Journal of Depression and Anxiety
Open Access
ISSN: 2167-1044
ISSN: 2167-1044
Research - (2023)Volume 12, Issue 1
Objective: Anxiety is a frequent symptom in Heart Failure with Reduced Ejection Fraction (HFrEF). By inhibition of the endopeptidase neprilysin, Sacubitril/Valsartan (S/V) blocks degradation of Atrial Natriuretic Peptide (ANP), which has anxiolytic-like effects in man. In patients suffering from HFrEF we presumed an acute anxiolytic action of S/V and its mediation via ANP.
Methods: 56 patients with decompensated HFrEF were treated with S/V (24 mg/26 mg b.i.d.) or Treatment As Usual (TAU) without S/V in an open, non-randomized observational pragmatic controlled trial. At days 0 and 7, anxiety was assessed using the Hospital Anxiety and Depression Scale (HADS) and plasma levels of ANP and heart failure biomarker N-Terminal pro B-type Natriuretic Peptide (NT-proBNP) were measured.
Results: 35 patients participated on both days. Within one week, HADS-anxiety ratings were significantly decreased under S/V, but not TAU. Plasma ANP and NT-proBNP levels did not influence this finding. A significant treatment group effect on the day 7/day 0-ratios of HADS-anxiety emerged when related to the changes in ANP levels.
Conclusion: First evidence for an acute decrease of anxiety under S/V in patients is provided. This interesting, preliminary finding and the respective role of ANP, but also of other neprilysin substrates, need further study in randomized controlled trials.
Sacubitril; Anxiety; Heart failure; Neprilysin; Atrial Natriuretic Peptide (ANP)
Sacubitril/Valsartan (S/V), an Angiotensin Receptor-Neprilysin Inhibitor (ARNI), is indicated in Heart Failure with Reduced Ejection Fraction (HFrEF). In patients hospitalized with acute decompensated HFrEF, S/V significantly decreases plasma levels of heart failure biomarker N-Terminal pro-B-Type Natriuretic Peptide (NT-proBNP) versus enalapril as early as the first week of treatment [1]. Via inhibition of neprilysin by sacubitrilat, Atrial Natriuretic Peptide (ANP) is dose-dependently elevated by S/V in plasma of rodents within hours [2]. Some benefits of ARNI therapy in man may be mediated by natriuretic, diuretic, vasorelaxatory and antifibrotic effects of therapeutically elevated ANP [3]. Beside its cardiovascular actions, ANP exerts acute anxiolytic-like effects in man [4]. An emerging role of ANP for innovative psychopharmacological treatments is under discussion [5]. Anxiety is a frequent, but often neglected symptom in heart failure [6]. Whether pathognomically elevated plasma ANP can reduce anxiety in heart failure is equivocal [7,8]. So far, no study has assessed behavioral effects of acute therapeutic elevation of ANP in cardiologic patients. In a pilot observational pragmatic controlled trial under real-world conditions in patients with decompensated HFrEF, we hypothesized that S/V acutely reduces anxiety, possibly mediated by ANP.
Subjects
56 adult subjects hospitalized with decompensated HFrEF were enrolled [8]. Recruitment at Herford Hospital took place from 5/29/2017 until 9/15/2020. Inclusion criteria were a clinical diagnosis and a Left Ventricular Ejection Fraction (LVEF) <40%. Subjects had to be proficient in German and without clinical signs of major organic brain syndrome. Subjects who had previously received S/V or taking psychotropic drugs had been excluded. Age, sex, body mass index, cardiovascular medical history, baseline vital signs, plasma creatinine and medication were recorded at admission. The protocol had been approved by the ethics committee of the Ruhr University Bochum at Bad Oeynhausen (#2017-151). This observational trial was recorded in the World Health Organization recognized German Register for Clinical Studies (#DRKS00012906). All subjects gave written informed consent prior to participation. They were informed that we intend to characterize the interaction of psychic condition and heart hormones during treatments, but were unaware of our hypotheses.
Treatments
Subjects received S/V 24 mg/26 mg b.i.d. (EntrestoR, Novartis Pharma GmbH, Nuremberg, Germany) or Treatment As Usual (TAU) without S/V according to the independent clinical decision of the attending cardiologists before inclusion of subjects in this prospective cohort trial.
Psychometric assessment
On days 0 and 7, symptoms of anxiety were assessed using the German version of the Hospital Anxiety and Depression Scale (HADS), which is a reliable and valid self-report questionnaire for patients in non-psychiatric hospital clinics [9]. It contains seven items for anxiety (each graded 0-3) and omits somatic items that may contaminate identification of symptoms in populations with physical diseases.
Endocrine measurements
For measurement of plasma ANP and NT-proBNP on days 0 and 7, venous blood was obtained after 15 minutes of rest in a supine position, transferred to prechilled tubes containing EDTA, plasma was immediately separated by centrifugation at 4°C and stored in aliquots at -80°C until analysis. ANP was determined using a commercially available radioimmunoassay (Phoenix Europe, Karlsruhe, Germany) after extraction; intraand interassay coefficients of variation were below 12%, as reported previously [8]. NT-proBNP was analyzed in the Department of Clinical Chemistry of the University Hospital Hamburg-Eppendorf; intra-and interassay coefficients of variation were below 8%.
Statistical analyses
Subjects participating on days 0 and 7 were included. Continuous variables are presented as mean ± Standard Error of the Mean (SEM), nominal or categorical variables as absolute or relative frequencies. Demographic and clinical characteristics were exploratively compared between groups using Mann- Whitney U-tests for continuous or chi-square tests for categorical variables. Non-parametric Wilcoxon matched-pairs tests were first applied to test within each treatment group the pre/posttreatment (i. e. time) effects on HADS-anxiety ratings, furthermore on ANP and NT-proBNP concentrations. Multivariate Analysis of Covariance (MANCOVA) with repeated measures was subsequently performed for testing time effects of HADS-anxiety ratings by considering ANP and/or NT-proBNP as time-dependent covariates. For testing treatment group effects on the day 7/day 0 ratios of HADS-anxiety, one-factorial analysis of variance or covariance with or without covariates was applied. As nominal level of significance, α=0.05 was accepted and corrected according to Bonferroni procedure.
Subjects
Of the 56 subjects, 38 had started with S/V (age 67.2 ± 2.3 years, LVEF 31.2 ± 1.0%, 30 men, 8 women) and 18 with TAU (age 68.1 ± 3.2 years, LVEF 31.2 ± 1.4 %, 9 men, 9 women). In the S/V group reasons for drop-out (n=15, i. e. 39.9%) were stop of S/V due to hypotension (n=4), transport problems after discharge (n=4), non-availability by telephone (n=4), and refusal due to illness (n=3). Under TAU six patients (33.3%) dropped out, because of scheduling difficulties (n=3), non-accessibility (n=2), or withdrawn consent (n=1). 35 subjects participated on both study days; 23 had received S/V and 12 under TAU (Table 1).
| Subjects | S/V | TAU | P value |
|---|---|---|---|
| N | 23 | 12 | |
| Men/Women | 19/4 | 07/5 | 0.118 |
| Age (years) | 66.1 ± 2.7 | 66.3 ± 4.3 | 0.74 |
| LVEF (%) | 29.6 ± 1.2 | 30.6 ± 1.6 | 0.543 |
| BMI (kg/sqm) | 27.3 ± 1.4 | 28.7 ± 2.4 | 0.555 |
| Heart rate (/min) | 76.4 ± 3.8 | 79.2 ± 2.9 | 0.229 |
| Syst. BP (mm Hg) | 117.7 ± 3.7 | 126.9 ± 7.5 | 0.354 |
| Diast. BP (mm Hg) | 76.4 ± 3.8 | 74.2 ± 2.8 | 0.238 |
| Creatinine (mg/dl) | 1.4 ± 0.2 | 1.5 ± 0.3 | 0.578 |
| Hypertension (%) | 65 | 67 | 0.931 |
| Diab. mellitus (%) | 38 | 8 | 0.089 |
| Smoking (%) | 48 | 33 | 0.728 |
| Atrial fibrillation (%) | 39 | 25 | 0.403 |
| Myoc. infarction (%) | 48 | 33 | 0.41 |
| Beta blockers (%) | 96 | 100 | 0.463 |
| ACE inhibitors (%) | 26 | 67 | 0.020* |
| Diuretics (%) | 83 | 92 | 0.467 |
| AT-II blockers (%) | 13 | 17 | 0.771 |
| Digitalis (%) | 0 | 8 | 0.16 |
Note: Groups were exploratively compared using Mann-Whitney U-tests for continuous variables or chi-square tests for categorical variables (* denotes statistical significance on the nominal level of significance (α=0.05)). Please be informed that ACE inhibitors must be paused for at least 36 hours before starting S/V, which may explain the significantly lower percentage of ACE inhibitors in patients who we assigned to S/V treatment according to independent clinical decision.
Table 1: Demographic and clinical characteristics at admission of the subjects of the Sacubitril/Valsartan (S/V) and treatment as usual (TAU) groups, who participated on days 0 and 7 (given as mean ± SEM or absolute or relative frequencies).
Anxiety ratings and pre/post effects
In the S/V group HADS-anxiety significantly decreased from 6.30 ± 0.89 to 4.61 ± 0.60 within one week (Wilcoxon matchedpairs test: z=-2.600, n=23, p=0.009), while the respective ratings for the TAU group (6.67 ± 1.18 on day 0 and 6.17 ± 1.07 on day 7) did not change (z=-0.672, n=12, p=0.501). All individual HADS-anxiety ratings are depicted in Figure 1.
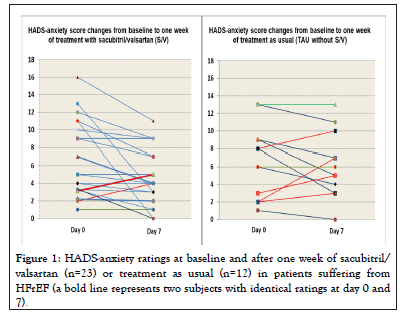
Figure 1: HADS-anxiety ratings at baseline and after one week of sacubitril/ valsartan (n=23) or treatment as usual (n=12) in patients suffering from HFrEF (a bold line represents two subjects with identical ratings at day 0 and 7).
Endocrine results in the S/V group and their impact on anxiety ratings
ANP concentrations did not increase significantly in the S/V group (1047.3 ± 196.8 pg/ml on day 0 vs. 1313.8 ± 312.2 pg/ml on day 7; Wilcoxon matched-pairs test: z=-1.186, n=23, p=0.236). Respective NT-proBNP levels marginally decreased from 4337.8 ± 1842.5 pg/ml at baseline to 3842.3 ± 2134.7 pg/ml after one week (z=-1.885, p=0.059). When NT-proBNP levels were considered as a time-dependent covariate in a MANCOVA with dependent variables HADS-anxiety and ANP, a significant pre/post effect was detected (F(2;20)=6.132, p=0.008), which was localized with univariate F-tests at HADSanxiety (F(1;21)=7.358, p=0.013), but not at ANP (F(1;21)=2.860, p=0.106). No significant effect of the covariate NT-proBNP emerged (F(2;20)=0.705, p=0.506). When considering ANP levels at days 0 and 7 as a covariate, the previously found significant time effect on HADS-anxiety during S/V persisted (F(1;21)=8.940, p=0.007), but the covariate effect did not show statistical significance (F(1;21)=0.990, p=0.330).
Group effects on changes of anxiety ratings
While no significant group effect on the HADS-anxiety ratios (day 7/day 0) was seen (0.90 ± 0.10 for S/V, 1.19 ± 0.26 for TAU; ANOVA: F(1;31)=1.798, p=0.190), when additionally considering the respective ANP ratios (1.24 ± 0.11 for S/V, 0.94 ± 0.14 for TAU) as a covariate, a significant group effect on the HADS-anxiety ratio emerged (ANCOVA: F(1;31)=8.771, p=0.006) and the covariate ANP ratio showed a significant effect, too (F (1;30)=26.065, p<0.0001). The group effect on the HADS-anxiety ratio remains significant, when additionally to ANP ratio also age, sex and LVEF were considered as covariates (F(1;27)=5.854, p=0.023). When we formed the quotient of the HADS-anxiety and ANP ratios (0.74 ± 0.07 for S/V vs. 1.11 ± 0.13 for TAU), ANOVA also indicated a significant group effect on this quotient (F(1;33)=7.047, p=0.012).
After one week of S/V treatment anxiety ratings were significantly lower, but not in the control group (TAU). This time effect persisted when severity of heart failure (measured via NT-proBNP) or plasma ANP levels were considered as covariates and no significant effect of the covariates was detected. Significant differences between the treatment groups emerged when changes of HADS-anxiety were related to changes of ANP.
After chronic (four months) treatment with S/V significant improvement of anxiety symptoms in HFrEF patients was reported [10], but the applied anxiety scale measured many somatic symptoms associated with heart failure, no comparison group was included and no control for amelioration of heart failure was performed. In an open study about potential chronic antidepressant effects of S/V in patients with advanced heart failure, improved physical functioning was closely accompanied with decrease of depression [11]. Another recent observational study reported significantly reduced HADS-anxiety ratings after six months of S/V in patients with HFrEF, but interestingly not in patients with heart failure with preserved ejection fraction [12]. However, no control of HADS results for improvement of heart failure symptoms, documented by significantly reduced NYHA class and significantly prolonged 6-minute walking test distance in the HFrEF group at six-month follow-up, was undertaken. The authors speculated that S/V caused a suppressed inflammatory response and thus improved psychological symptoms.
Data about plasma ANP after initiation of S/V are limited in man, so far significant increases were reported at earliest after two weeks [3]. Another human study failed to detect increased plasma ANP by S/V after one week [13]. Besides ANP, various other, also behaviourally active peptides, such as adrenomedullin, substance P, angiotensin II, bradykinin, endothelin-1, neurotensin and oxytocin, are catalyzed by neprilysin [14,15]. Interestingly, neprilysin also controls the synaptic activity of enkephalin in the amygdala [16] and inhibition of enkephalinases was proposed as an anxiolytic mechanism in patients with anxiety disorders [17].
Although we were unable to show an influence of ANP on time effects under S/V, our analyses indicate an interaction between treatments and mutual influences of HADS-anxiety and ANP, which needs further research. Limitations of our pilot protocol include a relatively low sample size, its open design, lacking randomization, a low fixed dose of S/V, no standardized control therapy, no additional external assessment of anxiety symptoms, no supplemental anxiety challenge, and no measurement of plasma S/V levels. Future more sophisticated studies, i. a. with dose-response evaluations and longer treatment times, also in patients with anxiety disorders, are needed to replicate and extend our promising, but preliminary finding of acutely reduced anxiety under S/V and should further clarify its underlying mechanism.
We thank Mrs. Iris Remmlinger-Marten, Mrs. Kirsten Dammann and Mrs. Sandra Schwentesius (Dept. of Psychiatry and Psychotherapy, University Hospital Hamburg-Eppendorf) for expert technical assistance and Mrs. Birgit Kleinen, Dr. Monika Neuhaeuser and Dr. Martin Eversmeyer for their friendly help and valuable support at Herford Hospital.
The authors declare no conflict of interest.
This research did not receive any specific grant from funding agencies in the public, commercial, or non-for-profit sector.
All authors consent to publication of the work.
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
Citation: Kellner M, Yassouridis A, Górski D, Waheed S, Kähler J, Wiedemann K (2023) Acute Anxiolytic Effects of Sacubitril/Valsartan in Patients with Heart Failure. J Dep Anxiety. 12:499.
Received: 27-Feb-2023, Manuscript No. JDA-23-22055; Editor assigned: 02-Mar-2023, Pre QC No. JDA-23-22055 (PQ); Reviewed: 16-Mar-2023, QC No. JDA-23-22055; Revised: 23-Mar-2023, Manuscript No. JDA-23-22055 (R); Published: 30-Mar-2023 , DOI: 10.35248/2167-1044.23.12.499
Copyright: © 2023 Kellner M, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.