Journal of Pharmaceutical Care & Health Systems
Open Access
ISSN: 2376-0419
ISSN: 2376-0419
Research Article - (2023)Volume 10, Issue 3
Background: Analgesics are known to have many side effects with mild to serious impacts. After fracture surgery analgesics are commonly prescribed, but literature on their adverse events specifically in ankle and hind foot fractures is scanty. This study aimed to explore the current practice of prescribing oral analgesics for ankle, and hind foot fractures post-surgery, the incidence of adverse events, and possible risk factors.
Methods: The study was initiated in June 2022. A total of 19 adult patients with traumatic ankle and hind foot fractures were recruited at a tertiary care hospital. Oral analgesics prescribed at discharge and 1-week follow-up were stratified with potential adverse events recorded at 1- and 2-week follow-ups respectively. Adverse events incidence was calculated. The relationship between adverse events, age, and gender was assessed by logistic regression and correlation coefficients.
Results: The overall adverse events incidence in ankle and hind foot fractures was 1.1 events/people-year recorded at 1 and 2 weeks follow-ups. On stratification, high-risk analgesics identified are acetaminophen exclusively or in combination with diclofenac or tramadol predisposing to cardiovascular risk (N=4.21%). Naproxen combined with tramadol, orphenadrine added acetaminophen, or diclofenac predisposing to gastrointestinal and central nervous system complications (N=3.16%).
Conclusion: Current data on adverse event incidence and stratification to prescribed oral analgesics in ankle and hind foot fractures could be helpful in the optimal safe analgesics selection. Further data from the ongoing study will provide us with better insight into the safe analgesic selection and establishing a fracture-specific optimal pain management protocol as per patient needs.
Adverse events incidence; Analgesics; Ankle fractures; Hind foot fractures; Trauma
Fractures due to injury initially require effective analgesia to control pain and for patient wellbeing. Thereafter, continuing pain management is for controlling rehabilitation pain and improving the patient’s quality of life. If severe pain is inappropriately controlled, it may lead to serious systemic consequences like tachycardia, vasoconstriction with visceral ischemia, myocardial infarction in patients with underlying cardiac problems, increases respiratory rate and volume, and predisposes to develop chronic pain [1-4]. These complications may lead/to patient morbidity, and mortality. To overcome these health risks and for achieving optimal fracture treatment outcomes, adequate pain control is essential. In routine fracture care, orthopedic surgeons prescribe various analgesics either alone or in combination with pain for control. Analgesics may cause several Adverse Events (AE) and Serious Adverse Events (SAE) like delayed fracture union, nerve or vascular damage, nausea, local infection, etc [5]. Acetaminophen is reported to be safe and an alternative treatment to NSAIDs in managing orthopedic surgery pain [6,7]. Although safety has been reported, gastrointestinal, cardiovascular, renal, and acute hepatic failure has been reported particularly at higher than recommended doses [8-12]. Liver disease, excess alcohol intake, fasting, and psychiatric problems like depression, increased impulsivity, etc. predispose individual to acetaminophen-induced acute liver toxicity. Thus, besides acetaminophen safety and recommendation, patient-related risk factors should be considered with special dose adjustments [13].
As far as opioid analgesics are concerned, many pain management guidelines recommend opioid use in orthopedic injuries that might lead to undesired adverse consequences like fatigue, insomnia, dry mouth, nausea, vomiting, addiction, etc [14,15]. The risk of bone non-union significantly increased if opioids were used in tarsal fractures and opioids with NSAIDs were used in ankle fractures [16]. In orthopedic care, acetaminophen in combination with the anticholinergic orphenadrine is used which acts synergistically for pain as well as injury site muscle spasm relief. Orphenadrine is relatively safe with few anticholinergic-related side effects like blurred vision, increased intraocular pressure, nausea, dry mouth, tachycardia, palpitation, gastric irritation, restlessness, itching, drowsiness, etc [17]. It is reported that patients experienced only orphenadrine-related mild AEs with no SAEs [18,19]. To minimize analgesic risks and to optimize fracture pain management, the National Institute for Health and Care Excellence prepared National clinical guidelines in the year 2016 that was derived from available evidence on oral analgesics treatment in trauma fractures. For ages 16 and above, the guidelines recommendation is to use oral acetaminophen in mild and oral acetaminophen added codeine in moderate pain. If required, Nonsteroidal Anti- Inflammatory Drugs (NSAIDs) could be added as a supplement for pain control except in elderly or frail adults due to evidence of severe gastrointestinal bleeding, renal function impairment, delayed fracture bone healing, mortality due to existing cardiovascular problems, etc [20-25].
Additionally, the National Institute for Health and Care Excellence (NICE) guideline segregated the pain treatment according to the fracture type, site involved, age, etc. As per guidelines, oral acetaminophen is the treatment of choice in adult patients with dose adjustments who are underweight [26,27]. In ankle and long bone fractures, oral acetaminophen is the initial treatment of choice and if the pain remains uncontrolled, could be combined with codeine initially and then NSAIDs if required Special caution should be taken for elderly and frail adults while adding codeine or NSAIDs [28]. These guidelines indicate a limited choice of analgesics in the elderly age group because of more health risks. Post-surgical pain management recommendation of the Centers for Disease Control and Prevention (CDC) is to use acetaminophen and NSAIDs with selected opioids for inadequate pain control [29]. For acute traumatic or post-surgical pain, several guidelines recommend multimodal analgesic treatment in which different types of analgesics are combined at low doses than normally recommended like acetaminophen, NSAIDs, muscle relaxants, etc. These combinations act synergistically and reduce the risk of analgesics-associated AEs as well as spare opioid overuse or addiction [30]. Additionally, using multimodal analgesics mutually with non-drug pain management strategies aids in better pain control and minimizing opioid use like in tibia shaft fractures [31].
Several research studies have been conducted to develop pain management standards in acute and long-term trauma care but still, firm evidence on implementing safe trauma fracture pain management through analgesics is under exploration. For optimum pain management, it is essential to better understand the associated AEs with potential predictors like fracture anatomical site, patient-related factors, and patient response to different analgesics. Unfortunately, scarce evidence is available to assist clinicians in optimal fracture-specific safe analgesic selection. In a tertiary care hospital, there is no standard protocol for fracture pain management. Analgesics are prescribed on the surgeon’s choice, pain, injury severity, etc. The current research aims to identify post-surgery routinely prescribed oral analgesics in the ankle, and hind foot trauma fractures at the time of hospital discharge and 1-week follow-up. Additionally, to ascertain AEs incidence at 1- and 2-week follow-ups after analgesic consumption and to relate potential patient or injuryrelated factors that predispose to these AEs. The study might assist in providing a clear picture of the current trend of selecting analgesics or their combinations in daily ankle and hind foot fracture care with potentially associated AEs in our set patient population. Stratifying prescribed analgesics to AEs may give us better insight into the benefit versus risks approach of analgesic selection in ankle and hind foot fractures and contribute to ongoing research on a safe analgesic selection strategy. A future standard protocol for selecting safe and appropriate analgesics in adult ankle and hind foot fracture patients could be developed as per the patient’s need.
After obtaining National Bioethics Committee (Ref No: 4-87/ NBC-725/22/1398), Ethical Review Committee (Ref No: 2022-5370-22345), and Institutional approvals, the study was initiated at a tertiary care hospital in June 2022. Inclusion criteria were the patients more than or equal to 18 years of age, of any gender, presenting with trauma-associated ankle, hind foot, and proximal femoral fractures operated at a tertiary care hospital, and voluntary participation. Patients treated with amputations, having severe psychiatric illnesses, or were unable to consent voluntarily were excluded from the study. Following the patient eligibility assessment, written informed consent was obtained as per Good Clinical Practice guidelines. Patients’ data were obtained from their medical record files and the online hospital medical record system. All routinely prescribed oral analgesics were recorded at the time of discharge, and postdischarge follow-ups at 1 week ± 1 day and 2 weeks ± 2 days. Potential AEs were identified and recorded at 1 and 2 week follow-ups for analgesics that were prescribed at the time of patient discharge and 1-week follow-up. To observe drug compliance, a daily analgesic diary was provided to all patients at the time of discharge and the 1-week follow-up together with verbal confirmation at follow-ups.
For the current analysis, only patients presenting with ankle, and hind foot fractures were selected from the study. Descriptive analysis was performed for quantitative (mean ± SD or median, IQR) and qualitative (frequency and percentage) variables. The routinely prescribed oral analgesics at the time of discharge and follow-ups were stratified with potentially analgesic-related AEs. The incidence of AEs was also calculated by using the formula:
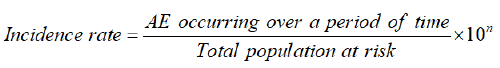
The relationship between AEs and gender or age was assessed using binary logistic regression analysis and correlation coefficient. A p-value less than 0.05 were considered statistically significant with a 95% confidence level.
In the entire study, a total of 67 eligible patients were recruited from June 5 to December 5, 2022, time. Only ankle and hind foot fracture cases were included in the current analysis to identify their AE incidence. A total of 19 cases were identified whose demographics with a mechanism of injury, fracture site, and treatment procedures are given in Table 1 along with cases that experienced side effects potentially due to analgesics in Table 2. Stratification was done for potential SAE/AE due to oral analgesics prescribed at discharge and 1-week follow-up and was assessed for up to 2-week follow-ups. An overall SAE/AE incidence calculated was 1.1 events/people-year that were recorded at the 1 and 2 week follow-ups (Table 3). Some potentially harmful or safe analgesics or their combinations were identified on stratification (Tables 2-4). It was identified that acetaminophen alone or in combination with diclofenac or tramadol is harmful and predisposing to cardiovascular risk (N=4, 21% of 19). An important finding recorded in these patients was hypotension (N=2) and hypertension (N=2). One of these patients who developed hypertension also developed angina. Furthermore, naproxen combined with tramadol, orphenadrine added acetaminophen, or diclofenac cause gastrointestinal and Central Nervous System (CNS) complications (N=3, 16% of 19) (Table 3). One of the patients on these oral analgesics also received parenteral ketorolac at home. This observation indicates a need for a safe selection of analgesic combinations in ankle and hind foot fracture treatment.
| Number of cases=N (% of 67 cases) | ||
|---|---|---|
| Total cases | 19 | |
| Age | 41 ± 16 years | |
| Gender | Males=10 (53%) | |
| Females=09 (47%) | ||
| Fracture anatomical site | Ankle fracture=16 (84%) | |
| Bilateral ankle fracture N=2 | ||
| Malleolar fracture N=8 | ||
| Bimalleolar fractures N=4 | ||
| Tri-malleolar fracture N=2 | ||
| Hindfoot fracture N=3 (16%) | ||
| Talus with calcaneus fracture N=1 | ||
| Calcaneus fracture N=1 | ||
| Talus fracture N=1) | ||
| Mechanism of injury | Road traffic accidents N=9 (47%) | |
| Ground level fall N=6 (32%) | ||
| Twisting N=2 (11%) | ||
| Fall from height=1 (5%) | ||
| Firearm injury=1 (5%) | ||
| Surgical | procedures | ORIF=17 (89%) |
| performed | Screw with k-wires and tension band wiring=1 (5%) | |
| Herbert screw fixation=1 (5%) | ||
Table 1: Patient demographics, injury characteristics, and treatment provision.
| Patient characteristics, injury site, and management having AE due to D/C analgesics (N=07) | Characteristics, injury site, and management in patients having AE due to 1 week analgesics (N=03) | |
|---|---|---|
| Mean age | 37 ± 9 years | 36 ± 8.5 years |
| Gender | Females=4 (57%) | Females=2 (67%) |
| Males=3 (43%) | Males=1 (33%) | |
| Fracture type | Ankle malleolar fracture=3 (43%) | Bilateral ankle fracture=1 (33%) |
| Bilateral ankle fracture=1 (14%) | Trimalleolar ankle fracture=1 (33%) | |
| Trimalleolar ankle fracture=1 (14%) | Talus with calcaneus fracture=1 (33%) | |
| Talus with calcaneus fracture=1 (14%) | ||
| Talus fracture=1 (14%) | ||
| Surgery | ORIF=6 (86%) | ORIF=3 (100%) |
| performed | Herbert screw fixation=1 (14%) | |
| Comorbid | None=4 (57%) | None=2 (67%) |
| condition | HTN=1 (14%) | Multiple sclerosis, left uveitis=1 (33%) |
| DM+HTN=1 (14%) | ||
| Multiple sclerosis, left uveitis=1 (14%) | ||
Note: D/C: At the time of hospital discharge, ORIF: Open Reduction Internal Fixation, HTN: Hypertension, DM: Diabetes Mellitus.
Table 2: Patient characteristics, injury site in patients who experienced adverse events.
| Side effects recorded | AE/SAE=07 | AE/SAE=03 | ||
| No AE=11 | No AE=13 | |||
| Lost to follow-up=01 | Lost to follow-up=02 and Missing data=01 | |||
| AE incidence rate | 1.1 events/people-year | |||
| Fracture type (N) | Analgesics prescribed at hospital discharge | AE in 7 patients (age years, comorbid) | Analgesics prescribed at 1-week follow-up | AE in 3 patients (age years, comorbid) |
| Talus+calcaneus (N=1) | Diclofenac 50 mg+misoprostol 200 µg BD | HTN+Angina (36, none) | Naproxen 550 mg BD | Thirst (36, none) |
| Tramadol 37.5 mg+acetaminophen 325 mg TID | Diclofenac 50 mg+misoprostol 200 mcg SOS | |||
| Acetaminophen 1000 mg QID | Tramadol 37.5 mg+acetaminophen 325 mg SOS | |||
| Ankle trimalleolar (N=1) | Ketorolac 30 mg IM 8 hr | Diarrhea+dry mouth+dyspnea+headache (45, multiple sclerosis) | Naproxen 550 mg BID | Diarrhea+dizziness+vomiting (45, multiple sclerosis) |
| Naproxen 550 mg BD | Orphenadrine 50 mg+acetaminophen 650 mg BD | |||
| Pregabalin 75 mg HS | Ketorolac IM 30 mg 8 hourly SOS | |||
| Acetaminophen 650 mg+orphenadrine 50 mg TID | ||||
| Bilateral ankle fracture (N=1) | Meloxicam 7.5 mg BID | Constipation (28, none) | Naproxen 550 mg SOS | Nausea+Difficult swallowing+Constipation (28, none) |
| Aspirin 75 mg OD | ||||
| Tramadol 37.5 mg+acetaminophen 325 mg SOS | ||||
| Ankle (N=1) | Acetaminophen 1000 mg QID or TID | Diarrhea+HTN (high SBP and DBP)+insomnia (50, DM+HTN) | ||
| Tramadol 37.5 mg+acetaminophen 325 mg BID | ||||
| Ankle (N=1) | Acetaminophen 1000 mg QID | Hypotension (low DBP), (24, none) | ||
| Talus (N=1) | Acetaminophen 1000 mg QID | Hypotension (low DBP), (38, HTN) | ||
| Diclofenac 50 mg+misoprostol 200 µg BD | ||||
| Gabapentin 300 mg HS | ||||
| Ankle (N=1) | Tramadol 37.5 mg+acetaminophen 325 mg BD | Insomnia (38, none) | ||
| Diclofenac 50 mg+misoprostol 200 µg BD | ||||
Note:D/C: At the time of hospital discharge, AE: Adverse Event, SAE: Serious Adverse Event, HTN: Hypertension, DM: Diabetes Mellitus.
Table 3: Analgesics prescribed and recorded Adverse Events (AE) with their incidence.
| Fracture type (N), (age years, comorbid) | Analgesics prescribed on hospital discharge with no AE/SAE | Fracture type | Analgesics were prescribed on a 1-week follow-up with no AE/SAE |
|---|---|---|---|
| Ankle (N=1),Bimaleolar, (68, Parkinson's disease+HTN+DM) | Diclofenac 50 mg+misoprostol 200 µg BID; Aspirin 75 mg OD; Acetaminophen 1000 mg QID | Ankle (N=1); Bimaleolar, (68, Parkinson's disease+HTN+DM) | Acetaminophen 1000 mg QID; Diclofenac 50 mg+misoprostol 200 µg BID; Aspirin 75 mg OD |
| Ankle (N=1), (58, CKD+DM+HTN+Hypothyroidism) | Tramadol 37.5 mg+acetaminophen 325 mg HS; Aspirin 75 mg OD; Acetaminophen 1000 mg TID | Ankle (N=2), Talus (N=1), (33, none), (24, none), (38, HTN) | Acetaminophen 1000 mg TID/BID/SOS |
| Ankle (N=2) One bilateral, (30, none), (80, none) |
Acetaminophen 1000 mg QID; Celecoxib 100 mg; BID; Aspirin 75 mg OD | Ankle (N=1); Bilateral, (30, none) | Acetaminophen 1000 mg QID; Celecoxib 100 mg BID; Aspirin 75 mg OD |
| Ankle (N=4); One trimaleolar, (33, none), (38, HTN), (56, none), (37, none) | Acetaminophen 1000 mg QID/TID; Diclofenac 50 mg+misoprostol 200 µg BD/TID | Ankle (N=1), (80, none) | Acetaminophen 1000 mg TID; Aspirin 75 mg OD |
| Ankle (N=1); Bimaleolar, (20, none) Calcaneus (N=1), (48, none) |
Etoricoxib 60 mg OD ; Tramadol 37.5 mg+acetaminophen 325 mg HS Tramadol 37.5 mg+acetaminophen 325 mg TID Diclofenac 50 mg+misoprostol 200 µg BD Aspirin 75 mg OD |
Ankle (N=2);One trimalleolar, (42, DM+HTN), (58, CKD+DM+HTN+Hypothyroidism) Calcaneus (N=1), (48, none) |
Acetaminophen 1000 mg BID; Tramadol 37.5 mg+acetaminophen 325 mg HS/PRN; Diclofenac 50mg+misoprostol 200 µg PRN; Acetaminophen 650 mg+Orphenadrine 50 mg BD; Aspirin (75 mg OD) |
| Ankle (N=1), (30, none) | Meloxicam 15 mg OD ; Tramadol 37.5 mg+acetaminophen; 325 mg TID | Ankle (N=1), (30, none); Ankle (N=1), (38, none); Ankle (N=1), (50, DM+HTN); Ankle (N=1); Bimaleolar, (56, none) | Meloxicam 7.5 mg BID-PRN; Tramadol 37.5 mg+acetaminophen 325 mg SOS; Orphenadrine citrate 50 mg+ Acetaminophen 650 mg BID; Acetaminophen 1000 mg QID; Diclofenac 50 mg+misoprostol 200 µg BID |
| Total number of cases 11 | Total number of cases 13 |
Note: AE: Adverse Event, SAE: Serious Adverse Event, HTN: Hypertension, DM: Diabetes Mellitus, CKD: Chronic Kidney Disease.
Table 4: Analgesics prescribed with no AE/SAE.
To identify safe analgesics on stratification, it was observed that except for one patient, most of the patients (N=3, 16% of 19) who were prescribed acetaminophen as a single drug did not suffer from any SAE/AE. Interestingly, the prescription of acetaminophen either in combination with celecoxib/etoricoxib (N=3, 16% of 19) or tramadol in a PRN/HS regimen (N=3, 16% of 19) was safe. Patients who were prescribed meloxicam alone were also observed to be safe as patients experienced either no AE or only mild event of constipation (N=2, 10% of 19). Thus, on stratification, the above-described potentially safe analgesic combinations were identified for ankle and hind foot fractures. To further assess the relationship of AE to age and gender, binary logistic regression analysis and correlation coefficient were applied. No significant relationship was found between AE recorded at 1- and 2-week follow-ups to gender (OR=1.6, p=0.6), (OR=2.3, p=0.5), and age (OR=0.9, p=0.2), (OR=0.9, p=0.3) respectively. Further analysis of ongoing research might provide us with concrete results on a larger sample size.
The study explored the current practice with variation in prescribing oral analgesics the ankle and hind foot fractures postoperatively at the time of patient discharge and 1-week follow-up. It was identified that there was no uniform analgesic treatment strategy and selection is the surgeon’s choice. Some analgesic combinations either exceeded the normally recommended doses like acetaminophen or the same drug class analgesics prescribed together like NSAIDs, causing analgesic overdose or enhanced drug effect that results in serious consequences. The incidence of AE recorded at 1 and 2 week follow-ups was 1.1 in the selected patient cohort. It was observed that acetaminophen in combination with celecoxib/etoricoxib or tramadol at HS/PRN regimen was identified safe with no AE/SAE detected when prescribed at discharge or 1-week follow-up. Meloxicam was also safe in terms of none or only mild AE if any. These findings support the previous literature and suggest that acetaminophen is safe for ankle, and talus fractures [6,7,26,27]. Notably, caution must be taken while prescribing acetaminophen in patients with cardiovascular problems as SAEs like hypotension, hypertension, and angina were recorded in patients who were taking acetaminophen either exclusively or in combination with tramadol added acetaminophen and diclofenac sodium. This finding supports the previous studies in which acetaminophen potentially develops hypo or hypertension, diclofenac increases the risk of cardiovascular events and tramadol develops AEs in patients with compromised cardiovascular function [32-36]. Moreover, intravenous, as well as oral acetaminophen is known to produce hypotension that might need sufficient treatment [32]. Evidence shows that Systolic Arterial Pressure (SAP) and Mean Arterial Pressure (MAP) was reduced significantly due to acetaminophen and the possible mechanism was a reduction of both cardiac outputs, systemic vascular resistance, or a decrease in arterial tone [37,38]. For diastolic blood pressure, it was reported that mean diastolic blood pressure along with MAP and SBP significantly decreased by acetaminophen infusion [39]. This finding indicates periodic blood pressure monitoring and cardiovascular assessment in patients taking these analgesics. Additionally, caution should be taken while prescribing to patients with underlying cardiovascular disease or who are on risk stratification and predisposed to develop such problems.
Other analgesic combinations like naproxen with orphenadrine, tramadol-added acetaminophen, or diclofenac were also identified as harmful. One of these patients was also prescribed ketorolac parenteral. As naproxen, diclofenac, and ketorolac belong to the NSAID class of analgesics, this combination should be avoided for minimizing undesired side effects in patients. Thus, these analgesics or their combinations were found to be harmful in ankle and hind foot fractures. As the purpose of drug combination is to increase therapeutic efficacy and minimize risks, it is important for treating clinicians to understand safe analgesic selection with appropriate dose adjustments to avoid serious consequences. Thus, these analgesics require caution while being prescribed. Interestingly, no significant relationship was observed between SAE/AE and age or gender. Incidents were recorded even in those patients who were of the younger age group with no comorbid condition and both genders developed side effects almost equally. The current data on identified potentially safe oral analgesics as well as harmful analgesic combinations could be helpful and contribute to the ongoing research to further explore optimal safe pain management in ankle and hind foot fractures. This might assist in developing a future safe pain management strategy for ankle and hind foot fractures.
In daily practice, oral analgesics prescription for ankle and hind foot fractures is as per the clinician’s choice. With an overall incidence of SAE/AE events/people-year, it is determined that inappropriate analgesic combinations with higher than recommended doses or combined with analgesics that belong to the same drug category produce undesirable analgesics-related adverse events. Acetaminophen combined with celecoxib, etoricoxib, tramadol at HS/PRN dose, or meloxicam is potentially safe with fewer side effects. Acetaminophen, though seemingly safe may have potentially harmful effects if prescribed alone or in combinations like diclofenac, and tramadol that could be serious and mainly predispose to develop hypotension, hypertension, or angina. Thus, these drugs require careful monitoring and caution while being prescribed to patients with underlying cardiovascular problems. Naproxen combined with tramadol, orphenadrine added acetaminophen or diclofenac also seemed harmful analgesic combination that produced gastrointestinal CNS side effects. Further ongoing study data might assist in exploring safe analgesic selection in ankle and hind foot fractures with due consideration of patient-related factors.
Dr. Tashfeen Ahmad and Dr. Zehra Abdul Muhammad contributed to the study concept, Data interpretation, questionnaire design, and critical manuscript review, Dr. Zehra Abdul Muhammad contributed to the study design, Literature search, manuscript writing, data collection with analysis, data interpretation, Dr. Yasir Mohib, Dr. Naveed Baloch, and Dr. Rizwan Haroonrashid provided feedback with a critical manuscript review.
The study was generously supported by the Orthopedic Trauma Association (proposal ID #6704).
The authors have no conflicts of interest to declare.
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
Citation: Muhammad ZA, Ahmad T, Mohib Y, Haroonrashid R, Baloch N (2023) Adverse Events in Analgesia for Fractures: A Study for Pharmacovigilance. J Pharma Care Health Sys. 10:276.
Received: 02-May-2023, Manuscript No. JPCHS-23-23824; Editor assigned: 04-May-2023, Pre QC No. JPCHS-23-23824 (PQ); Reviewed: 23-May-2023, QC No. JPCHS-23-23824 ; Revised: 02-Jun-2023, Manuscript No. JPCHS-23-23824 (R); Published: 12-Jun-2023 , DOI: 10.35248/2376-0419.23.10.276
Copyright: © 2023 Muhammad ZA, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.