Journal of Chromatography & Separation Techniques
Open Access
ISSN: 2157-7064
ISSN: 2157-7064
Research Article - (2014) Volume 5, Issue 5
Batch adsorption of Cd (II) onto orange peel, a residue of the fruits processing industry, has been studied. Equilibrium isotherms, kinetic data, and thermodynamic parameters have been evaluated. Equilibrium data fit well with Langmuir isotherm model. The kinetic data were found to follow the pseudo-second-order model. The negative ΔHº value indicates the exothermic nature of the adsorption process. Orange peel was shown to be a promising adsorbent for Cd (II) removal from aqueous solutions.
Keywords: Adsorption; Isotherms; Kinetic models; Thermodynamics; Orange peel
A Constant describing the energy of interaction between solute and adsorbent surface;
β Desorption constant (g/mg) during any experiment;
C0 Initial concentration (mg/l);
CeEquilibrium concentration (mg/l);
KF & n Freundlich constants;
k1 Pseudo first-order adsorption rate constant (l/min);
k2Pseudo second-order adsorption rate constant (g/mg. min);
KD Distribution coefficient (cm3.g-1)
KLLangmuir constants (L mg-1);
kid Intraparticle diffusion rate constant (min-1);
MWeight of adsorbent (mg);
q Adsorption capacity,(mg of Cd (II) /g adsorbate);
qe Adsorption capacity at equilibrium, (mg of Cd (II) /g adsorbate);
qmax Maximum adsorption (mg of Cd (II) /g adsorbate);
qt Adsorption capacity at time t, (mg of Cd (II) /g adsorbate);
R Gas constant (8.314 J/mol/K);
Rc Percent of Cd (II) adsorbed (mg/g);
ReThe removal efficiency of Cd (II) (mg/g);
r2 Correlation coefficient
TTemperature (K)
tContact time (min);
V Volume of the solution;
α Initial adsorption rate (mg of Cd (II)/g adsorbate. min);
Cadmium is one of the toxic heavy metals released to environment from a number of industries such as, electroplating process, metallurgy, pigments, plastic, fertilizers and batteries production. The maximum concentration of cadmium ions in drinking water is 0.003 mg/L according to the World Health Organization [1]. Conventional heavy metal clean-up technologies cover precipitation, ion exchange, chemical oxidation/reduction, reverse osmosis, electrodialysis, ultra filtration, solvent extraction, etc.[2]. However some disadvantages of the technologies, such as high cost, sensitive operating conditions and production of secondary sludge [3,4]. Adsorption process has received much interest and become an alternative to conventional precipitation and other techniques, especially for wastewaters that contain low concentrations of metals and its effectiveness [5]. Activated carbon is considered to be a highly effective adsorbent for heavy metal removal from wastewater, but it is readily solubilized under extreme pH conditions [6], and is also very high cost [7]. Low-cost agricultural waste byproducts, such as sugarcane bagasse [8], rice husks [9], sawdust [10], coconut husks [11], and oil palm shell [12], have been investigated to eliminate heavy metals from wastewater. Agricultural residues are usually composed of lignin and cellulose as the major constituents with other polar functional groups such as alcohols, aldehydes, ketones, carboxylic acids and ethers that facilitate metal complexation resulting biosorption of heavy metal ions[13], from wastewaters. The objective of this research was to investigate the removal of Cd(II) from aqueous solutions by orange peel. The effect of adsorbent dosage, initial metal concentration, contact time, temperature and pH were examined. Equilibrium isotherms, kinetic data, and thermodynamic parameters have been studied.
Preparation of adsorbent
Orange fruits were purchased from a local market and peeled manually. The peels was washed thoroughly with distilled water to remove the dirt, and dried at 80°C for 24 h, finally crushed and sieved to obtain a particle size of 0.355 mm and used as such.
Preparation of adsorbate solutions
1000 mg/L of Cd (II) was prepared as stock solution by dissolvingthe desired quantity of CdCl2·2H2O (Aldrich, USA) in distilled water. The required solutions were prepared by diluting the stock solution tothe desired Cd (II) concentrations. The Cd (II) concentrations were determined by an atomic adsorption spectrophotometer.
Adsorption experiments
Batch experiments were carried out to investigate the parametric effects of adsorbent dose, contact time, pH, temperature, and initial Cd(II) concentration foradsorption ontoorange peel. 50mL of different concentrations (50-150 mg/L) of Cd (II) solutions (Co) with a range of pH values from 2 to10 was transferred in a conical flaskwith a required amount of adsorbent. The solution was agitated at 150 rpm in a thermostatic shaker water bathfor different time (10 to120 min) at different temperature (30, 40, 50 and 60°C). The samples were withdrawn and centrifuged at 5000 rpm for 5 min and the supernatant solutions were analyzed. The pH of the solutions was adjusted with0.1 N NaOH or 0.1 N HCl.
The removal efficiency of Cd (II) was defined as:
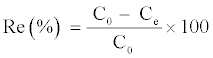 (1)
(1)
In addition, the adsorption capacity (q) is calculated according to the following equation:
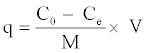 (2)
(2)
Adsorption Isotherms Study
Equilibrium studies that give the capacity of the adsorbent and adsorbate are described by adsorption isotherms, which is usually the ratio between the quantity adsorbed and that remained in solution at equilibrium at fixed temperature [14,15]. Freundlich and Langmuir isotherms are the earliest and simplest known relationships describing the adsorption equation.
Langmuir isotherm
The Langmuir equation is used to estimate the maximum adsorption capacity corresponding to complete monolayer coverage on the adsorbent surface and is expressed by [16]:
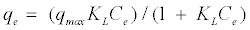 (3)
(3)
The linear form of the above equation after rearrangement is given by:
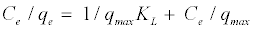 (4)
(4)
The experimental data is fitted into the above equation for linearization by plotting Ce/qe against Ce. The constants qmax and KL can be determined from the slope and intercept, respectively.
Freundlich isotherm
The Freundlich model [17], is an empirical equation used to estimate the adsorption intensity of the sorbent towards the adsorbate and is given by:
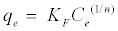 (5)
(5)
Also, the value of n indicates the affinity of the adsorbate towards the adsorbent. The above equation is conveniently used in linear form as:
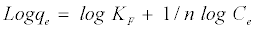 (6)
(6)
A plot of ln Ce against lnqe yielding a straight line indicates the conformation of the Freundlich adsorption isotherm. The constants 1/n and ln KF can be determined from the slope and intercept, respectively.
Adsorption Dynamics Study
The study of adsorption dynamics describes the adsorbate uptake rate and evidently, this rate controls the residence time of adsorbate uptake at the solid-solution interface. Kinetics of Cd (II) adsorption on the orange peel was analyzed using pseudo first-order, pseudo secondorder, Elovich and intra particle diffusion kinetic models [18,19].
Pseudo-first-order model
The pseudo first order kinetic model [20], was given by equation:
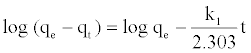 (7)
(7)
Values of k1 and qe were calculated from the slope and intercept values of the straight line of plotting log (qe-qt) versus t.
Pseudo-second-order model.
The sorption data were also analyzed in terms of pseudo-second order model [20,21],given by the equation:
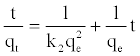 (8)
(8)
If the initial adsorption rate, h (mg/g. min) is:
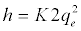 (9)
(9)
then Equations (11) and (12) become:
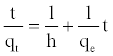 (10)
(10)
The plot of t/qt versus t should give a straight line and the pseudo second order rate constant, K2 and equilibrium adsorption capacity, qe, were calculated from the values of intercept and slope, respectively.
The Elovich model
The Elovich model equation is generally expressed as [19]:
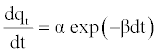 (11)
(11)
To simplify the Elovich equation, assumed αβt >> t and by applying the boundary conditions t=0 to t=t and qt=0 to qt=qt, Equation (9) becomes:
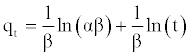 (12)
(12)
A plot of qt vs. ln(t) should yield a linear relationship with a slope of (1/β) and an intercept of (1/β) ln(αβ).
The intraparticle diffusion model
The intraparticle diffusion model is expressed as [16]:
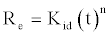 (13)
(13)
A linearized form of the equation is obtained as:
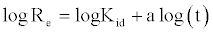 (14)
(14)
If Cd (II) adsorption fits the intraparticle model, a plot of log R vs. log t should yield a linear relationship with a slope of a and an intercept of log kid.
Characterization of adsorbent
Figure 1a and 1b represent the SEM photographs ofadsorbent before and after adsorption with 1000x magnification. It became apparent from the surface texture ortopology of adsorbent before adsorption that the adsorbent surface isrough, porous and irregular shapes. After adsorption, Figure 1b shows that the metal ion being adsorbed on the adsorbent site. In addition,adsorbent surface was stuffed with Cd (II) ion resulting in loss of surface porosity and roughness.
Adsorption dynamics
Effect of pH: The effect of pH has been studied by varying it in the range of 2-10 at 30°C, 2 hrs, 0.5 adsorbent and particle size of 0.335 mm (Table 1). It was observed that the removalofCd (II) increases with the increase of pHfrom 2 to8. After that, the capacity of adsorption decreases slightly in pH range of 8-10. The highest adsorption efficiency is observed at pH 5. These observations can be explained by the fact that at lower pH values, the competing of H+ with Cd(II) for the adsorption sites of on orange peel, lead to decreasing the removal percent of Cd(II). But with increasing pH there were fewer H+ ions present in the solution and consequently more negatively charged sites were made available and this facilitated greater Cd (II) ions uptake by electrostatic attraction. Decreasing in adsorption at high pH may be due to the formation of soluble hydroxyl complexes [22].
| Parameter | Removal efficiency (Re %) | q (mg /g) | |
|---|---|---|---|
| pH: | 2 | 25.53 | 1.277 |
| (Condition: 50mg L-1, mass = 0.5 g, 3 h, 30°C) | 3 | 84.45 | 4.252 |
| 4 | 92.33 | 4.616 | |
| 5 | 97.33 | 4.889 | |
| 6 | 97.58 | 4.875 | |
| 7 | 97.73 | 4.886 | |
| 8 | 97.70 | 4.888 | |
| 9 | 89.34 | 4.457 | |
| 10 | 68.54 | 3.427 | |
| Time (min): | 10 | 49.92 | 2.460 |
| (Condition: 50 mg L-1, mass = 0.5 g, pH= 5 , 30 °C) | 20 | 56.21 | 2.811 |
| 30 | 77.33 | 3.867 | |
| 40 | 89.50 | 4.475 | |
| 50 | 93.19 | 4.659 | |
| 60 | 97.76 | 4.889 | |
| 80 | 97.71 | 4.885 | |
| 100 | 97.65 | 4.882 | |
| 120 | 97.75 | 4.887 | |
| Initial concentration (mg /l): | 50 | 97.76 | 4.889 |
| Condition: pH= 5, mass = 0.5 g, 60 min, 30 °C) | 75 | 94.29 | 7.12 |
| 100 | 90.68 | 9.36 | |
| 125 | 76.36 | 9.35 | |
| 150 | 62.28 | 9.34 | |
| Adsorbent dose (g) : | 0.3 | 78.12 | 4.075 |
| Condition: 50 mg L-1, 60 min, pH= 5, 30 °C) | 0.4 | 91.34 | 4.567 |
| 0.5 | 97.76 | 4.889 | |
| 0.6 | 97.69 | 4.073 | |
| 0.7 | 97.71 | 3.489 | |
| Temperature (°C) : | 30 | 97.76 | 4.889 |
| Condition: 50 mg L-1, 60 min, mass = 0.5 g, pH=5) | 40 | 96.50 | 4.825 |
| 50 | 95.38 | 4.769 | |
| 60 | 92.24 | 4.612 | |
Table 1: Adsorption data of adsorption process.
Effect of initialCd (II) concentrations and contact time: The removal of Cd (II)ontoorange peel was found to increase with time and attained a maximum value at 60 min. On changing the initial concentration of Cd (II) solution from 50 to 150 mg/l at 30°C, 0.5 g adsorbent, pH 5 and particle size of 0.335 mm. At low concentrations, metal ions are easily adsorbed on vacant sites. Table 1 shows the effect of metal ion concentration on percent removal of Cd (II). As the metal ion concentration increases, the percent removal decreases. Table 1 shows the effect of metal ion concentration on percent removal of Cd (II). As the metal ion concentration decreases, the percent removal increases. This may be due to the vacant sites are filled up and no further adsorption occurs due to saturation of vacant sites of adsorbent.
Effect of adsorbent dosage: The adsorption percent at various doses of orange peel from 0.3 to 0.7 g is shown in Table 1. Increasing the adsorbent dose to 0.5 g increase the adsorption percent of metal ions, which is due to the increasing in adsorption sites of adsorbent material resulting from increasing of surface area of adsorbent. However, further increase of adsorbent dosage does not afford exhaustive adsorption of Cd (II). This may be due to overlapping of adsorption sites as a result of overcrowding of adsorbent particles
Adsorption isotherms
The results of this study show that orange peel was effective, in the adsorption of Cd (II) as its removal reached 97.33% at 30°C, pH 5,60 minutes contact time, 0.5 g of adsorbent and Cd (II) initial concentration of 50 mg/l. The Experimental data were applied in the two isotherms (Figures 2 and 3), which results indicate that the adsorption of Cd (II) onto orange peel fits the Langmuir isotherm model because it gives a higher correlation coefficient (r2= 0.983) value than Freundlich isotherm model (r2= 0.883), verifying the assumption that the adsorbate molecules could be adsorbed in monolayer coverage on the surface of the adsorbent. The adsorption constants evaluated from the isotherms with the correlation coefficients are given in Table 2.
| Langmuir constant | Freundlich constant | ||||
|---|---|---|---|---|---|
| KL | qmax | r2 | Kf | n | r2 |
| 0.516 | 4.90 | 0.983 | 6.150 | 4.032 | 0.883 |
Table 2: Langmuir and Freundlich constants of adsorption system.
Adsorption kinetics
From Table 3 and Figures 4-7 , show that the pseudo second order model is the best fitting model because it gives a higher correlation coefficient (r2=0.988) than the other kinetic models [pseudo-first-order (r2= 0.925), Elovich(r2= 0.901) and intraparticle diffusion (r2=0.805) models.
| Experimental qe (mg/g) | Pseudo-first-order model | Pseudo-second-order model | Elovich model | intraparticle diffusion model | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| K1 (1/min) | qe (mg/g) | r2 | qe (mg/g) | K2 (g/mg.min) | r2 | β (mg/g) | α (g/mg.min) | r2 | Kid(min-1) | a | r2 | |
| 15.91 | 0.0759 | 6.999 | 0.925 | 4.950 | 0.0971 | 0.988 | 0.759 | 1.231 | 0.901 | 14.79 | 1.366 | 0.805 |
Table 3: Adsorption kinetic model rate constants.
Thermodynamic Studies
The effect of the temperature on the adsorption ofCd (II) ions was studied in the range of 30-60°C. The result shows that temperature has anegative effect on the adsorptionofCd (II) onto orange peels. Thermodynamic parameters, such as enthalpy variation (ΔHo), entropy variation (ΔSo)and change in Gibbs free energy (ΔGo),were calculated from the curve relating the distribution coefficient (KD) as a function of temperature (Figure 8), using the equations [16]:
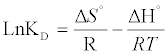 (15)
(15)
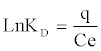 (16)
(16)
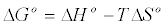 (17)
(17)
From Figure 8, the values of ΔHo, ΔSo were determined from the slope and intercept values of the straight line of plotting ln KD versus 1/T(K), respectively, (Table 4). Negative value of ΔGo indicates the feasibility of the process and indicates the spontaneous nature of the adsorption. Decreasing ΔGo values with decreasing temperature, suggests that lower temperaturemakes the adsorption easier.The negative ΔHº value indicates the exothermic nature of the adsorption process. The magnitude of ΔHº may give an indication about the type of sorption. Two main types of adsorption are physical and chemical. Basically, the heat evolved during physical adsorption is of the same order of magnitude as the heats of condensation, i.e., 2.1-20.9 kJ/mol, while the heats of chemisorption generally falls into a range of 80- 200 kJ/mol [23]. From Table 4, the absolute value of ΔHº are 34.935, which therefore indicate that Cd (II) adsorption by orange peelcould be attributed to a physic-chemical adsorption process rather than a pure physical or chemical adsorption process. Negative value of ΔSo indicate a decrease in randomness at the solid/solution interface during the adsorption process while low value of ΔSo indicates that no remarkable change on entropy occurs [24].
| Temperature(K) | LnKD | Thermodynamic parameters | ||
|---|---|---|---|---|
| ΔHo(kJ/mol) | ΔSo(KJ/mol.K) | ΔGo(kJ/mol) | ||
| 303 | 1.480 | -34.935 | -0.102 | -4.039 |
| 313 | 1.014 | -3.009 | ||
| 323 | 0.724 | -1.9989 | ||
| 333 | 0.170 | -0.969 | ||
Table 4: Thermodynamic data of adsorption process.
Orange peel was effective, as a Cd (II) adsorbent, for which the removal reached 97.33% at 30°C. The highest adsorption efficiency is observed at pH 5. Increasing temperature and initial concentration of Cd (II)lead to decreasing the removal of Cd (II). The Langmuir isotherm model appears to be the best fitting model for adsorption process. The kinetics of Cd (II) adsorption on the orange peelwas found to follow a pseudo second-order rate equation. Thermodynamic parameters (ΔGo, ΔHo and ΔSo) showed that the adsorption process is spontaneous and exothermic in nature.