244 (211-278) | 0.14 | |
| Change of AST | 48 | 7 (-51-59.5) | 4 | 140 (29.5-184) | 7 | 8 (-78-59) | 9 | 8 (-47-60) | 9 | 41 (3-108) | 8 | -51 (-85.5--27.5) | 11 | 25 (-36-43) | 0.12 | |
| Change of ALT | 48 | -47 (-98.5-76.5) | 4 | 153.5 (11-183) | 7 | 67 (-155-191) | 9 | 54 (28-111) | 9 | -23 (-55-99) | 8 | -156.5 (-211--93.5) | 11 | -59 (-97--48) | 0.01 | |
Table 3: Post treatment laboratory values.
Histologic findings
All mice except for three exhibited histologic evidence of alcohol injury at death either prior to the 4 weeks or at euthanasia. The different findings that were seen included necrosis or varying degrees of steatosis. Table 4 demonstrates the various findings according to dose of activated cells and survival to the end date of the study.
Of the 35 mice that did not survive, 23 had necrosis, 25 had steatosis and 3 had not significant findings at death. Of the surviving mice, none had necrosis and 27 had 1 to 2+ steatosis. None of the surviving animals had no findings (Table 4) (Figure 2) (Supplementary Table 1).
| Dose (Activated stem cells) | Survived to end date | Number | Necrosis present | 1-2+ Steatosis present | 3+ Steatosis present | No significant findings |
|---|---|---|---|---|---|---|
| 1 million | No | 3 (27%) | 2 | 0 | 3 | 0 |
| 1 million | Yes | 8 (73%) | 0 | 8 | 0 | 0 |
| 5,00,000 | No | 6 (60%) | 4 | 2 | 2 | 0 |
| 5,00,000 | Yes | 4 (40%) | 0 | 4 | 0 | 0 |
| 2,50,000 | No | 5 (50%) | 3 | 2 | 3 | 0 |
| 2,50,000 | Yes | 5 (50%) | 0 | 5 | 0 | 0 |
| 1,00,000 | No | 6 (54%) | 5 | 5 | 0 | 0 |
| 1,00,000 | Yes | 5 (46%) | 0 | 5 | 0 | 0 |
| 28,000 | No | 8 (80%) | 5 | 2 | 0 | 2 |
| 28,000 | Yes | 2 (20%) | 0 | 2 | 0 | 0 |
| Placebo | No | 7 (70%) | 4 | 5 | 1 | 1 |
| Placebo | Yes | 3 (30%) | 0 | 3 | 0 | 0 |
Table 4: Histologic findings of explanted livers after treatment.
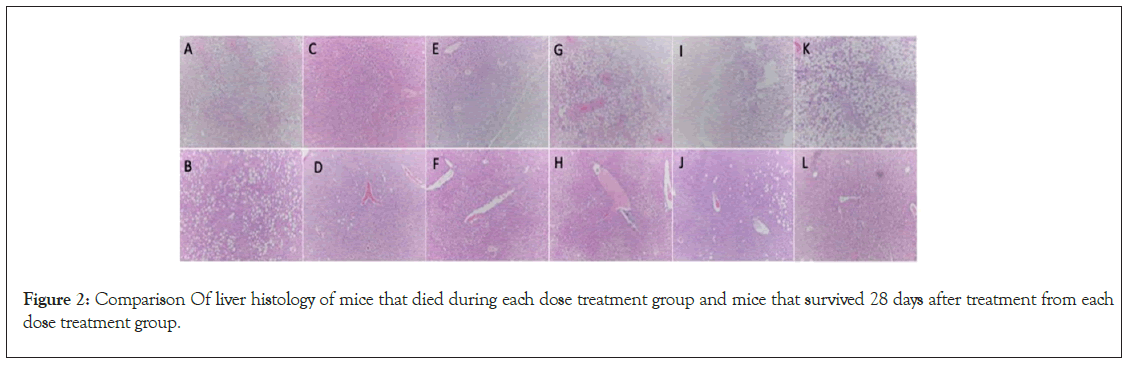
Figure 2: Comparison Of liver histology of mice that died during each dose treatment group and mice that survived 28 days after treatment from each dose treatment group.
From the above Figure 2, (A): Placebo mouse #604 died showing moderate (2+) steatosis, Zone 3 patchy necrosis HE × 100x; (B): Placebo mouse #606 survived 28 days after treatment showing moderate (2+) steatosis, HE × 100x; (C): 28,000 activated MSC mouse #645 died showing moderate (2+) steatosis, zone 3 patchy necrosis HE × 100x; (D): 28,000 Activated MSC mouse #654 survived 28 days after treatment with mild (1+) steatosis, HE × 100x; (E): 100,000 activated MSC mouse #658 died showing moderate (2+) steatosis and marked areas of confluent necrosis, HE × 100x; (F): 100,000 activated MSC mouse #119 survived 28 days after treatment showing mild (1+) steatosis, HE × 100x; (G): 250,000 activated MSC mouse #701 died showing marked (3+) steatosis and areas of confluent necrosis, HE × 100x; (H): 250,000 activated MSC mouse #607 survived 28 days after treatment showing mild (1+) steatosis, HE × 100x; (I): 500,000 activated MSC mouse #691 died showing marked (3+) steatosis and areas of confluent necrosis, HE × 100x; (J): 500,000 activated MSC mouse #632 survived 28 days after treatment showing mild (1+) steatosis, HE × 100x; (K): 1,000,000 activated MSC mouse #727 died showing marked (3+) steatosis, focal zone 3 necrosis HE × 100x; (L): 1,000,000 activated MSC mouse #601 survived 28 day after treatment with mild (1+) steatosis, HE × 100x.
Safety
Measuring food and liquid consumption during the study period, Figure 3A demonstrates that there was no statistical difference in consumption between the study groups. The same is true of body weight (Figure 3).
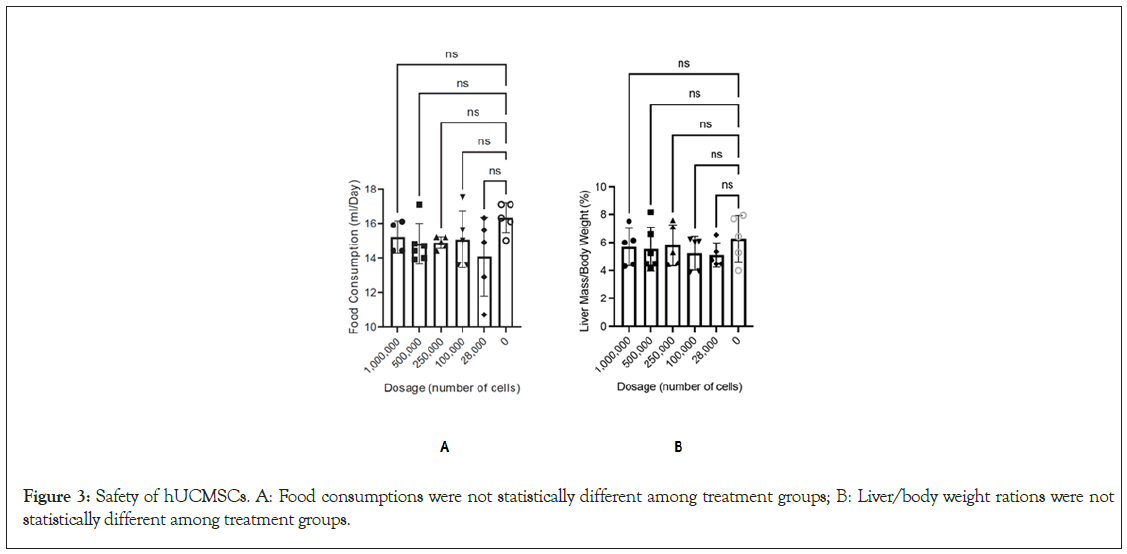
Figure 3: Safety of hUCMSCs. A: Food consumptions were not statistically different among treatment groups; B: Liver/body weight rations were not statistically different among treatment groups.
Explant alcoholic hepatitis patient liver have low BCL2 while MSC-indirect coculture induced BCL2
To further validate mechanistic link between tissue protection mechanisms by MSC injection, explant liver tissues from alcoholic hepatitis patients were cocultured with MSCs. MSC conditioning media have almost no effect, while MSC indirect coculture for 48 hours induced BCL2 proteins in MSC-coculture groups in Boyden chamber. These results indicate that alcoholic hepatitis patient liver have residual BCL2 protein expression while MSC secreted factors may induce BCL2 proteins (Figure 4).
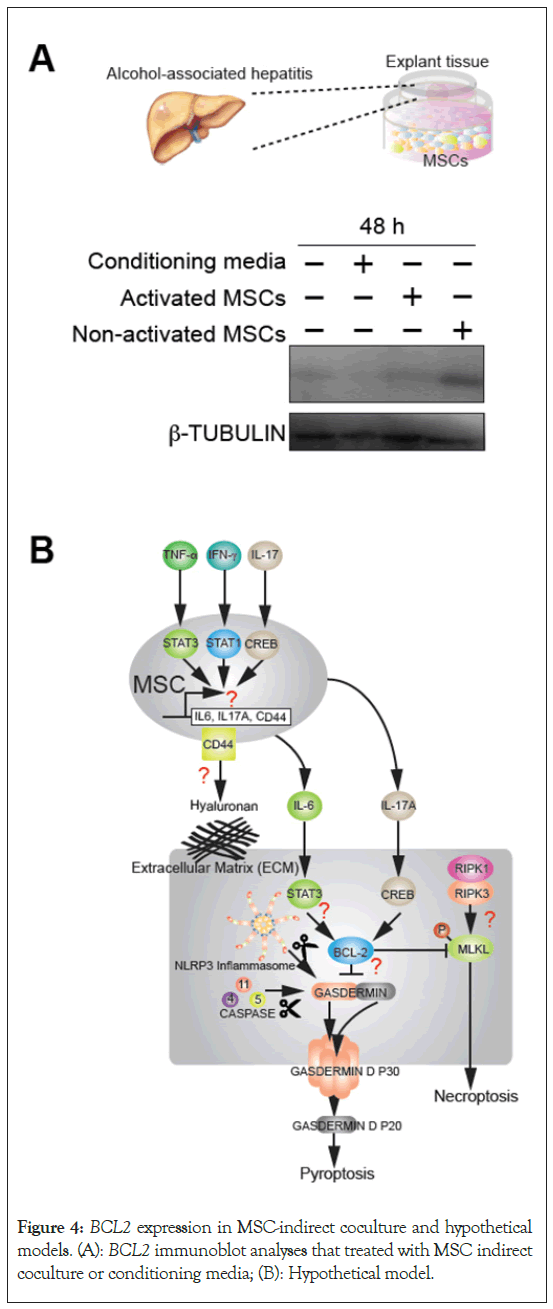
Figure 4: BCL2 expression in MSC-indirect coculture and hypothetical models. (A): BCL2 immunoblot analyses that treated with MSC indirect coculture or conditioning media; (B): Hypothetical model.
Alcohol-associated liver disease continues to represent a major health crisis in the United States and has only worsened during the COVID-19 pandemic [1,9]. Unfortunately, treatment strategies for this disease are limited to supportive care or liver transplantation as salvage for those without liver recovery. Given the persistent organ shortage and need for equitable distribution of organs, durable treatments for alcohol-associated liver disease are needed. In this study we have demonstrated that frozenthawed activated hUCMSCs at high doses are associated with a survival advantage in mice that have undergone a binge drinking protocol. In addition, we have utilized frozen-thawed activated hUCMSC demonstrating a ready to use efficacious therapy for a potential practical approach for acute liver failure treatment.
Alcohol, through its activation of both the innate and adaptive immune response, has been shown induce inflammation in the liver via multiple signaling pathways [10]. Unlike normal hepatocyte apoptosis, alcohol-related hepatocyte death is related to inflammation and mediated by necroptosis and pyroptosis pathways [11]. One strategy currently employed to reduce inflammation and liver disease in alcohol associated liver disease is the use of high dose corticosteroids. This is predicated on the hypothesis that TNF-induced inflammation is the cause of hepatocyte dysfunction [12]. While recommended in certain scenarios by the American Association for the Study of Liver Disease, the use of corticosteroids remains controversial with no clear level-one evidence supporting its efficacy [13,14]. Additionally, in a recent clinical trial where corticosteroids were combined with direct TNF inhibitors, no survival advantage was noted [15]. More recently, MSCs have garnered attention as a possible therapeutic strategy given their ability to not only reduce liver inflammation but also their ability to differentiate into hepatocyte-like cells to promote liver regeneration [16]. While hepatocyte-like cells do not fully differentiate into fully mature hepatocytes, they do have a similar morphology as well as the ability to carry out similar synthetic liver function [17]. In our study, we demonstrate that multiple high-dose activated MSC treatments were associated with a significant survival advantage, thus replicating our results from a previous study [5]. We have also demonstrated that with decreasing dosage the survival benefit was lost, indicating that there could be a potential dosedependent response. In a study by Shi, et al. in which hUCMSC were used with success in adults with acute-on-chronic liver failure, a dose of approximately 0.5 × 106 hUCMSCs per kilogram was utilized without any clear methodology as to the dosing decision [18]. Additionally, when hUCMSCs have been studied in liver cirrhosis, dosages have ranged from single fixed administration; single weight-based administration, and repeated weight-based administration [19-21]. Given our findings in a mouse models of decreased efficacy with multiple lower dosage of cells and the lack of any clear dosing guidelines in previous studies, it will be paramount that appropriate dosing strategies are employed in human studies in order to adequately evaluation the clinical response to activated hUCMSCs.
Liver necrosis, much like the presence of inflammation, in alcohol associated liver disease has been thought to be due to upregulation of TNF-α as well as other pro-inflammatory cytokines and Damage Associated Molecular Patterns (DAMPs) [22]. As previously stated, the goal of high-dose corticosteroids is to diminish this inflammatory response and prevent hepatocyte necrosis. Unfortunately, in steroid non-responders, there is clear mitochondrial damage and the liver lacks the ability to undergo hepatocyte proliferation [23]. Further, when the immune system is under constant stimulation by DAMPs there is a switch from a proinflammatory state to an immunodeficient stage which is believed to contribute to the underlying physiology of acute-onchronic liver failure [24]. While direct TNF-α inhibition has not been successful, hUCMSCs have been shown to both ameliorate the inflammatory response as well as DAMP stimulation [15,25,26]. In previous work we have demonstrated that activated hUCMSC downregulate one such DAMP, Receptor-Interacting Protein Kinase (RIPK3), a key mediator in necroptosis, which when inhibited has been shown to markedly decrease hepatic necrosis in animal nodels [5,27]. While previous in human studies have inferred pathologic liver recovery when utilizing hUCMSCs through improvement in laboratory studies such as AST and ALT, we did not observe any laboratory value changes in our mouse model [18]. We did, however, observe a clear lack of necrosis on histologic examination in mice treated with high dose activated hUCMSCs, further supporting our previous findings of a protective effect from necroptosis. In future work, we plan to elucidate the mechanism in which activated hUCMSCs facilitate this protective response.
One of the many challenges of cell-based therapies is the implementation into clinical practice. Harvesting, isolating, and expanding hUCMSCs requires both significant infrastructure and technical expertise. One way to make this therapy more widely available is through cryopreservation in an “off the shelf” model. Within the literature, the use of thawed cryopreserved hUCMSCs has been met with mixed success due to what has been described as a “cryo-stun effect,” in which thawed hUCMSCs do not regain full potency [28]. In a recent systemic review of 41 studies of bone marrow-derived, thawed MSCs, the MSCs maintain their general morphology but there did appear to be a loss in metabolic activity when cells were thawed [29]. Despite this theorical limitation, thawed MSCs have previously been shown to be effective in animal models for both allergic airway disease as well as tendon rupture [30,31]. In this study we have demonstrated that multiple high doses of thawed activated hUCMSCs were associated with improved survival as well as decreased liver necrosis in our mouse models. These findings were consistent with our previous work which was conducting using fresh cells [5]. Additionally, this work serves as an important practical translational application for activated hUCMSCs’ use. Historically, the majority of animal studies have involved fresh cells while studies in humans are often undertaken with thawed cell preparations [32]. Importantly, when we look at the overall safety of the thawed cells, we did not observe any changes in body weight or food intake between the two groups. A recent review by Vimalesvaran, et al., examined 21 trials using MSCs in liver disease spanning acute liver failure, cirrhosis, and posttransplant recovery that MSCs had no significant adverse events [33]. Through the use of thawed activated hUCMSCs, we have demonstrated a potential for an “off the shelf” immediate use therapy for patients with acute alcohol hepatitis that is potentially associated with minimal, if any adverse events.
This study demonstrates promising data for a potential treatment for acute alcohol hepatitis as well as addresses two key questions.First, we have demonstrated that efficacy of treatment with activated hUCMCSs is dose-dependent and that high doses are required to see a survival advantage. Secondly, we have demonstrated that thawed activated hUCMSCs appear efficacious and did not yield any adverse effects, providing the possibility of future broad clinical application. Our future studies will focus on further understanding the protective mechanism of activated hUCMSCs to eventually move towards clinical applications. The multiple high-dose thawed activated hUCMSCs are associated with improved survival and histologic changes in mice with alcohol induced liver disease.
The study is fully funded by PrimeGenUS, INC. Joel Marh Karina Zaragoza and Julia Kim are employed by PrimeGenUS INC, Linda Sher and Leonard Makowka have been consultants for PrimeGenUS INC.
This work was supported by grants UL1TR001855 and UL1TR000130 from the National Center for Advancing Translational Science (NCATS) of the U.S. National Institutes of Health. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Juan Carlos Hernandez, Yicheng Aiden Zhu and Sean P. Martin authors contributed equally.
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
Citation: Hernandez JC, Zhu YA, Martin SP, Kohrman N, Yeh DW, Marh J, et al. (2024) Activated Frozen Mesenchymal Stem Cells in Humanized Mice after Induction of Acute Liver Injury through Alcohol Binging. Int J Phys Med Rehabil. 12:723.
Received: 16-Feb-2024, Manuscript No. JPMR-24-29603; Editor assigned: 19-Feb-2024, Pre QC No. JPMR-24-29603 (PQ); Reviewed: 06-Mar-2024, QC No. JPMR-24-29603; Revised: 14-Mar-2024, Manuscript No. JPMR-24-29603 (R); Published: 22-Mar-2024 , DOI: 10.35248/2329-9096.24.12.723
Copyright: © 2024 Hernandez JC, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.