Journal of Physical Chemistry & Biophysics
Open Access
ISSN: 2161-0398
ISSN: 2161-0398
Research Article - (2013) Volume 3, Issue 2
The removal of 4-Chloro-2-nitrophenol (4C2NP) from aqueous solutions using zero valent iron nanoparticles (nZVI) and Pd-doped zero valent iron nanoparticles (Pd-nZVI) was investigated in terms of initial pH, adsorbent dosage, contact time and temperature. The maximum adsorption occurred at the pH value of 5. The adsorbent studied exhibits high efficiency for 4C2NP adsorption and the equilibrium states could be achieved in 10 min in both the adsorbents. Equilibrium data were analyzed by two isotherms, namely the Freundlich isotherm and the Langmuir isotherm. The best fit to the data was obtained with the Langmuir isotherm. Adsorption kinetics data were modeled using the first order, the pseudo-first and pseudo-second order and Elovich equations. Results show that the pseudo-second order kinetics model was found to correlate the experimental data well.
<Keywords: Adsorption; Kinetic models; nZVI; Pd-nZVI; 4-Chloro- 2-nitrophenol
Polluted water has always been a serious problem to the environment. Besides, various water pollutants including pesticides , dyes and surfactants, pharmaceuticals are emerging classes of aquatic contaminants. Industrial use of phenol and its derivatives over the past decades had led to severe environmental pollution. Out of this, around 190.3 ton per month constitute phenolic wastes disposed mainly by petrochemicals, pharmaceuticals and polymer industries [1,2]. 4-Chloro-2-nitrophenol (4C2NP) is the one most common isomer of chloronitrophenol that have been detected in various industrial effluents [3,4]. A number of physicochemical methods have been used for treatment of wastewater containing 4C2NP. There are some reports to destruction of 4-chloro-2-nitrophenol such as ozonation [4], coimmobilized mixed culture system [5,6], various advanced oxidation processes [7] and adsorption onto nano-TiO2 [8], CNTs [9].
Zero valent iron (ZVI) materials were proposed as a reactive material in permeable reactive barriers (PRBs) due to its great ability in reducing and stabilizing different types of pollutants [10,11].
Pd-doped nZVI (Pd-nZVI) is known to exhibit higher reactivity than the other reported bimetallic systems [12,13].
Therefore, the present objective of this study is to evaluate the adsorption ability of 4C2NP using nZVI and Pd-nZVI surfaces were investigated. Adsorption isotherms parameters were also calculated and discussed. Finally, the rates and mechanism of the adsorption process were investigated. Various kinetic evaluations have been used to describe the adsorption process. Here we attempted to apply a simple first order kinetic model for changing the bulk concentration, and pseudo first-order rate equation and pseudo second-order and Elovich model for the adsorbent phase concentration [14,15].
Materials
Pd-Fe bimetallic nanoparticles were produced by adding 0.54 mol/L NaBH4 aqueous solution dropwise to a 1-L three-necked flask containing equal volume of 0.27 mol/L FeSO4·6H2O aqueous solution with mechanical stirring at 30°C for 10 min. First of all, the ferrous iron was reduced to zero-valent iron according to the following reaction:
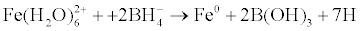
The Fe0 nanoparticles were then rinsed 3 times with 400 ml deoxygenated deionized water under nitrogen atmosphere. Subsequently, the Pd-Fe bimetallic nanoparticles were synthesized by reacting the wet iron particles with desired amount of potassium hexachloropalladate aqueous solution under stirring and nitrogen atmosphere according to the bimetallic nanoparticles were following equation:
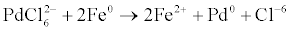
Then rinsed 3 times with 400 ml deoxygenated deionized water under nitrogen atmosphere to remove chloride ions.4-Chloro-2- nitrophenol (C6H4ClNO3, Mw= 173.56 g/mol) was supplied by Fluka, Germany (Table 1).
Table 1: Physicochemical properties of the nZVI and Pd-nZVI.
| Isotherm | Langmuir | Freundlich | ||||
| qm(mg/g) | KL(L/mg) | r2 | KF | n | r2 | |
| nZVI | 4.8476 | 0.4677 | 0.9957 | 3.6838 | 0.5705 | 0.9916 |
| Pd-nZVI | 6.6181 | 0.5488 | 1.0000 | 7.6278 | 0.6477 | 0.9904 |
Table 2: Isotherm constants for the adsorption of 4C2NP.
| Simple 1st order | Pseudo 1st order | Pseudo 2nd order | Elovich | ||||||||
| k1 | r2 | k1 | qe | r2 | k2 | qe | r2 | β | α | r2 | |
| nZVI | 0.0369 | 0.9861 | 0.1005 | 2.030 | 0.9859 | 0.2250 | 4.914 | 0.9983 | 1.583 | 115.66 | 0.9753 |
| Pd-nZVI | 0.0395 | 0.9839 | 0.1101 | 2.231 | 0.9891 | 0.2112 | 5.414 | 0.9969 | 1.495 | 48.996 | 0.9785 |
Table 3: Comparison of the simple first order, pseudo first- and second-order and Elovich model.
Batch adsorption experiments
All adsorption experiments were carried out at room temperature (25°C). Solutions were placed in glass flasks and gently agitated on a rotary shaker. After agitating the flasks forpredetermined time intervals samples were withdrawn from the flasks. The adsorbent was separated from the solution by centrifugation (REMI make) at 1000 rpm for 1 minute.The final concentrations of the 4C2NP solutions were analyzed using a UV–Vis spectrophotometer (Shimadzu UV–Vis, UV-160). The amount of 4C2NP adsorbed (qe) in mg/g at time t was computed by using the following equation:
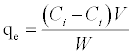 (1)
(1)
Where Ci and Ct are the 4C2NP concentrations in mg/L initially and at a given time t, respectively, V is the volume of Cd and Pb solutions in L, and w is the weight of sorbent in g.
Effect of contact time
The removal of 4C2NP increased with time and attains saturation in about 10 min. basically, the removal of adsorption is rapid, but it gradually decreases with time until it reaches equilibrium. Figure 1 represents the amount removal of 4C2NP versus the contact time for the constant initial concentration and by using the optimum pH value which was obtained for the two adsorbents [16].
Effect of pH
The experiments carried out at different pH values showed that there was a change in the quantity of adsorbed 4C2NP on the solid phase of nZVI and Pd-nZVI over the entire pH range of 2 to 12, as shown in Figure 2. This Figure indicated the strong force of interaction between the cadmium and lead ions and the adsorbent powder that, H+ ion could influence the adsorption capacity. Electrostatic repulsion decreases with the increasing in pH due to reduction of positive charge density on the adsorption edges thus resulting in an increase 4C2NP adsorption [17] (Table 4). Here the interaction is more at pH=5 due to the competence of acidic H+ ion with 4C2NP for the adsorption sites.
| Adsorbent | qm(mg/g) | Ref. |
| SWCNTs | 1.4440 | [9] |
| MWCNTs | 4.4260 | [9] |
| nZVI | 4.8476 | This study |
| Pd-nZVI | 6.6181 | This study |
Table 4: Comparison of 4C2NP adsorption with different adsorbents.
Effect of adsorbent dose
The effect of adsorbent dose on the removal of 4C2NP for different concentrations (3 and 9 mg/L) were investigated by agitating with different adsorbent dosage over the range of 0.1-0.9 g/L. The study reveals that amount adsorption increases with increase in the nZVI and Pd-nZVI (Figure 3). This attributes the increased adsorbents surface area and availability of more adsorption sites.Almost complete 4C2NP removal was achieved within 10 min from a different 4C2NP concentration solution at pH 5.0 in the presence of 0.5 g/L amount of adsorbents.
Effect of temperature
To study the effect of temperature on the adsorption of 4C2NP by the adsorbents, the experiments were performed at temperatures of 298, 308, 318, 328, and 338 K. Figure 4, shows the adsorption capacity of 4C2NP by these adsorbents was found to decrease with a rise in temperature, suggesting the process in this research has been exothermic.
Adsorption isotherms
From the various isotherm equations that may be used to analyze adsorption data in aqueous phase, the Langmuir [18]—the theoretical equilibrium isotherm and the Freundlich [19]—the empirical equilibrium isotherm are the most common models.The linear forms of these equations are displayed as equation (2) (Langmuir) and (3) (Freundlich):
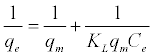 (2)
(2)
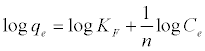 (3)
(3)
where qm (mg g-1) is the maximum adsorption capacity, qe (mg g-1) is the amount of adsorbed 4C2NP, Ce (mg L-1) is the equilibrium 4C2NP concentration, KF and n are the Freundlich constants, and KL (L mg-1) is the Langmuir constant. The linear Langmuir isotherms were fitted to the experimental data. The Langmuir and Freundlich parameters, along with the coefficients of determination (r2) of the linear plots, are presented in Table 2. Adsorption of 4C2NP on nZVI and Pd-nZVI can be fitted by Langmuir model (Figure 5).
Kinetic study
In order to examine the mechanism of adsorption process such as mass transfer and chemical reaction, a suitable kinetic model is needed to analyze the rate data. Many models such as homogeneous surface diffusion model and heterogeneous diffusion model (also known as pore and diffusion model) have been extensively applied in batch reactors to describe the transport of adsorbate inside the adsorbent particles.
Simple First Order Model: The sorption kinetics may be described by a simple first order equation [20]. The change in bulk concentration of the system using the followinga linear form simple first order rate Equation (4).
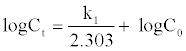 (4)
(4)
Where Ct and Co are the concentration of 4C2NP at time t and initially (mg/L), respectively, and k1 is the first order rate constant, (1/min).
The experimental results showed that the log Ct versus t (Figure 6(A)) for constant initial concentrations of 4C2NP was deviated considerably from the theoretical data.
Pseudo Firs-order Model: The adsorption kinetics may be described by a pseudo first order equation [21]. The linear form equation is the following:
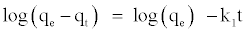 (5)
(5)
where qe and qt are amounts of 4C2NP adsorbed at equilibrium and at time (mg/g), respectively, and k1 is the equilibrium rate constant of pseudo first-order adsorption, (1/min). Figure 6(B) shows a plot of linearization form of pseudo first-order model. The correlation coefficients for the pseudo first order kinetic model were low. This suggests that this adsorption system is not a pseudo first-order reaction.
Pseudo Second-order Model: The adsorption kinetics may also be described by a pseudo second-order equation [22]. The linear form equation is the following:
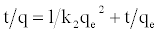 (6)
(6)
Where k2 is the equilibrium rate constant of pseudo second-order adsorption (g/mg.min). The slopes and intercepts of plots t/q versus t were used to calculate the pseudo second-order rate constants k2 and qe. The straight lines in plot of t/q versus t (Figure 6(C)) show good agreement of experimental data with the pseudo second-order kinetic model. Table 3 lists the computed results obtained from the pseudo second-order kinetic model. These indicate that the adsorption system studied belongs to the second order kinetic model.
Elovich Model: The Elovich model equation is generally expressed as [23]
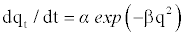 (7)
(7)
where α is the initial adsorption rate (mg/g.min) and β is the adsorption constant (g/mg) during any experiment.
To simplify the Elovich equation, Chien and Clayton assumed α β >> t and by applying the boundary conditions at and at equation (7) becomes qt = 0 at t = 0 and qt = qt att = tequation (8) become:
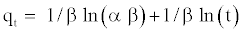 (8)
(8)
If 4C2NP adsorption fits the Elovich model, a plot of qt versus ln(t) (Figure 6(D)) should yield a linear relationship with a slope of 1/β and an intercept of 1/β ln(α β). The correlation coefficients for the Elovich kinetic model were low. This suggests that this adsorption system is not an acceptable for this system.
The Intra-particle Diffusion Model: The kinetic results were further analyzed by the intra-particle diffusion model to elucidate the diffusion mechanism [24]
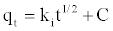 (9)
(9)
where C is the intercept and ki is the intra-particle diffusion rate constant (mg/g min0.5), which can be evaluated from the slope of the linear plot of qt versus t1/2 (Figure 7).
The first sharper portion is due to the diffusion of adsorbate through the solution to the external surface of the adsorbent and the second portion represents the gradual adsorption procedure, that is, the diffusion of adsorbate molecules inside the adsorbent.It is easy to find that ki of first region was higher than ki of second region. This indicates that the adsorption rate of 4C2NP is higher in the beginning owing to the large surface area of the adsorbent available for the adsorption. The adsorbate formed a thick layer in the exterior gradually due to the inter attraction and molecular association. This blocked the further adsorption and the uptake rate was limited by the rateat which the adsorbate was transported from the exterior to the interior sites of the adsorbent particles.
Adsorption kinetic and equilibrium parameters for 4C2NP on nZVI and Pd-nZVI were obtained in a batch system. Adsorption capacity was dependent on the contact time, pH of the solution, adsorbent dosage and temperature.The maximum amount of 4C2NP removal from the wastewater for nZVI and Pd-nZVI in the contact time = 10 min. The adsorption capacity of 4C2NP on Pd-nZVI is more than nZVI surfaces and the adsorption isotherms are fitted by Langmuir equation. The kinetics of adsorbents was experimentally studied and the obtained rate data were analyzed using the simple first order, pseudo first-order, the pseudo second-order and Elovich kinetic models. Based on the values of the correlation coefficient (r2) obtained for all tested models, pseudo second-order was found to best correlate the rate kinetic data of nZVI and Pd- nZVI.
The financial support of this work by Islamic Azad University Shahre-Qods Branchand Islamic Azad University Science and Research Branch is greatly acknowledged.