Research Article - (2021)Volume 9, Issue 4
Adaptations Associated with Non-Specific Chronic Low Back Pain: A Narrative Review
Joshua Brodie Farragher1*,
Gavin Paul Williams2,3,
Adrian Pranata4,
Doa El-Ansary4,5,6,
Selina Parry3 and
Adam Leigh Bryant1
*Correspondence:
Joshua Brodie Farragher, Centre for Health Exercise and Sports Medicine, The University of Melbourne,
Melbourne,
Australia,
Email:
Author info »
Abstract
Background: Non-specific chronic low back pain (NSCLBP) represents a growing global burden. Individuals with
LBP inherently adapt in a variety of ways, across psychological, behavioural and physical domains. However, adaptive
changes (e.g., altered lifting behaviour) may persist, becoming maladaptive, resulting in negative functional
consequences (i.e., persistence of pain, increased disability). Clinical practice guidelines lack specificity to direct the
type of interventions, dosage and treatment duration. A better understanding of how the maladaptive changes seen
in people with NSCLBP relate to meaningful outcomes (i.e., disability, function, quality of life) and defined subgroups
of people with NSCLBP may inform effective interventions. The aim of this review is to investigate the interrelationship
of psychological, behavioural and neuromuscular NSCLBP-related adaptations, and their clinical
significance with respect to disability, function, quality of life and pain.
Methods and findings: Three MEDLINE searches were conducted to investigate the psychological, behavioural and
neuromuscular adaptations in people with NSCLBP. The initial search returned 12972 articles and 238 were
identified for full-text review. A total of 93 articles were included in this review. Psychological and behavioural
maladaptations (i.e., fear-avoidance beliefs) are associated with poorer patient outcomes, whereas there is uncertainty
regarding the impact of maladaptations in the neuromuscular system on important clinical outcomes. Moreover, the
evidence is more supportive of the interrelationship between psychological and behavioural maladaptations than any
interrelation with neuromuscular maladaptations. To date, methodologies designed to assess NSCLBP-related
functional deficits lack ecological validity. Assessment of patients with NSCLBP should focus on psychological and
behavioural domains that relate to an individual’s disability and functional impairments. Individuals with NSCLBP
present with a variety of diverse adaptions that should focus intervention that aligns patient goals and functional
deficits.
Keywords
Non-specific chronic low back pain; Adaptations; Neuromuscular; Behaviour; Psychological factors
Introduction
Non-specific Chronic Low Back Pain (NSCLBP) is the leading
cause of disability worldwide [1]. At least 80% of the population
will experience Low Back Pain (LBP) in their lifetime [2] and
approximately 5%-10% of those will go on to develop chronic
symptoms [3]. The incidence of NSCLBP has increased by
19.6% since 2006 [4] and the enormity of this burden is
expected to continue to grow with the global increase in the
ageing population number [5]. The growth in NSCLBP is also in
line with a more general rise in chronic disease such as diabetes
[6] and dementia [7], but any association with these conditions
remains unclear. Sequelae observed in people with NSCLBP
occur across multiple domains, including psychological,
behavioural and neuromuscular [8]. These changes may be
adaptative (i.e., compensatory changes that decrease one’s
symptoms, allowing better coping in their environment) or
maladaptive (i.e., compensatory changes that increase one’s
symptoms, inhibiting their ability to cope in their environment)
[9]. Importantly, maladaptive changes commonly observed in
people with NSCLBP usually result from negative beliefs and a
poor understanding of the relationship between pain and harm
(e.g., sitting upright despite an increase in one’s symptoms by
doing so) [9]. Initial responses to LBP can be adaptive, but
persistent adaptive behaviours can become maladaptive and
contribute to the transition from acute to persistent pain [10].
Interventions for NSCLBP are primarily focused on reducing
disability and improving function [11]. This has been a mainstay
focus for almost a century [12]; however, the treatment approach
has changed dramatically over the past three decades, with the
introduction of the bio psychosocial model [12,13]. Clinical
practice guidelines throughout the world support the use of
exercise therapy and psychological interventions for the
management of NSCLBP [14]. However, guidelines lack detail
regarding delivery mode and specific exercises. This likely stems
from the fact that numerous published interventional studies
have reported minimal between-group effect sizes [15-19],
ultimately leading to conclusions that each modality is equally
effective. The clinical guidelines assert that the NSCLBP
population is heterogenous, however, they provide no current
recommendations as to how to distinguish or manage subgroups
within the broader NSCLBP population [14]. As a result
of these issues, it is difficult for clinicians to apply these
guidelines to people with NSCLBP [20]. Furthermore, there is
an absence of recommendations pertaining to behavioural and
neuromuscular assessments for people with NSCLBP, despite a
large portion of NSCLBP-related literature devoted to these
domains. Finally, the methods used to quantify NSCLBP-related
adaptations are diverse and, in many instances, the clinical
relevance of these adaptations has not been investigated.
Therefore, it is timely to consider the adaptive and maladaptive
changes that occur in people with NSCLBP, with the
anticipation that a better understanding of the clinical
significance of these changes will lead to the development of
better treatment strategies. As such, the aims of this paper are to
i) evaluate the appropriateness and ecological validity of
methods used to identify neuromuscular NSCLBP-related
maladaptations; ii) discuss the nature and inter-relationships of
psychological, behavioural and neuromuscular NSCLBP-related
maladaptations; iii) evaluate the clinical significance of these
maladaptations in relation to meaningful NSCLBP-related
outcomes (i.e., disability, function and quality of life); and iv)
propose a new model for multi-domain adaptations associated
with NSCLBP (Figure 1). In addressing this knowledge gap, this
review provides a narrative overview of the research investigating
NSCLBP-related adaptations.
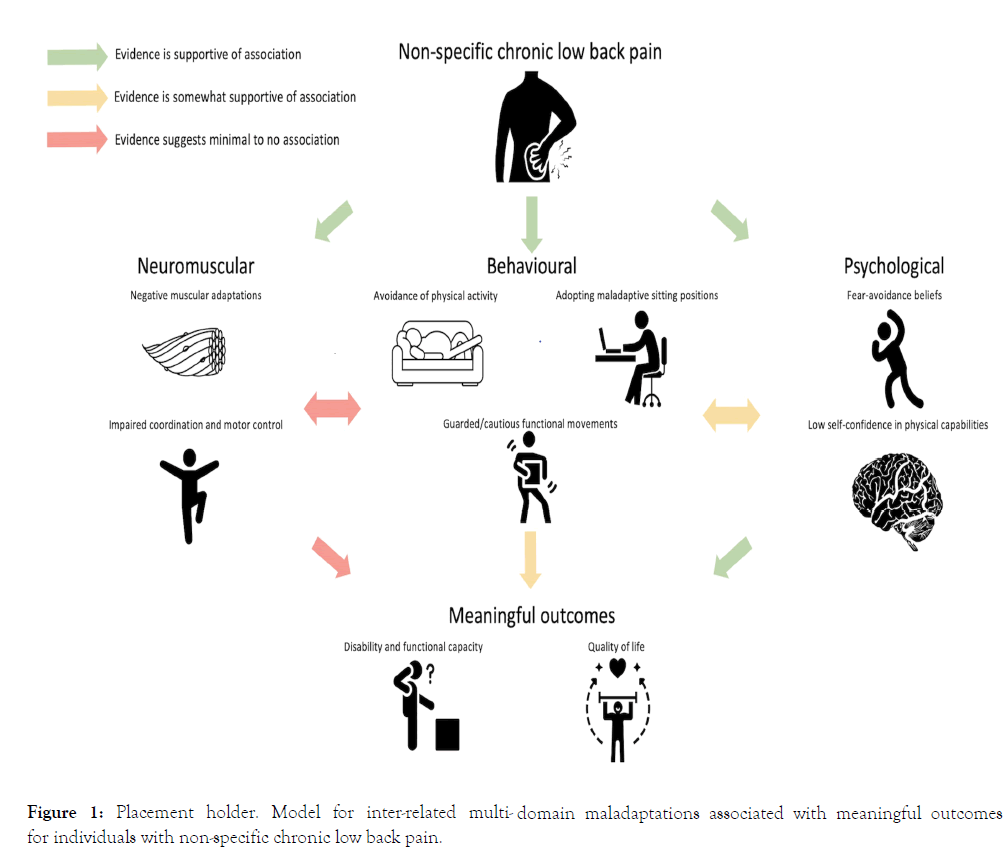
Figure 1: Placement holder. Model for inter-related multi- domain maladaptations associated with meaningful outcomes for individuals with non-specific chronic low back pain.
Methods and Findings
A series of literature searches were conducted on MEDLINE
(PubMed) between August 2020 and March 2021. Studies were
included that evaluated the outcomes of fear-avoidance beliefs,
pain self-efficacy, physical activity, lifting and sitting behaviour,
motor weakness, lumbar muscle morphology and motor control
in people with NSCLBP. Peer-review studies involving humans
and available in English were included in the review.
Randomised controlled trials, observational studies, systematic
reviews and meta-analyses were also included as they denote
higher levels of evidence [21]. All other study designs were
excluded from this review. Three separate searches were
conducted. Medical subject heading (MeSH) terms used to
perform the first search were: (CLBP OR Chronic Low Back
Pain OR LBP or Low Back Pain OR Non-Specific Chronic Low
Back Pain OR NSCLBP) AND (kinesiophobia OR fearavoidance
OR self-efficacy OR pain self-efficacy), yielding 1318
articles. MeSH terms used in the second search included: (CLBP
OR chronic low back pain OR LBP or low back pain OR nonspecific
chronic low back pain OR NSCLBP) AND (lifting OR
sitting OR physical activity), yielding 7640 articles. The third
search used MeSH terms including: (CLBP OR chronic low
back pain OR LBP or low back pain OR non-specific chronic
low back pain OR NSCLBP) AND (weakness OR strength OR
lumbar multifidus OR erector spinae OR motor control OR
neuromuscular control), yielding 4014 articles.
In total, the searches returned 12972 articles. After removing
duplicates, 10711 remained. Of the 10711 studies, 538 were not
available in English and 8605 were excluded based on study
design, leaving 2106 for title and abstract review. The first
author reviewed the titles and abstracts for relevance, resulting
in 238 studies remaining for full-text review. As a result of the
full-text review, 93 articles were included in the final review.
Psychological Adaptations
Psychological maladaptations are widely reported in people with
NSCLBP [22-24]. Importantly, maladaptive psychological
changes are strongly associated with functional disability [25-27]
and development of chronic LBP [23].
Fear-avoidance beliefs
Pain is one of the most aversive stimuli and, as such, has a close
relationship with fear [28]. Therefore, it is not surprising that
the tendency to avoid painful tasks is a common adaptive
mechanism seen in people with LBP [29,30]. Fear-avoidance
beliefs have been described as both adaptive and maladaptive in
that behaviours adopted in the short-term may be protective but
when sustained in the longer-term, may be deleterious [28]. Fearavoidance
beliefs are generally sustained as a result of
‘catastrophising’ beliefs and negative interpretations of painful
stimuli and their association with harm [31]. In those with LBP,
there is limited evidence to suggest the commonality of these
negative beliefs; however, it is well understood that they are
associated with the persistence of LBP [32-35]. This could
explain why fear-avoidance beliefs are sustained into the chronic
phase of NSCLBP.
The longevity of behavioural adaptations resulting from fearavoidance
beliefs may be responsible for changes in the
neuromuscular system [36]. Whilst it has long been
hypothesised that people with NSCLBP who demonstrate high
levels of fear-avoidance beliefs would demonstrate more
protective behaviours [37], the evidence pertaining to this is
unclear. Specifically, the uncertainty regarding the relationship
between fear-avoidance beliefs and behavioural changes is partly
due to conflicting results [37-44]. Furthermore, a proportion of
the uncertainty is due to varied assessment methods of fearavoidance
beliefs. For example, there is recent evidence
suggesting that data derived from task-specific fear-avoidance
questionnaires [45] are more strongly associated with
behavioural changes than data from general fear-avoidance
questionnaires [46].
Whilst a body of literature has investigated the relationship
between fear-avoidance and behavioural changes [37,39-41],
there is a paucity of evidence investigating the relationship with
neuromuscular-related deficits in NSCLBP. Of the muscular
properties investigated, high fear-avoidance beliefs are associated
with reduced lumbar extension strength [47]. The evidence is
conflicting with respect to the relationship between fearavoidance
beliefs and lower limb muscle strength [48,49].
Importantly, studies investigating relationships between fearavoidance
beliefs and NSCLBP-related muscle deficits are in
short supply with considerable measurement variability [48,49]
and small sample sizes [50]. Thus, there is uncertainty about the
relationship between fear-avoidance beliefs and neuromuscular
maladaptations. Furthermore, there is evidence to suggest
neuromuscular maladaptations and fear-avoidance beliefs
contribute to disability independently [51].
Pain self-efficacy
Pain self-efficacy is defined as the belief in one’s ability to
perform painful or perceived painful tasks or movements in
order to achieve a desirable outcome [52]. Self-efficacy beliefs are
modifiable through an individual’s experiences [53]. Indeed,
longstanding improvements in pain self-efficacy have been
identified in people with NSCLBP as a result of education and
exercise [54]. By contrast, it is also possible that experiencing
pain can negatively impact one’s perception of their ability to
perform painful tasks, thereby resulting in lower pain selfefficacy.
Low pain self-efficacy during the acute phase of LBP
could also lead to avoidance of provocative tasks, not because of
fear-avoidance itself, but due to the lack of confidence in their
ability to cope with pain. The continued avoidance of painful
tasks may perpetuate the existence of low pain self-efficacy and
lead to the persistence of LBP. In those with NSCLBP, higher
levels of pain self-efficacy are associated with decreased pain
intensity, reduced disability scores [55, 56] and greater
functional capacity [57-59]. Moreover, low pain self-efficacy and
high fear-avoidance beliefs typically coexist in people with
NSCLBP [60]. Despite greater research emphasis on fearavoidance
beliefs, there is evidence to suggest that pain selfefficacy
is a more important psychological mediator in the
relationship between pain and disability in the NSCLBP
population [61]. Furthermore, pain self-efficacy appears to be a
critical mediator in the relationship between fear-avoidance and
NSCLBP-related outcomes, such as pain and disability [62].
However, to date, the impact of pain-self efficacy on physical
measures such as muscle weakness, muscle morphology and
neuromuscular control is currently unknown.
Behavioural Adaptations
Functional behaviours are impacted by pain or the threat of
pain in people with NSCLBP. Physical activity, lifting and sitting
are examples of behaviours that are commonly reported as
problematic in this population [63-65]. Importantly, changes in
these behaviours in response to pain or fear of pain vary
amongst individuals with NSCLBP [66].
Physical activity
It is a commonly held belief by clinicians that people with
NSCLBP are less physically active than healthy individuals.
Arguably, this belief is attributed to the theoretical fearavoidance
model which indicates that decreases in physical
activity are the result of fear of pain or harm [23]. Indeed,
theoretical models have identified physical inactivity following
the onset of LBP as a perpetuating factor for chronicity [65].
However, these theoretical models are only partially supported
as there is conflicting evidence to suggest that physical activity
deficits are commonplace in those with NSCLBP [67,68]. One
limitation to our understanding of physical activity behaviours
in people with NSCLBP stems from the outcome measures used
to quantify physical activity that are unable to differentiate
between different types of activities (e.g., walking or lifting).
Therefore, whilst some people with NSCLBP demonstrate levels
of physical activity similar to healthy individuals, they may
actually modify or avoid certain provocative tasks (e.g., lifting).
Indeed, no previous studies have performed time and motion
analyses of people with NSCLBP. Importantly, time and motion
analyses has led to a paradigm shift in the early rehabilitation
phase following stroke [69,70]. The nature and extent of
adaptations to physical activity in NSCLBP need to be
established before appropriate interventions and education can
be developed.
The heterogenic nature of the NSCLBP population has likely
contributed to findings presented in previous studies that
indicate negligible to no differences in physical activity-related
behaviours compared with healthy control participants. The
avoidance-endurance theoretical model suggests that a
proportion of people with NSCLBP will decrease their physical
activity-related behaviours whilst others will persevere despite
their LBP [71]. Recent evidence supports this model by
demonstrating that people with NSCLBP exhibit avoidance,
persistence or a combination of both behaviours, towards tasks
that reproduce pain [72]. Thus, people with NSCLBP display
considerable diversity in their behaviours relating to physical
activity, thereby supporting the notion that this population is
heterogenous.
Lifting
Lifting is a risk factor for the development of LBP [73]. A
popular belief held by people with NSCLBP, and clinicians is
that a safe lifting technique should be characterised by a
‘straight’ back with movement and force produced from the
lower limbs [74,75]. This squat-lift technique is also commonly
advocated for the prevention of LBP in the workplace [64];
however, from a biomechanical and scientific perspective, ‘best’
lifting technique is equivocal [76-78] with spinal load (i.e.,
compression and shear) comparisons between ‘squat’ (i.e., bent
knee and straight back) and ‘stoop’ (i.e., minimal knee bend and
bent back) lifting techniques demonstrating mixed results
[79-82]. Moreover, biomechanical comparisons of lifting
techniques between those with and without NSCLBP have
shown i) considerable variability, and ii) conflicting results with
increased [83], and reduced to no differences [84,85] in lumbar
range of motion. These inconsistent findings also provide
support to the notion that people with NSCLBP are not
homogenous with respect to their lifting behaviour [86]. Subclassification,
based on observed motor control impairments
(i.e., flexion pattern or active extension pattern), has
demonstrated significant differences in spinal kinematics
between healthy controls and people with NSCLBP, as well as
significant differences between NSCLBP sub-groups [87].
Therefore, the commonly held community perceptions together
with general clinical recommendations suggesting that there is a
singular ‘safe’ way of lifting are not evidence-based.
Coordination deficits between the trunk and lower limbs during
lifting has also been associated with NSCLBP [88,89].
Individuals with NSCLBP display differences in trunk and lower
limb coordination and reduced movement variability compared
with healthy controls [90]. Coordination deficits extend beyond
the musculoskeletal system as dysfunction of the respiratory
system during lifting has been identified in individuals with
NSCLBP. Specifically, individuals with NSCLBP perform lifts
with greater inhaled lung volume than those without LBP [91].
The increase in inspired lung volume is associated with
increased spinal stability and requires further thoracic spine
extension [92,93]. Furthermore, altered breathing patterns may
be associated with psychological factors commonly seen in
people with NSCLBP, such as fear and apprehension [94].
Sitting
People with NSCLBP often identify sitting as a task that
exacerbates their symptoms [63]. There are two key components
regarding sitting: i) time and ii) posture. Similar to lifting,
clinicians and people with NSCLBP commonly share the
misconception that an upright sitting posture is optimal [95-97].
However, like lifting, there is a lack of evidence to support the
notion that there is a singular ‘best’ universal sitting posture. In
reality, people with NSCLBP tend to adopt sitting positions at
either extreme of range (i.e., lordotic or kyphotic positions)
compared to healthy individuals [98,99]. Adopting end of range
sitting postures is a NSCLBP-related maladaptive trait as
evidence demonstrates that when patients are positioned at the
opposing end of range position or in a more neutral sitting
position, they report reductions in pain intensity during sitting
[98]. When it comes to sitting posture, it is apparent that a ‘one
size fits all’ approach fails to address the complex and highly
specific needs of people with NSCLBP. The evidence relating to
the impact of sitting time on NSCLBP, however, is unclear due
to inconsistencies in the literature [100-104], which likely result
from differences in outcome measures used (e.g., patient
reported measures vs. accelerometry).
Neuromuscular Adaptations
Maladaptations within the neuromuscular system including
muscle weakness, muscle atrophy and motor control
impairments, have long been described in those with NSCLBP
[105,106]. Exercise interventions aimed at reducing disability in
people with NSCLBP have targeted these neuromuscular deficits
[15,16].
Morphological adaptations
Adaptations in NSCLBP-related muscle morphology have been
investigated for almost 30 years [107]. These studies have
primarily focussed on the morphology of the lumbar extensors
and trunk muscles given their involvement in lumbar spine
stability [107,108]. Indeed, numerous imaging studies have
reported altered morphology and decreased size of the lumbar
multifidus in NSCLBP people compared to healthy controls
[109-112]. People with unilateral NSCLBP display significantly
reduced multifidus size on the symptomatic side with a
moderate positive relationship with pain duration. However, no
associations with weakness or self-reported disability have been
identified [113,114]. Importantly, these studies have focused on
people with minimal disability and may not be representative of
a more disabled NSCLBP population.
In addition to muscle size, numerous studies have investigated
NSCLBP-related changes in muscle composition. Specifically,
magnetic resonance imaging studies have demonstrated that
people with NSCLBP exhibit increased fat infiltration in their
erector spinae and lumbar multifidus muscles [115-120].
Surprisingly, the severity of fat infiltration in the lumbar
muscles is only weakly associated with the severity of NSCLBPFarragher
related disability [117,118]. Whilst similar to the
abovementioned evidence pertaining to lumbar muscle size, no
studies have investigated relationships between lumbar muscle
morphology, weakness and performance of functional tasks.
Considering the weak associations between lumbar muscle
morphology and disability, it is unlikely that interventions
aimed at augmenting muscle size and composition would yield
clinically relevant improvements in people with NSCLBP
Motor weakness
The impact of motor weakness on function in people with
NSCLBP is contentious [121]. Even the existence of NSCLBPrelated
motor weakness is debated in the literature. In this
respect, there are inconsistent findings regarding the presence of
motor weakness in the lumbar extensors of those with NSCLBP
when compared with healthy controls [122-130].
The disparity of findings between studies may be partly due to
the testing methods employed as some protocols limit pelvic
rotation in a seated position [129,130] in order to isolate lumbar
extension [131], whilst others permit posterior pelvic rotation
[122-128]. Studies that restricted pelvic rotation reported no
difference in lumbar extensor force-output between people with
and without NSCLBP [129,130]. In contrast, six of the seven
trials that did not restrict pelvic rotation found significant
evidence of motor weakness during lumbar extension between
participants with NSCLBP and healthy controls [122-127].
Restriction of pelvic rotation may lack ecological validity. Some
studies have attempted to address this issue by testing lumbar
extensor muscle strength in standing [122,123,126]. However,
this test is ‘static’ in nature and, as such, does not test the types
of isotonic muscle contractions utilised during functional tasks,
such as lifting.
Despite the known involvement of the posterior pelvic rotators
(i.e., gluteal and hamstring muscles) in functional tasks [132],
there is a paucity of evidence evaluating them in people with
NSCLBP. Of the single study identified, no difference between a
NSCLBP group and healthy controls was identified [133].
However, obvious methodological flaws create uncertainty, as
muscle strength testing has been performed i) using manual
muscle testing, which is inherently inaccurate [134,135]; ii) using
maximal effort contractions which are rarely required in day-today
activities, iii) are conducted in non-functional positions (i.e.,
side-lying), or iv) are quantified using isometric contractions that
are not representative of the types of isotonic (i.e., concentric
and eccentric) muscle contractions involved in functional,
closed kinetic chain movements. Therefore, it is not surprising
that neuromuscular impairments including motor weakness
exhibit limited associations with clinical and functional
outcome measures (i.e., disability and functional performance)
given that the testing methods lack ecological validity.
Assessment protocols designed to quantify muscle force output
though coordinated functionally relevant movements are likely
to be more relatable to functional deficits in this population.
Motor control deficits
Motor control is defined as the ability of the central nervous
systems to produce purposeful and coordinated movements
[136]. In people with LBP, motor control deficits have been
described in the literature for decades. A landmark study by
Hodges and Richardson [105] indicated that spinal stability and
control are altered with LBP. Early studies focussed on the deep
trunk muscles (i.e., transversus abdominus and multifidus
muscles) [137-139]. However, there are conflicting results
regarding differences in recruitment patterns of the transversus
abdominus between those with and without NSCLBP [105,
140-143]. Potential reasons for the disparity between studies may
be due to low participant numbers and differing assessment
methods (e.g., lower-limb tasks in supine [143], bilateral upper
limb tasks whilst standing [105] or performing abdominal
drawing-in in a hook-lying position and in supine [140-142]).
Only one study has reported an association, albeit weak,
between improvements in the ability to contract the transversus
abdominus muscle and LBP-related disability [144].
Motor control deficits have also been explored via other
methods. One prospective cohort study assessed LBP-related
motor control impairments via three different methods. Firstly,
visual observation of thoracolumbar dissociation was used to
assess motor control, but visual observation is inherently
inaccurate. Secondly, passive extension of the lumbar spine was
assessed, but this measures range of movement rather than
motor control. Thirdly, deep muscle contraction, which is
similar to the abovementioned methods, lacks ecological validity
[145]. Overall, none of these clinical tests were able to predict
changes in NSCLBP-related disability or pain over an 8-week
period [145]. In an attempt to implement a more ecologically
valid assessment of motor control, Pranata, Perraton [129] found
isometric assessment of force control of the lumbar extensor
muscles was predictive of disability level. Whilst this protocol
has some functional relevance in that it examined i) a
provocative task (sitting) and ii) a sub-maximal force output of a
muscle group associated with lumbar spine stability and
movement, data collected in sitting may not translate to other
functional tasks such and walking and lifting. It is apparent that
testing muscle strength and motor control in static, isometric or
non-functional tasks limits the strength of associations with
NSCLBP-related disability.
Conclusion
Maladaptations in multiple domains can lead to disability,
impaired function and health related quality of life for people
with NSCLBP. However, current evidence suggests that
maladaptations in the psychological and behavioural domains
have a greater impact than those in the neuromuscular domain.
Furthermore, the interrelationship between behavioural and
psychological factors is stronger than any interrelationships with
neuromuscular factors. Based on the available evidence, it is
clear that the neuromuscular system is impacted by NSCLBP;
however, studies evaluating the measures of these impairments
have failed to identify meaningful relationships with clinically
important outcomes. This lack of association is arguably
attributed to variability in methods. Future investigation could
consider: i) developing novel ecologically valid tests for
neuromuscular impairments in relation to functional deficits
commonly experienced in NSCLBP; ii) identifying and/or
validating already proposed heterogenous sub-groups within the
NSCLBP population; and iii) if NSCLBP sub-groups exist,
identify discriminating features between them in order to tailor
specific interventions towards these sub-groups. This will more
importantly inform targeted and specific interventions for
NSCLBP.
Funding
There was no funding source for this review.
Competing Interests
The authors declare they have no competing interests.
References
- Hoy D, March L, Brooks P, Blyth F, Woolf A, Bain C, et al. The global burden of low back pain: Estimates from the Global Burden of Disease 2010 study. Ann Rheum Dis. 2014;73(6): 968-674.
- Rubin DI. Epidemiology and risk factors for spine pain. Neurol Clin. 2007;25(2): 353-71.
- Meucci RD, Fassa AG, Faria NM. Prevalence of chronic low back pain: Systematic review. Rev Saude Publica.2015;49.
- Global burden of disease, injury incidence, prevalence collaborators. Global, regional, and national incidence, prevalence, and years lived with disability for 328 diseases and injuries for 195 countries, 1990–2016: A systematic analysis for the Global Burden of Disease Study. 2016. 2017.
- Gatchel RJ. The continuing and growing epidemic of chronic low back pain. Healthcare (Basel). 2015;3(3): 838-45.
- Pozzobon D, Ferreira PH, Dario AB, Almeida L, Vesentini G, Harmer AR, et al. Is there an association between diabetes and neck and back pain? A systematic review with meta-analyses. PLoS One, 2019;14(2): e0212030.
- Whitlock EL, Diaz-Ramirez LG, Glymour MM, Boscardin WJ, Covinsky K E, Smith AK. Association between persistent pain and memory decline and dementia in a longitudinal cohort of elders. JAMA Intern Med. 2017;177(8): 1146-1153.
- Alhowimel A, AlOtaibi M, Radford K, Coulson N. Psychosocial factors associated with change in pain and disability outcomes in chronic low back pain patients treated by physiotherapist: A systematic review. Sage Open Medicine. 2018;6(1): 1-8.
- DiNapoli EA, Craine M, Dougherty P, Gentili A, Kochersberger G, Morone NE, et al. Deconstructing chronic low back pain in the older adult--step by step evidence and expert-based recommendations for evaluation and treatment. Part V: Maladaptive Coping. Pain Med. 2016;17(1): 64-73.
- Nees F, Becker S. Psychological processes in chronic pain: influences of reward and fear learning as key mechanisms-Behavioral evidence, neural circuits, and maladaptive changes. Neuroscience. 2018;387: 72-84.
- Gatchel RJ, Mayer TG. Evidence-informed management of chronic low back pain with functional restoration. Spine J. 2008;8(1): 65-69.
- Allan DB, Waddell G. An historical perspective on low back pain and disability. Acta Orthopaedica Scandinavica. 1989;60:1-23.
- Harland N, D Lavallee. Biopsychosocial management of chronic low back pain patients with psychological assessment and management tools. Physiotherapy. 2001;89(5): 305-312.
- Oliveira CB, Maher CG, Pinto RZ, Traeger AC, Lin CC, Chenot JF, et al. Clinical practice guidelines for the management of non-specific low back pain in primary care: An updated overview. Eur Spine J. 2018;27(11): 2791-2803.
- Hayden JA, Van Tulder MW, Malmivaara A, Koes BW. Exercise therapy for treatment of non-specific low back pain. Cochrane Database Syst Rev. 2005;3: CD000335.
- veira CB, Maher CG, Pinto RZ, Traeger AC, Lin CC, Chenot JF, et al. Clinical practice guidelines for the management of non-specific low back pain in primary care: An updated overview. Eur Spine J. 2018;27(11): 2791-2803.
- Saragiotto BT, CG Maher, Yamato TP, Costa LO, Menezes Costa LC, Ostelo RW, et al. Motor control exercise for chronic non-specific low-back pain. Cochrane Database Syst Rev. 2016(1): CD012004.
- Vitoula K, Venneri A, Varrassi G, Paladini A, Sykioti P, Adewusi J, et al. Behavioral therapy approaches for the management of low back pain: An up-to-date systematic review. Pain Ther. 2018;7(1): 1-12.
- Hajihasani A, Rouhani M, Salavati M, Hedayati, Kahlaee AH. The influence of cognitive behavioral therapy on pain, quality of life, and depression in patients receiving physical therapy for chronic low back pain: A systematic review. PMR. 2019;11(2): 167-176.
- Brouwers MC, Kho ME, Browman GP, Burgers JS, Cluzeau F, Feder G, et al. AGREE II: Advancing guideline development, reporting and evaluation in health care. CMAJ. 2010;182(18): E839-42.
- Wood L, Hendrick PA. A systematic review and meta-analysis of pain neuroscience education for chronic low back pain: Short-and long-term outcomes of pain and disability. Eur J Pain. 2019;23(2): 234-249.
- Gatchel RJ, Neblett R, Kishino N, Ray CT. Fear-avoidance beliefs and chronic pain. J Orthop Sports Phys Ther. 2016;46(2): 38-43.
- Vlaeyen JW, Linton SJ. Fear-avoidance and its consequences in chronic musculoskeletal pain: A state of the art. Pain. 2000;85(3): 317-332.
- Burns PB, Rohrich R J, Chung KC. The levels of evidence and their role in evidence-based medicine. Plast Reconstr Surg. 2011;128(1): 305-310.
- Crombez G, Vlaeyen JW, Heuts PH, Lysens R. Pain-related fear is more disabling than pain itself: Evidence on the role of pain-related fear in chronic back pain disability. Pain. 1999;80: 329-339.
- Boersma K, Linton SJ. Psychological processes underlying the development of a chronic pain problem a prospective study of the relationship between profiles of psychological variables in the fear–avoidance model and disability. Clin J Pain. 2006;22(2): 160-166.
- Boersma K, Linton SJ. How does persistent pain develop? An analysis of the relationship between psychological variables, pain and function across stages of chronicity. Behav Res The. 2005;43:1495-1507.
- Lundberg M, Frennered K, Hagg O, Styf O. The impact of fear-avoidance model variables on disability in patients with specific or nonspecific chronic low back pain. Spine (Phila Pa 1976). 2011;36(19):1547-53.
- Grotle M, Vollestad NK, Veierod MB, JI Brox. Fear-avoidance beliefs and distress in relation to disability in acute and chronic low back pain. Pain, 2004;112(3): 343-52.
- Leeuw M, Goossens ME, Linton SJ, Crombez G, Boersma K, JW Vlaeyen. The fear-avoidance model of musculoskeletal pain: current state of scientific evidence. J Behav Med. 2007;30(1): 77-94.
- Turk DC, Wilson HD. Fear of pain as a prognostic factor in chronic pain: Conceptual models, assessment, and treatment implications. Curr Pain Headache Rep. 2010;14(2): 88-95.
- Picavet HS, Vlaeyen JWS, Schouten JS. Pain catastrophizing and kinesiophobia: Predictors of chronic low back pain. Am J Epidemol. 2002;156: 1028-1034.
- Gron S, Jensen RK, Jensen TS, Kongsted A. Back beliefs in patients with low back pain: A primary care cohort study. BMC Musculoskelet Disord. 2019;20(1): 578.
- Barbosa FM, Vieira ÉB d M, Garcia JBS. Beliefs and attitudes in patients with chronic low back pain. Brazil J Pain. 2018;1(2).
- Ng SK, Cicuttini FM, Wang Y, Wluka AE, Fitzgibbon B, Urquhart DM. Negative beliefs about low back pain are associated with persistent high intensity low back pain. Psychol Health Med. 2017;22(7): 790-799.
- van Dieen JH, Flor H, Hodges PW. Low-back pain patients learn to adapt motor behavior with adverse secondary consequences. Exerc Sport Sci Rev. 2017;45(4): 223-229.
- Urquhart DM, Bell RJ, Cicuttini FM, Cui FM, Forbes A, Davis SR. Negative beliefs about low back pain are associated with high pain intensity and high level disability in community-based women. BMC Musculoskelet Disord. 2008;9:148.
- Verbunt JA, Seelen HA, Vlaeyen JW, van der Heijden GJ, Knottnerus JA. Fear of injury and physical deconditioning in patients with chronic low back pain. Arch Phy MedRehabil. 2003;84(8):1227-1232.
- Geisser ME, Haig AJ, Wallbom AS, Wiggert EA. Pain-related fear, lumbar flexion, and dynamic emg among persons with chronic musculoskeletal low back pain. Clin J Pain. 2004;20(2): 61-69.
- Thomas JS, France CR, Sha D, Wiele NV. The influence of pain-related fear on peak muscle activity and force generation during maximal isometric trunk exertions. Spine. 2008;33: e342-e348.
- Panhale VP, Gurav RS, Nahar SK. Association of physical performance and fear-avoidance beliefs in adults with chronic low back pain. Ann Med Health Sci Res. 2016;6(6): 375-379.
- Karayannis NV, Jull GA, Nicholas GA, Hodges P W. Psychological features and their relationship to movement-based subgroups in people living with low back pain. Arch Phys Med Rehabil. 2018;99(1): 121-128.
- Yahia A, Yangui N, Mallek A, Ghroubi S, Elleuch MH. Kinesiophobia, functional disability and physical deconditioning evaluation in chronic low back pain. Ann Phy Rehabil Med. 2017;60.
- Demoulin C, Huijnen IP, Somville PR, Grosdent S, Salamun I, Crielaard JM, et al. Relationship between different measures of pain-related fear and physical capacity of the spine in patients with chronic low back pain. Spine J. 2013;13(9): 1039-1047.
- Jette NG, Lim, Lim HL, Mokhtar SA, Gan SA, Singh DKA. Lumbar kinematics, functional disability and fear avoidance beliefs among adults with nonspecific chronic low back pain. Sultan Qaboos Univ Med J. 2016;16(4): e430–e436.
- Matheve T, Baets LDe, Bogaerts K, Timmermans A. Lumbar range of motion in chronic low back pain is predicted by task‐specific, but not by general measures of pain‐related fear. Eur J Pain. 2019;23(6):1171-1184.
- Al-Obaidi SM, Nelson RM, Al-Awadhi S, Al-Shuwaie N. The role of anticipation and fear of pain in the persistence of avoidance behavior in patients with chronic low back pain. Spine (Phila Pa 1976). 2000;25(9): 1126-1131.
- Leeuw M, Goossens ME, van Breukelen GJ, Boersma K, Vlaeyen JW. Measuring perceived harmfulness of physical activities in patients with chronic low back pain: The Photograph Series of Daily Activities--short electronic version. J Pain. 2007;8(11): 840-849.
- Verbunt JA, Seelen HA, Vlaeyen JW, Bousema EJ, van der Heijden GJ, Heuts PH, et al. Pain-related factors contributing to muscle inhibition in patients with chronic low back pain: An experimental investigation based on superimposed electrical stimulation. Clin J Pain. 2005;21(3): 232-240.
- Wesselink E, Raaij E de, Pevenage P, van der Kaay N, J Pool. Fear-avoidance beliefs are associated with a high fat content in the erector spinae: A 1.5 tesla magnetic resonance imaging study. Chiropr Man Therap. 2019;27: 14.
- Lee J, Park S. The relationship between physical capacity and fear avoidance beliefs in patients with chronic low back pain. J Phys Ther Sci. 2017;29(10): 1712-1714.
- Dubois JD, J Abboud, C St-Pierre, M Piche, M Descarreaux. Neuromuscular adaptations predict functional disability independently of clinical pain and psychological factors in patients with chronic non-specific low back pain. J Electromyogr Kinesiol. 2014;24(4): 550-557.
- Ferrari S, Vanti C, Pellizzer M, Dozza L, Monticone M, Pillastrini P. Is there a relationship between self-efficacy, disability, pain and sociodemographic characteristics in chronic low back pain? A multicenter retrospective analysis. Arch Physiother. 2019;9: 9
- Bandura A. Self-efficacy: Toward a unifying theory of behavioral change. Psychol Rev. 1977;84(2): 191-215.
- Levin JB, Lofland KR, Cassisi JE, Poreh AM, Blonsky ER. The relationship between self-efficacy and disability in chronic low back pain. Inter J Rehabil Hea. 1996;2(2): 19-28.
- Nicholas MK, Wilson PH, Goyen J. Comparison of cognitive-behavioral group treatment and an alternative non-psychological treatment for chronic low back pain. Pain. 1992;48(3): 339-347.
- Karasawa Y, Yamada K, Iseki M, Yamaguchi M, Murakami Y, Tamagawa T, et al. Association between change in self-efficacy and reduction in disability among patients with chronic pain. PLoS One. 2019;14(4): e0215404.
- La Touche R, Grande-Alonso M, Arnes-Prieto P, Paris-Alemany A. How does self-efficacy influence pain perception, postural stability and range of motion in individuals with chronic low back pain? Pain Physician. 2019;22: E1-E13.
- Lackner JM, Carosella AM, Feuerstein M. Pain expectancies, pain, and functional self-efficacy expectancies as determinants of disability in patients with chronic low back disorders. J Consul Clin Psychol. 1996;64(1): 212-220.
- de Moraes Vieira EB, de Goes Salvetti M, Damiani LP, de Mattos Pimenta CA. Self-efficacy and fear avoidance beliefs in chronic low back pain patients- coexistence and associated factors. Pain Manag Nurs. 2014;15(3): 593-602.
- Asghari A, Nicholas MK. Pain self-efficacy beliefs and pain behaviour. A prospective study. Pain. 2001;94(1): 85-100.
- Woby SR, Urmston M, Watson PJ. Self-efficacy mediates the relation between pain-related fear and outcome in chronic low back pain patients. Eur J Pain. 2007;11(7):711-718.
- Costa LCM, Maher CG, McAuley JH, Hancock MJ, Smeets RJ. Self-efficacy is more important than fear of movement in mediating the relationship between pain and disability in chronic low back pain. Eur J Pain. 2011;15(2): 213-219.
- Garg A, Moore JS. Prevention strategies and the low back in industry. Occupa Med. 1992;7(4): 629-640.
- Williams MM, Hawley JA, McKenzie RA, van Wijmen PM. A comparison of the effects of two sitting postures on back and referred pain. Spine. 1991;16(10).
- Vlaeyen JWS, Kole-Snijders AMJ, Boeren RGB, van Eck H. Fear of movement/(re)injury in chronic low back pain and its relation to behavioral performance. Pain. 1995;62(3): 363-372.
- O'Sullivan PB, Caneiro JP, O'Keefe M, Smith A, Dankaerts W, Fersum KV, et al. Cognitive functional therapy: An integrated behavioral approach for the targeted management of disabling low back pain. Phys Ther. 2018;98(5): 408-423.
- Griffin DW, Harmon DC, Kennedy NM. Do patients with chronic low back pain have an altered level and/or pattern of physical activity compared to healthy individuals? A systematic review of the literature. Physiotherapy. 2012;98(1): 13-23.
- Bernhardt J, Dewey H, Thrift A, Donnan G. Inactive and alone: Physical activity within the first 14 days of acute stroke unit care. Stroke. 2004;35(4): 1005-1009.
- Efficacy and safety of very early mobilisation within 24 h of stroke onset (AVERT): A randomised controlled trial. The Lancet. 2015;386(9988): 46-55.
- Hasenbring M, Hallner D, Klasen B. Psychological mechanisms in the transition from acute to chronic pain-over-or underrated? Schmerz. 2001;15(6): 442-447.
- van Weering M, Vollenbroek-Hutten MMR, Kotte EM, Hermens HJ. Daily physical activities of patients with chronic pain or fatigue versus asymptomatic controls. A systematic review. Clin Rehabil. 2007;21:1007-1023.
- Huijnen IP, Verbunt JA, Peters ML, Smeets RJ, Kindermans HP, Roelofs J, et al. Differences in activity-related behaviour among patients with chronic low back pain. Eur J Pain. 2011;15(7):748-755.
- de Looze MP, Dolan P, Kingma I, Baten CTM. Does an asymmetric straddle-legged lifting movement reduce the low-back load? Hum Move Sci. 1998;17(2): 243-259.
- Nolan D, O'Sullivan K, Stephenson J, O'Sullivan P, Lucock M. How do manual handling advisors and physiotherapists construct their back beliefs, and do safe lifting posture beliefs influence them? Musculoskelet Sci Pract. 2019;39: 101-106.
- Hsiang SM, Brogmus GE, Courtney TK. Low back pain (LBP) and lifting technique- A review. Intern J Indus Ergonomics. 1997;19(1):59-74.
- van Dieen JH, Hoozemans MJ, Toussaint HM. Stoop or squat: A review of biomechanical studies on lifting technique. Clin Biomech (Bristol, Avon). 1999;14(10): 685-696.
- Saraceni N, Kent P, Ng L, Campbell A, Straker L, O'Sullivan P. To flex or not to flex? is there a relationship between lumbar spine flexion during lifting and low back pain? a systematic review with meta-analysis. J Orthop Sports Phys Ther. 2020;50(3): 121-130.
- Bazrgari B, Shirazi-Adl A, Arjmand N. Analysis of squat and stoop dynamic liftings: muscle forces and internal spinal loads. Eur Spine J. 2007;16(5): 687-699.
- Singh G, Newton C, K O'Sullivan, Soundy A, Heneghan NR. Exploring the lived experience and chronic low back pain beliefs of English-speaking Punjabi and white British people: A qualitative study within the NHS. BMJ Open. 2018;8(2): e020108.
- Dolan P, Earley M, Adams MA. Bending and compressive stresses acting on the lumbar spine during lifting activities. J Biomech. 1994;27(10): 1237-1248.
- Kingma I, Faber GS, Bakker AJ, van Dieen JH. Can low back loading during lifting be reduced by placing one leg beside the object to be lifted? Phys Ther. 2006;86(8): 1091-1105.
- McGregor AH, Mc Carthy ID, Hughes SP. Lumbar spine motion during freestyle lifting and changes in this motion with time. J Back Musculoskelet. Rehabil. 1997;9(1): 35-37.
- Shin G, Mirka G. The effects of a sloped ground surface on trunk kinematics and L5/S1 moment during lifting. Ergonomics. 2004;47(6): 646-659.
- Sanchez-Zuriaga D, Lopez-Pascual J, Garrido-Jaen D, de Moya M F, Prat-Pastor J. Reliability and validity of a new objective tool for low back pain functional assessment. Spine (Phila Pa 1976). 2011;36(16): 1279-1288.
- Lariviere C, Gagnon D, Loisel P. A biomechanical comparison of lifting techniques between subjects with and without chronic low back pain during freestyle lifting and lowering tasks. Clin Biomech. (Bristol, Avon), 2002;17(2): 89-98.
- Hemming R, Sheeran L, van Deursen R, Sparkes V. Non-specific chronic low back pain: differences in spinal kinematics in subgroups during functional tasks. Eur Spine J. 2018;27(1): 163-170.
- Coenen P, Gouttebarge V, van der Burght AS, van Dieen JH, Frings-Dresen MH, van der Beek AJ, et al. The effect of lifting during work on low back pain: A health impact assessment based on a meta-analysis. Occup Environ Med. 2014;71(12): 871-877.
- Kim MH, Yi CH, Kwon OY, Cho SH, Cynn HS, Kim YH, et al. Comparison of lumbopelvic rhythm and flexion-relaxation response between 2 different low back pain subtypes. Spine (Phila Pa 1976). 2013;38(15): 1260-1267.
- Slaboda JC, Boston JR, Rudy TE, Lieber SJ. Classifying subgroups of chronic low back pain patients based on lifting patterns. Arch Phys Med Rehabil. 2008;89(8): 1542-1549.
- Hagins M, Lamberg EM. Individuals with low back pain breathe differently than healthy individuals during a lifting task. J Orthop Sports Phys Ther. 2011;41(3): 141-148.
- Pranata A, Perraton L, El-Ansary D, Clark R, Mentiplay B, Fortin K, et al. Trunk and lower limb coordination during lifting in people with and without chronic low back pain. J Biomech, 2018.
- McGill S, Seguin J, Bennett G. Passive stiffness of the lumbar torso in flexion, extension, lateral bending, and axial rotation. Effect of belt wearing and breath holding. Spine. 1994;19(6): 696-704.
- Chaitow L. Breathing pattern disorders, motor control, and low back pain. J Osteo Med. 2004;7(1): 33-40.
- Shirley D, Hodges PW, Eriksson AEM, Gandevia SC. Spinal stiffness changes throughout the respiratory cycle. J App Physiol. 2003;95(4): 1467-1475.
- O'Sullivan K, O'Sullivan P, O'Sullivan L, Dankaerts W. What do physiotherapists consider to be the best sitting spinal posture? Man Ther. 2012;17(5): 432-437.
- O'Sullivan K, O'Keeffe M, O'Sullivan L, O'Sullivan P, Dankaerts W. Perceptions of sitting posture among members of the community, both with and without non-specific chronic low back pain. Man Ther. 2013;18(6): 551-556.
- Dankaerts W, O'Sullivan P, Burnett AF, Straker LM. Differences in sitting postures are associated with non-specific chronic low back pain disorders when sub-classified. Spine. 2006;31(6): 698-704.
- Korakakis V, O'Sullivan K, O'Sullivan PB, Evagelinou V, Sotiralis Y, Sideris A, et al. Physiotherapist perceptions of optimal sitting and standing posture. Musculoskelet Sci Pract. 2019;39: 24-31.
- Roffey DM, Wai EK, Bishop P, Kwon BK, Dagenais S. Causal assessment of occupational sitting and low back pain: Results of a systematic review. Spine J. 2010;10(3): 252-261.
- O'Sullivan K, O'Sullivan L, O'Sullivan P, Dankaerts W. Investigating the effect of real-time spinal postural biofeedback on seated discomfort in people with non-specific chronic low back pain. Ergonomics. 2013;56(8): 1315-1325.
- Chen SM, Liu MF, Cook J, Bass S, Lo SK. Sedentary lifestyle as a risk factor for low back pain: A systematic review. Int Arch Occup Environ Health. 2009;82(7): 797-806.
- Park SM, Kim HJ, Jeong H, Kim H, Chang BS, CK Lee, et al. Longer sitting time and low physical activity are closely associated with chronic low back pain in population over 50 years of age: A cross-sectional study using the sixth Korea National Health and Nutrition Examination Survey. Spine J. 2018;18(11): 2051-2058.
- Hanna F, Daas RN, El-Shareif TJ, Al-Marridi HH, Al-Rojoub ZM, Adegboye OA. The relationship between sedentary behavior, back pain, and psychosocial correlates among university employees. Front Public Health. 2019;7: 80.
- Hodges PW, Richardson CA. Inefficient muscular stabilization of the lumbar spine associated with low back pain. A motor control evaluation of transversus abdominis. Spine (Phila Pa 1976). 1996;21(22): 2640-2650.
- Gupta N, Christiansen CS, Hallman DM, Korshoj M, Carneiro IG, Holtermann A. Is objectively measured sitting time associated with low back pain? A cross-sectional investigation in the NOMAD study. PLoS One. 2015;10(3): e0121159.
- Hides JA, Stokes MJ, Saide M, Jull GA, Cooper DH. Evidence of lumbar multifidus muscle wasting ipsilateral to symptoms in patients with acute/subacute low back pain. Spine (Phila Pa 1976). 1994;19(2): 165-172.
- Nourbakhsh MR, Arab AM. Relationship between mechanical factors and incidence of low back pain. J Ortho Sports Phys Ther. 2002;32(9): 447-460.
- Hides J, Gilmore C, Stanton W, Bohlscheid E. Multifidus size and symmetry among chronic LBP and healthy asymptomatic subjects. Man Ther. 2008;13: 43-49.
- Cholewicki J, VanVliet JJ. Relative contribution of trunk muscles to the stability of the lumbar spine during isometric exertions. Clin Biomech (Bristol, Avon). 2002;17(2): 99-105.
- Barker K L, Shamley DR, Jackson D. Changes in the cross-sectional area of multifidus and psoas in patients with unilateral back pain: The relationship to pain and disability. Spine. 2004;29: E515-519.
- Kader DF, Wardlaw D, Smith FW. Correlation between the MRI changes in the lumbar multifidus muscles and leg pain. Clin Radiol. 2000;55:145-149.
- Danneels LA, Vanderstraeten GG, Cambier DC, Witvrouw E, De Cuyper HJ. CT imaging of trunk muscles in chronic low back pain patients and healthy control subjects. Euro Spine J 2000;9: 266-272.
- Rezazadeh F, Taheri N, Okhravi SM, Hosseini SM. The relationship between cross-sectional area of multifidus muscle and disability index in patients with chronic non-specific low back pain. Musculosk Sci Pract. 2019;42: 1-5.
- Hides J, Stanton W, Mendis MD, Sexton M. The relationship of transversus abdominis and lumbar multifidus clinical muscle tests in patients with chronic low back pain. Manu Ther. 2011;16: 573-577.
- Kjaer P, Bendix T, Sorensen JS, Leboeuf-Yde C. Are MRI-defined fat infiltrations in the multifidus muscles associated with low back pain? BMC Medicine, 2007;5: 2.
- Hildebrandt M, Fankhauser G, Meichtry A, Luomajoki H. Correlation between lumbar dysfunction and fat infiltration in lumbar multifidus muscles in patients with low back pain. BMC Musculoskelet Disord. 2017;18(1): 12.
- Wan Q, Lin C, Li X, Zeng W, Ma C. MRI assessment of paraspinal muscles in patients with acute and chronic unilateral low back pain. Br J Radiol. 2015;88(1053): 20140546.
- Teichtahl AJ, Urquhart DM, Wang Y, Wluka AE, Wijethilake P, O'Sullivan R, et al. Fat infiltration of paraspinal muscles is associated with low back pain, disability, and structural abnormalities in community-based adults. Spine J. 2015;15(7): 1593-1601.
- Urquhart DM, Berry P, Wluka AE, Strauss BJ, Wang Y, Proietto J, et al. 2011 Young Investigator Award winner: Increased fat mass is associated with high levels of low back pain intensity and disability. Spine (Phila Pa 1976), 2011;36(16): 1320-1325.
- Verbunt JA, Smeets RJ, Wittink HM. Cause or effect? Deconditioning and chronic low back pain. Pain. 2010;149: 428-430.
- Mengiardi B, Schmid MR, Boos N, Pfirrmann CWA, Brunner F, Elfering A, et al. Fat content of lumbar paraspinal muscles in patients with chronic low back pain and in asymptomatic volunteers: Quantification with MR spectroscopy. Radiol. 2006;240: 786-792.
- Bayramoglu M, Akman MN, Kilinc S, Cetin S, Yavuz N, Ozker R. Isokinetic measurement of trunk muscle strength in women with chronic low-back pain. Am J Phys Med Rehabil. 2001;80: 650-655.
- Crossman K, Mahon M, Watson PJ, Oldham PJ, Cooper RG. Chronic low back pain-associated paraspinal muscle dysfunction is not the result of a constitutionally ‘‘adverse’’ fiber-type composition. Spine. 2004;29(6): 628-634.
- Hultman G, Nordin M, Saraste H, Ohlsèn H. Body composition, endurance, strength, cross-sectional area, and density of MM erector spinae in men with and without low back pain. J Spi Dis. 1993;6: 114-123.
- Humphrey AR, Nargol AV, Jones AP, Ratcliffe AA, Greenough CG. The value of electromyography of the lumbar paraspinal muscles in discriminating between chronic-low-back-pain sufferers and normal subjects. Eur Spine J. 2005;14(2): 175-84.
- Demoulin C, Grosdent S, Debois I, Mahieu G, Maquet D, Jidovsteff B, et al. Inter-session, inter-tester and inter-site reproducibility of isometric muscle strength measurements. Isokine Exer Sci. 2006;14: 317-325.
- Renkawitz T, Boluki D, Grifka J. The association of low back pain, neuromuscular imbalance, and trunk extension strength in athletes. Spine J. 2006;6(6): 673-83.
- Kankaanpaa M, Taimela S, Laaksonen D, Hanninen O, Airaksinen O. Back and hip extensor fatigability in chronic low back pain patients and controls. Arch Phys Med Rehabil. 1998;79(4): 412-417.
- Lariviere C, Bilodeau M, Forget R, Vadeboncoeur R, Mecheri H. Poor back muscle endurance is related to pain catastrophizing in patients with chronic low back pain. Spine (Phila Pa 1976). 2010;35(22): E1178-1186.
- Graves JE, Webb DC, Pollock ML, Matkozich J, Leggett SH, Carpenter DM, et al. Pelvic stabilization during resistance training its effects on the development of lumbar extension strength. Arch Phys Med Rehabil. 1994;75(2): 210-215.
- Pranata A, Perraton L, El-Ansary D, Clark R, Fortin K, Dettmann T, et al. Lumbar extensor muscle force control is associated with disability in people with chronic low back pain. Clin Biomech (Bristol, Avon). 2017;46: 46-51.
- Cooper NA, Scavo KM, Strickland KJ, Tipayamongkol N, Nicholson JD, Bewyer JD, et al. Prevalence of gluteus medius weakness in people with chronic low back pain compared to healthy controls. Eur Spine J. 2016;25(4): 1258-1265.
- de Looze MP, Toussaint HM, van Dieën JH, Kemper HCG. Joint moments and muscle activity in the lower extremities and lower back in lifting and lowering tasks. J Biomech. 1993;26(9): 1067-1076.
- Anderson H, Jakobsen J. A comparative study of isokinetic dynamometry and manual muscle testing of ankle dorsal and plantar flexors and knee extensors and flexors. Europ Neurol. 1997;37(4): 239-242.
- Latash ML, Levin MF, Scholz JP, Schoner G. Motor control theories and their applications. Medicina (Kaunas). 2010;46(6): 382-392.
- Hodges PW, Richardson CA. Delayed postural contraction of transversus abdominis in low back pain associated with movement of the lower limb. J Spin Dis. 1998;11: 46-56.
- Bohannon RW. Manual muscle testing: Does it meet the standards of an adequate screening test? Clin Rehabil. 2005;19(6): 662-667.
- Hodges PW, Richardson CA. Relationship between limb movement speed and associated contraction of the trunk muscles. Ergonomics. 1997;40(11): 1220-1230.
- Ferreira PH, Ferreira ML, Hodges PW. Changes in recruitment of the abdominal muscles in people with low back pain: Ultrasound measurement of muscle activity. Spine. 2004;29: 2560-2566.
- Moseley GL, Hodges PW, Gandevia SC. Deep and superficial fibers of the lumbar multifidus muscle are differentially active during voluntary arm movements. Spine (Phila Pa 1976), 2002;27(2): E29-36.
- Pulkovski N, Mannion AF, Caporaso F, Toma V, Gubler D, Helbling D, et al. Ultrasound assessment of transversus abdominis muscle contraction ratio during abdominal hollowing: a useful tool to distinguish between patients with chronic low back pain and healthy controls? Eur Spine J. 2012;21 Suppl 6: S750-9.
- Pinto RZ, Ferreira PH, Franco MR, Ferreira MC, Ferreira ML, Teixeira-Salmela LF, et al. The effect of lumbar posture on abdominal muscle thickness during an isometric leg task in people with and without non-specific low back pain. Man Ther. 2011;16(6): 578-584.
- Teyhen DS, Bluemle LN, Dolbeer JA, Baker SE, Molloy JM, Whittaker J, et al. Changes in lateral abdominal muscle thickness during the abdominal drawing-in maneuver in those with lumbopelvic pain. J Orthop Sports Phys Ther. 2009;39(11): 791-798
- Oliveira CB, Pinto RZ, Schabrun SM, Franco MR, Morelhao PK, Silva FG, et al. Association between clinical tests related to motor control dysfunction and changes in pain and disability after lumbar stabilization exercises in individuals with chronic low back pain. Arch Phys Med Rehabil. 2019;100(7): 1226-1233.
Author Info
Joshua Brodie Farragher1*,
Gavin Paul Williams2,3,
Adrian Pranata4,
Doa El-Ansary4,5,6,
Selina Parry3 and
Adam Leigh Bryant1
1Centre for Health Exercise and Sports Medicine, The University of Melbourne, Melbourne, Australia
2Department of Physiotherapy, Epworth Hospital,, Richmond, Australia
3Department of Physiotherapy, The University of Melbourne, Melbourne, Australia
4Department of Nursing and Allied Health, Swinburne University of Technology, Hawthorn, Australia
5Department of Surgery, The University of Melbourne, Melbourne, Australia
6Clinical Research Institute, Westmead Private Hospital, Westmead, New South Wales, Australia
Citation: Farragher JB, Williams G, Pranata A, El-Ansary D, Parry S, Bryant AL (2021) Adaptations Associated with Non-Specific Chronic Low Back Pain: A Narrative Review. Int J Phys Med Rehabil. 9:602.
Received: 06-Apr-2021
Accepted:
20-May-2021
Published:
27-May-2021
, DOI: 10.35248/2329-9096.21.9.602
Copyright: © 2021 Farragher JB, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
