Journal of Chromatography & Separation Techniques
Open Access
ISSN: 2157-7064
ISSN: 2157-7064
Research Article - (2014) Volume 5, Issue 6
Activity concentrations of Cs radioisotopes (137Cs and 134Cs) were measured in four soil samples at the proximity of Fukushima daiichi nuclear power plant (FDNPP), located in and off the radioactive plume direction. Activity of Cs was higher than 90Sr and 238U in the plume direction. Lowest 137Cs activity was found to be 950 ± 13 Bq/kg and highest was 62,200 ± 880 Bq/kg. In case of 90Sr, the lowest and highest activity concentrations were found to be 8.4 ± 1.5 Bq/kg and 21.2 ± 2.6 Bq/kg respectively. Activity concentration of 238U varied from 25.4 ± 0.1 Bq/kg to 45.2 ± 0.1 Bq/kg. Activity ratio of 134Cs to 137Cs was in range of 0.84-0.87 measured in 2012, indicates the origin from FDNPP accident. Activity ratio of 234U/238U varied from 0.996-1.029. To establish the fate and transfer of radionuclides, site specific distribution coefficient (Kd) were measured in the soil samples using standard method. Kd values for Cs, Sr and U were found to vary from (114 ± 3 to 404 ± 43), (65 ± 0.2 to 154 ± 7) and (1640 ± 22 to 8563 ± 458) L/kg respectively. Different soil parameters like particle size distribution, pH, organic content, cation exchange capacity, CaCO3, elemental and oxide composition of soil has been carried out to understand the geochemical behaviour of these radionuclides. A good correlation was observed for Kd (Cs) and Kd (Sr) with cation exchange capacity and fine particle concentration and Kd (U) with Fe and organic content of soil.
Keywords: 137Cs; 90Sr; 238U; Activity ratio; Distribution coefficient; FDNPP
A catastrophic earthquake of magnitude (9.0) followed by tsunami on 11 March 2011, caused a major nuclear accident at the Fukushima Daiichi Nuclear Power Plant (FDNPP) about 250 km north to Tokyo, capital of Japan and inundated the nuclear site with seawater. The damage by the flooding of the site resulted in loss of cooling to the three reactor units. This led to eventual overheating, hydrogen explosions and a probable partial melting of the core of the three reactors [1]. This accident resulted in a substantial release of radioactive materials to the atmosphere, ocean, and has caused extensive contamination to the environment [2-4]. The nuclear accident was eventually classified at Level 7, the highest on the International Nuclear and Radiological Event Scale (INES) [5], the same as given as the Chernobyl Nuclear Power Plant accident (CNPP) in 1986.
Environmental monitoring with respect to different radionuclides is very important to understand the cause and severity of accident. Similarly, radionuclides with long half lives and their high solubility in aqueous solution under normal environmental conditions are of major concern from radiological safety point of view. During the accident significant deposition of 137Cs occurred on surface soils of Fukushima prefecture [6,7] as fallout. Due to its relatively long halflife of 30.2 years and chemical behaviour similar to that of potassium, 137Cs is considered as one of the most significant radionuclide in the environmental radioactivity monitoring. Measurements of Chernobyl fallout clearly indicate that soil is the main reservoir of 137Cs but its migration behaviour and associated profile distribution are site-specific and depend on soil characteristics and environmental conditions [8]. High volatile fission products including 90Sr, 129mTe, 131I, 134Cs and 137Cs released into the atmosphere and caused radioactive contamination of soils over a wide area [7,9]. 90Sr is chemically similar to calcium and it has a high transfer rate to the skeletal system. It leads to internal irradiation which can cause bone cancer, cancer of soft tissues, leukemia inside the human bones [10] together with its decay product 90Y. Therefore 90Sr and 90Y belong to the most hazardous fission products. From environmental monitoring point of view 90Sr is also of major concern, due to its high solubility. The chemical toxicity of the uranium metal is the primary environmental health hazard, whereas radioactivity of uranium a secondary concern. Exposure to uranium can result in both chemical and radiological toxicity. The update of the toxicologic evidence on uranium adds to the established findings regarding nephrotoxicity, genotoxicity, and developmental defects. Additional novel toxicological findings include some at the molecular level that raise the biological plausibility of adverse effects on the brain, on reproduction, including estrogenic effects, on gene expression, and on uranium metabolism [11]. For a better radiological impact assessment due to radionuclide contamination in ecosystems apart from the information on radionuclide species, knowledge on their interactions in soil-water systems influencing mobility and biological uptake is essential. Uranium has significant importance in radio-ecology because of its high solubility as U (VI). For prediction of radionuclide-soil interaction and subsequently transportation of radionuclides in the soil column, site specific distribution coefficient, Kd plays an important role.
Present work emphasises monitoring of different radionuclides like Cs, Sr and U in soil samples affected by FDNPP accident in order to know the extent of contamination. Further to understand their geochemical behaviour, the site specific distribution coefficients (Kd) values for the respective nuclides have been determined experimentally. The sorption and migration of these nuclides in soil could be influenced by many factors such as the nature of the radionuclides in solution as well as chemical and mineralogical nature and physical environment of the soil. Therefore in the present study the soil samples were also chemically characterized with respect to different soil parameters controlling sorption of these stable/radionuclides. Laboratory batch method was used for measurement of Kd in soil collected around FDNPP. Expecting contamination of soil samples with radio isotopes of Cs, Sr and U as a consequence of accident, stable isotopes are used for Cs and Sr whereas depleted uranium is used as tracer for uranium Kd estimation.
Sampling location
Sampling sites have been selected based on contamination level measured by gamma dose rate, which was influenced by radioactive plume direction during accident. Two contaminated soil samples were collected in the plume direction, FS1 (N:37.4140°, E:141.0020°) and FS2 (N:37.4240°, E:141.0004°) with measured dose rate 71 and 85 μSv/h respectively on 24th Nov, 2011. Apart from these, two more soil samples have been collected from less contaminated area in the opposite direction, FS3 (N:37.1982°, E:140.9598°) and FS4 (N:37.1683°: E:140.9894°) with measured dose rate 0.50 and 0.45 μSv/h respectively on 23rd Nov, 2011. All the four samples were within 20 km from FDNPP as shown in Figure 1.
Sample collection and processing
Surface soil samples were collected by a core sampler from a depth of 0-10cm.The samples were transferred to laboratory and air dried at room temperature to a constant weight followed by sieving using a 2 mm sieve for estimation of Kd and soil parameters. Sieved samples were further oven dried at 1100C for 24 hours and were packed in a plastic U-8 standard cylindrical containers (diameter=48 mm; h=58 mm) for γ-spectrometry measurement. Sieved and oven dried samples were further powdered (<150 μm) for chemical characterization. Powdered soil samples were chemically digested using microwave digestion system (Milestone, MLS 1200 Mega) for elemental analysis. Since Kd is highly dependent on ionic strength of water [12], instead of using distilled water or synthetic water, ground water samples were collected from the corresponding sites of soils to measure Kd values experimentally. The samples were filtered using 0.45 μm membrane filter and divided into two parts. One part was acidified for elemental analysis and nonacidified part water was used for Kd estimation.
Instrumental analysis
Gamma spectrometry: A high-purity germanium detector (ORTEC GEM-100210) γ-spectroscopy coupled with a multi-channel analyzer (ORTEC-7700-010) with a range 0-4000 keV and gamma studio software (Seiko EG&G, 2000) was used for the measurement of 134Cs and 137Cs. The detector efficiency was determined using a 100 g multi-nuclide standard source supplied by Japan Radioisotope association with quoted gamma energies ranging from 60 to 1333 keV an overall uncertainty of less than 5%. The results were compared with a certified soil standard IAEA-375 to check the reproducibility of the method. The counting time was varied from 10,000 sec to 72,000 sec depending on the activity concentration. In the case of radioactivity concentration for 137Cs, it was measured at energy of 662 keV whereas for 134Cs concentration was derived from net peak areas due to 605 keV and 796 keV gamma rays [13].
Liquid scintillator: The 90Sr activity was determined by a Wallac 1414-003 liquid scintillation spectrometer. Separation of strontium took place on a column filled with Sr-Spec (Eichrom Ltd). The resin was loaded on a column of 6 mm in diameter and 10 cm in height. The column was conditioned with 50 ml of 8 M HNO3 and the sample was passed through the column. Strontium was later eluted afterwards together with some possible traces of lead from the column with 50 ml of double distilled water (DDW). Strontium was separated from lead by precipitation of lead iodide [14] and then filtration. Solution containing strontium was evaporated to dryness, iodine excess present in solution was destroyed by addition of concentrated HNO3, evaporation and then addition of a few drops of peroxide followed by next evaporation to dryness. Dry residue (almost invisible) was dissolved in a mixture of 1.5 ml of 1 M HNO3 and 1.5 ml of DDW and then transferred to a 15 ml scintillator plastic vial. Those samples were analyzed by an HPGe low background gamma spectrometer to determine the recovery of 85Sr. Then, this solution was mixed with 10 ml of a liquid scintillation cocktail (HiSafe 3) and, after waiting two weeks for the equilibrium between 90Sr and its daughter, 90Y (T1/2=64 h), was subject of LSC measurement. Typical counting time was 30,000 s.
Inductively coupled plasma mass spectrometry (ICP-MS): Inductively coupled plasma mass spectrometry (Agilent 7500, Agilent Technologies, USA) was used for the measurement of stable isotopes of Cs, Sr and U in both soil and water, which yielded detection limits of 0.03 μg/L for Cs, 1.17 μg/L for Sr and 0.001 μg/L for U. The ICPMS detection limit was calculated as three times the standard deviation of the calibration blank measurements (1:1 v/v HNO3: MilliQ water, n=10). The relative error of ICP-MS results for the reference sample (lake sediment JLK-1) for 238U was 0.56 %. The parameters for data acquisition and optimization conditions are reported elsewhere [15].
Thermal ionisation mass spectrometry (TIMS): A VG Sector 54-30 thermal ionization mass spectrometer (VG Isotopes Ltd., UK), equipped with nine Faraday cup collectors and Daly ion detection system positioned behind axial Faraday and wide aperture retardation potential (WARP) energy filter, was used for the isotopic measurement of uranium. U was separated and pre-concentrated using a combination of anion exchange and extraction chromatography from digested soil [15]. First column was prepared by using pre-cleaned anion exchange resins (Dowex 1X-8, 200-400 mesh, Cl- form) and packed into MUROMAC polypropylene column (10 x 120 mm size) up to a height of 8 cm. The second column was packed with 0.5 ml of pre-cleaned UTEVA resin (Eichrom industries Inc.) 100-150 μm particle size in MUROMAC polypropylene column (7 x 60 mm size). The UTEVA resin contains diamylamylphosphonate (DAAP) as a specific extractant. Anion exchange column was first conditioned by passing 50 ml of 8 M HNO3. The digested soil sample in 8 M HNO3 (5 ml) was transferred to anion exchange column followed by a washing of 80 ml 8 M HNO3. Last 50 ml washing was collected after passing through anion exchange column, discarding the initial 30 ml washing of 8 M HNO3 . The UTEVA column was conditioned with 1.5 ml of 4 M HNO3. The washing solution collected from the first column was evaporated completely and taken in 1 ml of 4 M HNO3 to pass through UTEVA followed by a washing of 5 ml of 4 M HNO3. Then column was washed with 6 ml of 5 M HCl to remove Th and finally uranium was eluted from UTEVA column with 6 ml of 0.1 M HNO3. Complete organic destruction was carried out in the eluent collected from UTEVA column by treating with H2O2 and HNO3 before loading onto TIMS filament. The recovery for uranium separation by this method was checked using ICP-MS and was found to be 80 ± 5 %.
Inductively coupled plasma optical emission spectrometry (ICPOES): Major cations from soil and water samples were analysed using an inductively coupled plasma optical emission spectrometry (CCD simultaneous ICP-OES, VISTA-PRO, Seiko Instrument Inc.)
X-ray fluorescence spectrometer (XRF): Major oxide compositions in soil was analysed by X-ray fluorescence spectrometer (Rigaku ZSX 100e, Rigaku Corporation).
Estimation of distribution coefficient: Distribution coefficient, Kd for Cs, Sr and U nuclides were measured using laboratory batch method [16]. 1 g of air dried, homogenized soil sample (<2 mm size) was taken in triplicate in a 50 ml polypropylene centrifuge tube with screw cap and was equilibrated with 30 ml of water for 12 hours in a shaker with a shaking rate of 0.8-1.2, oscillations per second at 200C. Solution mixture was centrifuged at 4500 rpm for 30 minutes and filtered through 0.45 μm filter paper and the supernatant solution was discarded and the procedure was repeated to ensure no change in the pH of the suspension as a result of the equilibration. 30 ml water, spiked with the radiotracer/stable nuclide, was then added to the soil and the suspension was kept in the shaker and equilibrated for 72 hours which have been determined earlier. 3.3 μg/ml of stable Cs (CsNO3), 1.6 μg/ ml of stable Sr (Sr(NO3)2) and 3.3 μg/ml of U (UO2 (NO3)2) were used as tracer. The amount of stable nuclides released during sorption already present in soil and water is negligible compared to the spike amount. Solution mixture was centrifuged at 4500 rpm for 30 minutes and filtered through a 0.45 μm filter paper. The amount of adsorbed Cs, Sr and U were estimated from their concentration in the aqueous phase before and after the adsorption. Three control solutions of ground water and river water containing tracers were prepared for the determination of initial activity (Ao). Kd has been calculated using the following equation:
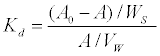
where, VW is the volume of water in the suspension, WS is the weight of soil, A0 and A are initial activity and activity at equilibration respectively. Activity sorbed on the wall of polypropylene tube was found to be negligible in the present case for the three nuclides.
Physico-chemical characterization of soil and water: Some selected properties were analysed in soils of <2 mm size fraction. Particle size analysis of soil samples was carried out using different mesh sieves for sand (2-0.05 mm), silt and clay (<0.05 mm). Soil parameters like pH was measured using 1:2.5 soil: water suspension for 1 hour using a pH meter and concentrations of exchangeable base cations (Ca+2, Mg+2, Na+ and K+) extracted with 1 N ammonium acetate at pH 7 were determined using ICP-OES. Organic content (OC) has been determined by loss of ignization method [17]. Major and trace elements were analysed in water samples using ICP-OES and ICP-MS respectively.
Distribution of Caesium, Strontium and Uranium radio isotopes and their activity ratio in soil affected by FDNPP
Activity concentrations for different radionuclides (Cs, Sr and U) in four soil samples are given in Table 1. A large variation in 137Cs activity was observed with lowest activity 950 ± 13 Bq/kg to highest activity 62,200 ± 880 Bq/kg. This is mainly because, the deposition of Cs released during nuclear power plant accident was local and strongly dependent on meteorological conditions-predominantly the radioactive plume direction during accident. For samples collected in the North West direction (FS-1 and FS-2), the caesium radioisotopes activity observed was much higher while compared to the samples collected from opposite direction (FS-3 and FS-4). However, 134Cs and 137Cs ratio was found to be same in all the samples (0.84-0.87 as measured in 8th May 2012), indicates that contamination comes from the accident. In case of 90Sr activity, it varied from 8.4 ± 1.5 Bq/kg to 22.3 ± 1.5 Bq/kg, which was much lower than Cs activity. The relatively low levels of 90Sr and a lack of any clear relation to caesium activity in samples (Table 1) suggest that predominantly strontium comes from global fallout, although one cannot exclude traces of FDNPP origin, which are masked by local variation of global fallout remains. It is very difficult to say something certain on 90Sr origin in samples (after decay of any possible addition of short lived 89Sr) since strontium generally is not conserved in soils, so its activity ratio to caesium as measured in soil samples even just a year after fallout can be much lower than it was in fallout originally. Activity concentration for 238U was found to vary from 25.4 ± 0.11 Bq/kg to 45.2 ± 0.1 Bq/kg.
| Sample | 134Cs (Bq/kg) | 137Cs (Bq/kg) | 90Sr (Bq/kg) | 238U (Bq/kg) | 134Cs/137Cs | 234U/238U |
|---|---|---|---|---|---|---|
| FS-1 | 53,400±770 | 62,200±880 | 18.4 ± 3.4 | 31.7±0.1 | 0.86±0.02 | 1.015±0.002 |
| FS-2 | 53,200±770 | 61,830±880 | 8.4 ± 1.5 | 49.2±0.2 | 0.86±0.02 | 1.029±0.001 |
| FS-3 | 850±20 | 1,010±20 | 21.2 ± 2.6 | 25.4±0.1 | 0.84±0.03 | 0.996±0.011 |
| FS-4 | 830±20 | 950±13 | 22.3 ±1.5 | 45.2±0.1 | 0.87±0.03 | 1.006±0.003 |
Table 1: Activity concentrations and activity ratio of Cs, Sr and U in soil around FDNPP.
Generally 234U/238U activity ratio is found to vary considerably due to natural causes in water, soil, sediment and uranium ore of different geographical origin. The mechanism of such variation is preferential leaching of 234U compared with 238U from solid phase, caused by radiation damage of crystal lattice upon alpha decay of 238U, oxidation of insoluble tetravalent 234U to soluble hexavalent 234U during decay, and alpha recoil of 234Th (and its daughter 234U) into solution phase [18]. 234U/238U, activity ratio, in soil typically ranges from 0.5 to 1.2 and also varies due to anthropogenic discharge. The activity ratio found to vary from 0.996-1.029 in the studied samples and did not show much disequilibrium. The distributions of 234U/238U are relatively uniform in soil with activity ratio not much different from 1.0 (indicating radiological equilibrium between 234U and 238U). This probably is because parent materials of these soils are relatively new volcanic ejecta, which initially had 234U/238U activity ratio near 1.0 and has not been subjected to leaching of 234U since its deposition.
Correlation of distribution coefficient and soil parameters
In order to investigate the fate of these nuclides under site specific conditions the distribution coefficients for the nuclides have been determined experimentally. The Kd values of three radionuclides at four sampling points are presented in Figure 2. Kd values, measured on triplicate analyses, for Cs, Sr and U were found to vary from (114 ± 3 to 404 ± 43), (65 ± 0.2 to 154 ± 7) and (1640 ± 22 to 8563 ± 458) L/kg respectively. In log scale, Kd values for Cs, Sr and U were found to be logKd(U) ~3>logKd(Cs) ~2>logKd(Sr) ~1-2. Kd values are highly dependent on site specific characteristics and the methodology adopted for analysis. Therefore in literature we can find 3 to 4 order of magnitude difference in the Kd values for these nuclides [19,20]. Kd being an important parameter, used as an input in contaminant transport model for long term prediction of radionuclide migration, needs to be accurately determined under specific conditions. During Fukushima accident a large area is contaminated, so these data can be used for prediction of long term transport of these radionuclides in soil water system. Kd values are site specific, therefore chemical characterization of the soil as well as water from the respective site has been carried out. The major oxide composition in soil sample and some important soil parameters are given in Tables 2 and 3.
| Sample | SiO2 | TiO2 | Al2O3 | Fe2O3 | MgO | CaO | Na2O | K2O | P2O5 | MnO | Total |
|---|---|---|---|---|---|---|---|---|---|---|---|
| FS-1 | 56.6 | 0.67 | 19.1 | 6.3 | 1.3 | 0.7 | 1.5 | 1.9 | 0.09 | 0.11 | 88.3 |
| FS-2 | 60.7 | 0.53 | 15.6 | 4.1 | 1.1 | 1.6 | 1.9 | 1.5 | 0.47 | 0.06 | 87.7 |
| FS-3 | 68.1 | 0.43 | 13.5 | 3.0 | 0.8 | 1.1 | 1.7 | 2.3 | 0.09 | 0.04 | 91.2 |
| FS-4 | 66.1 | 0.44 | 14.4 | 3.2 | 0.8 | 1.1 | 1.9 | 2.2 | 0.13 | 0.04 | 90.4 |
Table 2: Major oxide composition (mass %) in soil.
| Sample | pH | OC % | CEC mgeq/100g | CaCO3 % | Sand % | Silt+Clay % |
|---|---|---|---|---|---|---|
| FS-1 | 5.87 | 6.53 | 1.9 | 0.54 | 96.3 | 3.7 |
| FS-2 | 6.24 | 6.34 | 9.9 | 1.02 | 73 | 27 |
| FS-3 | 4.97 | 3.59 | 7.2 | 1.99 | 88.1 | 11.9 |
| FS-4 | 5.29 | 3.74 | 11.2 | 0.89 | 88.5 | 11.5 |
Table 3: Soil parameters affecting radionuclide sorption.
Soils are found to be richer in iron oxide content with minimum for FS-3 (3.0 %) and maximum for FS-1 (6.3 %). In FS-1 most of the major oxides are found to be at higher concentration than other soils. From the particle size distribution of soil, it is observed that FS-1 is mostly sandy having 96 % sand composition, whereas FS-2 is loamy sand with 27 % of silt and clay content. All four soil samples are acidic in nature having pH ranging from 4.97 to 6.24. A wide range for CaCO3 content have been observed in these four samples with a minimum of 0.54 % (FS-1) to a maximum 1.99 % (FS-3). In all the soil samples stable Cs, Sr and U varies from 3.11 to 5.03 μg/g, 107.5 to 154.5 μg/g and 2.04 to 3.95 μg/g respectively.
The pH of the water samples used for Kd determination found to vary from 6.61-8.03 at 20°C. The concentration of major ions e.g., Ca, Mg, Na and K in water vary from 9.2-15, 2.7-5.4, 3.7-7.4 and 1.4 to 20.6 mg/L respectively. Stable concentrations in water for Cs, Sr and U found to vary from 0.03-0.36, 37.7-43.0 and 0.004-0.026 μg/L respectively.
In order to understand the sorption behaviour, a correlation of Kd with respect to different soil parameters were carried out and is given in Table 4. Soils contain different cations with adsorbing components especially in the silt and clay fractions [21]. Important soil components affecting the sorption of radionuclides are minerals like smectite, illite and chlorite as well as oxides and hydroxides of iron, aluminum and manganese. In the present study we could find Kd (Cs) shows significantly good correlation with fine particles (silt + clay) (r=0.91, p=0.08), CEC (r=0.86, p=0.14) and exchangeable cations (K, Na, Mg, Ca) shown in Table 4, where r is represented by Pearson correlation coefficient and p is for significance level. Cs is readily adsorbed on the surfaces of soil mineral components as well as on those of colloids and suspended particles in soil solution. Ion exchange is the adsorption mechanism and the sorption behavior of Cs is dominated by highly selective interlamellar exchange sites between the 2:1 layers of clay and mica minerals called frayed edge sites (FES) on which adsorption has been reported to be virtually irreversible [22]. Sr shows similar behavior to that of Cs. Kd (Sr) shows significant correlation with Ex Ca (r=0.97, p=0.02) and can be attributed due to similar chemical properties. There is a good correlation of Kd (U) with organic content (OC), (r=0.8, p=0.2) and major oxides of soil components such as Fe, Al, Ti and Mn oxides and a significant reverse correlation is observed with soil CaCO3 (r=-0.96, p=0.03). The uranyl ion and its complexes adsorb onto clays, organics and oxides [20]. Organic complexes play an important role in uranium aqueous chemistry. The non-complex uranyl ion has a greater tendency to form complexes with fulvic and humic acids than many other metals with a +2 valence. This has been attributed mainly to greater “effective charge” of the uranyl ion compared to other divalent metals. As the ionic strength of an oxidized solution increases, other ions, notably Ca2+, Mg2+, and K+, will displace the uranyl ion from soil exchange sites, forcing it into solution. For this reason, the uranyl ion is particularly mobile in high ionic-strength solutions [20]. Other cations will not only dominate over the uranyl ion in competition for exchange sites, but also carbonate ions will form strong soluble complexes with the uranyl ion, thereby reducing the uranium sorption [20].
| Kd (U) | pH | OC | SiO2 | TiO2 | Al2O3 | Fe2O3 | MgO | MnO | CaCO3 | P2O5 |
| r | 0.72 | 0.8 | -0.92 | 0.87 | 0.92 | 0.88 | 0.88 | 0.85 | -0.96 | 0.10 |
| p | 0.28 | 0.2 | 0.07 | 0.12 | 0.07 | 0.12 | 0.12 | 0.15 | 0.03 | 0.89 |
| Kd (Cs) | Ex. Ca | Ex. K | Ex. Na | Ex. Mg | CEC | Sand | Silt +Clay | CaO | Na2O | P2O5 |
| r | 0.87 | 0.4 | 0.98 | 0.87 | 0.86 | -0.9 | 0.91 | 0.9 | 0.91 | 0.85 |
| p | 0.13 | 0.59 | 0.01 | 0.13 | 0.14 | 0.08 | 0.08 | 0.1 | 0.09 | 0.14 |
| Kd (Sr) | Ex. Ca | Ex. K | Ex. Na | Ex. Mg | CEC | Sand | Silt +Clay | CaO | Na2O | P2O5 |
| r | 0.97 | 0.78 | 0.79 | 0.98 | 0.97 | -0.75 | 0.75 | 0.82 | 0.91 | 0.52 |
| p | 0.02 | 0.21 | 0.21 | 0.02 | 0.02 | 0.24 | 0.25 | 0.18 | 0.03 | 0.47 |
Table 4: Pearson correlation coefficient (r) and significance levels (p) for Kd with soil parameters.
However, for a better correlation, sample size has to be increased which is in progress. Site specific Kd values could be used for contaminant transport model to predict the radionuclide migration and also the relationship between soil parameters and sorption behaviour of radionuclides will be helpful for soil remediation and long term dose assessment.
As a consequence of FDNPP accident a large area is contaminated with respect to Cs activity and is much higher than the global fallout. Cs activity ratio (134Cs/137Cs) indicates contamination from the accident. 90Sr contamination was also observed in the studied samples. When compared with Cs activity, about 3 order lower than Cs activity is observed in contaminated sites and 1 order lower in less contaminated site and likely coming at least in some part from global fallout. Uranium activity ratio (234U/238U) found to vary from 0.996 to 1.029. The Kd values for Cs, Sr and U found in the order of log Kd(U) ~3 > log Kd(Cs) ~2 > : logKd(Sr) ~1-2. Based on this study, Kd (Cs) and Kd (Sr) show a good correlation with CEC, exchangeable cations (K, Na, Mg, Ca) and with fine particles (silt+clay). Kd (Cs) is mostly associated with clay particles and the sorption is mainly controlled by ion exchange mechanism. Kd (Sr) shows significant correlation with Ex. Ca, Ex. Mg. and CEC, indicating the sorption may be controlled by cation exchange. Kd (U) shows a good correlation with organic content (OC) and major oxides of soil components such as Fe, Al, Ti and Mn oxides and a negative correlation was observed with CaCO3. The results obtained are preliminary in nature and systematic studies are in progress to ascertain a better correlation among distribution coefficient and soil parameters.
This research was supported by a Grant-in-Aid for Scientific Research (P- 11053) from the Japan Society for the Promotion of Science and Ministry of the Environment, Japan for sample collection. SM is thankful to the Japan Society for the Promotion of Science for the award of a research fellowship.