Clinical & Experimental Cardiology
Open Access
ISSN: 2155-9880
ISSN: 2155-9880
Case Report - (2023)Volume 14, Issue 7
Overview of the risks associated with Tricyclic Antidepressant (TCA) intoxication, along with an analysis of the relevant risk factors; including improper medication disposal and unsupervised access to expired medications; and treatment strategies. By presenting a case involving a comatose teenager who was diagnosed with amitriptyline intoxication (described in the literature as fatal), highlighting the severity and potentially fatal consequences of TCA overdose. If TCAs are not adequately disposed of after their expiration date or if they are not stored securely, they can become accessible to children, who may not be aware of the potential dangers associated with expired medications.
Amitriptyline; Cardiac arrhythmia; Vascular hypotension; Coma
While effective when used properly and under medical supervision, TCA intoxication can be a serious and potentially life-threatening medical emergency [1]. In this case, we will explore the risks associated with TCA intoxication and the relevant factors that contribute to it, including improper medication disposal and unsupervised access to expired medications. However, when taken in large quantities, TCAs can lead to a range of toxic effects on the body, primarily due to their impact on the cardiovascular and central nervous systems [2,3]. By addressing the risk factors associated with TCA intoxication, healthcare professionals and society at large can work together to enhance medication safety and prevent potential harm to individuals who use or come into contact with these medications.
Transport of a comatose 16-year-old girl by the emergency medical service, who had been previously announced that the 16-year-old was having a syncope over the phone [4]. Clinically noticeable at hospital: vertical up and down movements of the tongue, no motor manifestations, flaccid muscle tone, blood pressure 130/85, heart rate 130 bpm, temperature 35.5°C, no response to painful stimuli, constricted and non-reactive pupils, old self-inflicted cuts on the abdomen and on both thighs [5]. The parents, who arrived shortly after, reported a quarrel at home and statet that they had found their daughter in a state of impaired consciousness kneeling in front of her bed. Both parents were taking various medications for different illnesses and handed us a photo of all the medications (Figure 1).
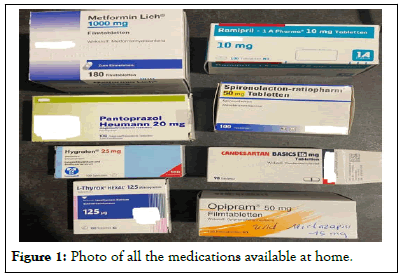
Figure 1: Photo of all the medications available at home.
According to the parent’s statement, there were no empty boxes in her room. The emergency CT scan and routine laboratory analysis were unremarkable, but the urine test for medications and drugs showed tricyclic antidepressants [6,7]. Serum was collected and later sent for quantitative analysis of the tricyclic antidepressants, followed by a transfer to the intensive care unit for monitoring. There, a severe arterial hypotension (nadir 80/50) and sinus tachycardia (max.157 bpm) developed, with persistent impairment of consciousness for almost 2 days and very slow recovery. Several 12-lead ECGs showed no further cardiac abnormalities [8]. Additional cerebral Magnetic Resonance Imaging (MRI) examination and lumbar puncture revealed evidence of inactive multiple sclerosis (positive oligoclonal bands, no enhancement of lesions, no pleocytosis in the Cerebrospinal Fluid (CSF), known maternal multiple sclerosis) (Figure 2).
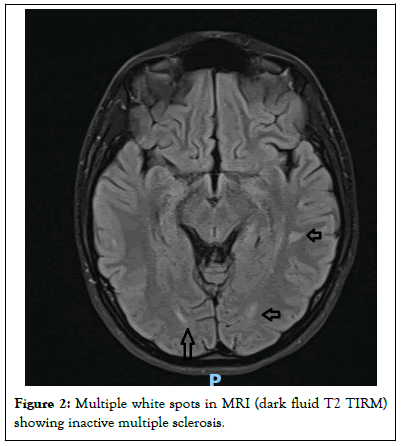
Figure 2: Multiple white spots in MRI (dark fluid T2 TIRM) showing inactive multiple sclerosis.
In the course of the treatment, inflammatory markers increased, and we found radiological evidence of pneumonia, which was interpreted as aspiration pneumonia and successfully treated with Piperacillin/Tazobactam. On the 6th day of hospitalization, we received the analysis of tricyclic antidepressants in the blood, showing a highly toxic (described as lethal in the literature) total level of amitriptyline and nortriptyline of 1044 ng/ml. The therapeutic range is 80-200 ng/ml, and the toxicity threshold is 300 ng/ml. However, amitriptyline was not among the medications reported by the parents to be present in the household. Explanation: The parents had disposed of expired medications and those with side effects they considered "too strong" in the household waste. Our patient had retrieved them and ingested an unknown amount on the evening of the quarrel.
Amitriptylin is metabolized into its active form through demethylation and has a high distribution volume due to its lipophilic nature. The recommended adult dosage is 25-75 mg/ day. In cases of amitriptyline poisoning, cardiovascular effects, such as arrhythmias and low blood pressure, are prominent, along with various central nervous and anticholinergic symptoms.
Sinus tachycardia, caused by the drug’s anticholinergic properties and inhibition of norepinephrine reuptake, is the most common cardiac rhythm disorder in amitriptyline overdose [9]. CNS toxicity is characterized by central nervous system depression with decreased consciousness leading to coma, seizures, and anticholinergic syndromes. Most complications are expected within 6 hours, but cardiac events can also occur later. Cardiological continuous monitoring is strongly recommended, and Multi-Lead ECGs should be recorded and measured, as a QRS duration of over 160 m/sec dramatically increases the risk of malignant cardiac arrhythmias [10]. Other ECG abnormalities, such as prolonged PQ and QT intervals, nonspecific ST-segment and T-wave changes, and atrioventricular block patterns, should be identified as predictors of complications. Even in the absence of evident cardiac arrhythmias, the prophylactic administration of Sodium Bicarbonate (NaBic) is recommended at a dosage of 1-2 mmol/ kg body weight, as it can reduce cardiac toxicity.
The use of sodium bicarbonate has benefits as it increases the serum pH and extracellular sodium concentration. By alkalizing the body, it favors the neutral form of amitriptyline, leading to a decrease in the active form of nortriptyline [11]. Nortriptyline can induce sodium channel blockades, which can result in cardiac rhythm disturbances and hemodynamic instability. The administration of sodium bicarbonate increases the electrochemical gradient across cardiac cell membranes by elevating extracellular sodium levels, thereby attenuating the nortriptyline-induced sodium channel blockade. Intravenous lipid emulsion therapy is a promising treatment for combating local anesthetic toxicity. There is evidence from animal studies and case reports in humans suggesting that lipid therapy may also be effective in cases of TCA overdose [12]. A bolus of 1.5 ml/kg of a 20% lipid emulsion is recommended within one minute, followed by an infusion rate of 0.25 ml/kg per minute (400 ml over 20 minutes). If hemodynamic instability persists, the bolus administration should be repeated after 5 minutes and the infusion rate doubled. The maximum cumulative dose is stated as 12 ml/kg.
In our case, the cause of the symptoms was only clarified on the 6th day of hospitalization, so no medications were administered. Since there is no clear correlation between amitriptyline plasma concentration and the prognosis of outcome after an overdose, the only reliable indicator is the QRS duration in the ECG. We believe that the patient survived despite having a lethal level of amitriptyline because the QRS duration in the recorded ECGs consistently remained within the normal range, and no malignant cardiac rhythm disturbances were observed.
• Send the collected serum for rapid determination of suspected substances using Liquid Chromatography with tandem Mass Spectrometry (LC-MS/MS).
• Continuous ECG monitoring in cases of tricyclic antidepressant poisoning (especially with amitriptyline).
• In addition to symptomatic intensive therapy (hemodynamic stabilization), the administration of NaBic and Lipid emulsion therapy may be lifesaving!
Citation: Elsayed K, Endmann M (2023) Adolescents and the Toxic Effects of Antidepressants: Understanding the Risks-Case Report. J Clin Exp Cardiolog. 14:817.
Received: 17-Jul-2023, Manuscript No. JCEC-23-25689; Editor assigned: 19-Jul-2023, Pre QC No. JCEC-23-25689 (PQ); Reviewed: 02-Aug-2023, QC No. JCEC-23-25689; Revised: 09-Aug-2023, Manuscript No. JCEC-23-25689 (R); Published: 16-Aug-2023 , DOI: 10.35248/2155-9880.23.14.817
Copyright: © 2023 Elsayed K, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.