
Journal of Clinical Trials
Open Access
ISSN: 2167-0870

ISSN: 2167-0870
Research Article - (2021)Volume 11, Issue 2
Background: Pueraria Decoction (PD) is a Japanese herbal medicine of Kampo tradition, which is used for acute
febrile diseases, inflammatory disease and allergic rhinitis. Moreover, PD is reported to have beneficial effects on
autonomic disturbance in patients.
Objective: The objective of the study is to investigate the effects of PD on autonomic nervous system of healthy adult
subjects using spectral analysis of heart rate and blood pressure variability during Head-Up Tilt Test (HUTT). Here,
we investigated the effects of PD on autonomic nervous system of healthy adults using spectral analysis of heart rate
and blood pressure variability during Head-Up Tilt Test (HUTT).
Methods: Twenty healthy subjects were divided into young and middle-aged groups, and examined twice with HUTT,
before and 5 minutes after taking 5 g of PD. Spectral analysis of RR interval and Systolic Blood Pressure (SBP)
variability was then used to measure the changes in autonomic functions.
Results: As for all study participants, low frequency power of the SBP (SBP-LF) was increased, and high frequency
power of RR interval (RR-HF) was decreased by tilt. However, PD did not show any effect on SBP-LF or RR-HF both
at supine and tilt positions. Tilting did not change the ratio of low and high frequency power of RR interval (RRLF/
HF) before taking PD, which was increased after taking it. When analyzed separately by age, RR-HF was decreased
in middle-aged group compared to the young counterpart in all conditions. Interestingly, PD increased RR-HF in
middle-aged group at supine position, and a significant reduction of the value appeared at tilt similar like young
group. After taking PD, tilt increased RR-LF/HF in both young and middle-aged groups.
Conclusion: Our study finds that PD has a function to stimulate sympathetic nerve, and simultaneously restores the
decreased parasympathetic functions in both young and elderly groups. Such findings suggest that PD might have a
combined effect to protect the whole autonomic nervous system.
Head-Up Tilt Test (HUTT); Kampo-medicine; Pueraria Decoction (PD); Autonomic nerve function; Sympathetic Nerve (SN); Parasympathetic Nerve (PN); Spectrum analysis
The Autonomic Nervous System (ANS) is the part of the nervous system, which acts largely at the unconscious level by supplying the glands, organs, and smooth muscle. As a consequence, the system plays an important role in controlling bodily functions including heart rate, blood pressure, respiration, body temperature, gastrointestinal motility, and urinary excretory functions [1]. ANS is divided anatomically and functionally into sympathetic and parasympathetic divisions, which often have opposing effects and a balance of their activity is required for the proper function of an organ [2]. Both sympathetic and parasympathetic divisions are composed of preganglionic and postganglionic neurons, where the neurotransmitters act by binding to receptors at the target area. Autonomic nerve dysfunctions are known to induce postural hypotension, syncope, and sweating abnormalities [3,4]. Alteration of autonomic balance could be an important risk factor for chronic diseases including cardiovascular and neurological diseases due to its important regulatory functions on those systems [5,6]. Moreover, both sympathetic and parasympathetic responses decline, and cardiovascular and neurodegeneration risk increases with aging [7,8]. These findings suggest a relation of autonomic balance with those disease pathologies, and restoration of such imbalance could provide some protection during the processes.
To evaluate changes of autonomic functions, several noninvasive tests are developed. These testing procedures rely on the response of the autonomic system against various types of maneuvers, thus specific test can provide information on a specific aspect of the autonomic system [9,10]. Among the tests, the Head-Up Tilt Test (HUTT) is important, because it is a standardized test that can be used to evaluate cardiovascular regulation of both sympathetic and parasympathetic systems [11]. Here, the subjects are tilted to 60° angle from the supine position to induce a shift of blood volume due to gravity, and autonomic response to that shift is evaluated by recording heart rate and blood pressure [12]. However, evaluation of overall changes in blood pressure and heart rate might not provide the precise status of autonomic regulation. Heart rate is regulated through the innervation of the sinoatrial node with both sympathetic and parasympathetic nerves. Although both parasympathetic and sympathetic nerves exert opposite action on the heart, the effects are not symmetrical physiologically resulting in variability of heart rate or RR interval in the ECG [13,14]. Three major components of RR interval have been recognized [15,16], where oscillation frequencies centered around 0.04 Hz (Very Low Frequency: VLF), 0.1 Hz (Low Frequency: LF) and 0.25 Hz (High Frequency: HF). Similarly, arterial pressure also shows the LF and HF components of variability [17]. Such an analysis of the variability of heart rate and blood pressure may allow a more detailed interpretation of autonomic function. Indeed, in a previous report, we have shown the usefulness of the incorporation of such spectral analysis of heart rate and blood pressure variability with HUTT in differentiating autonomic dysfunctions in Parkinson’s disease and multisystem atrophy [18].
Kampo is a branch of traditional Japanese herbal medicine, which has been developed from Chinese herbal medicine and practiced for more than 1500 years. Recently, Kampo is gaining popularity because of its methodical formulations and no side effects. Several studies have reported the beneficial effects of Kampo on the autonomic disturbance in patients [19,20]. Among the Kampo, Pueraria Decoction (PD) is a popular formulation used as a remedy for cold/influenza, the initial stage of febrile diseases, and inflammatory disease [21,22]. PD formulation is a mixture of 7 components: Pueraria (4.0 g, dried root of Leguminous Kudzu), ephedra (3.0 g, dried root of Ephedraceae), Cinnamomum cassia (2.0 g, dried twig of Cinnamomaceae), Poria (2.0 g, dry sclerotium of Poria cocos Wolf), Zingiber (2.0 g, fresh root of Zingiber officinale Roscoe), Glycyrrhiza (2.0 g, root and stolon of Glycyrrhiza uralensis Fisher) and Ziziphus (3.0 g, fruits of Rosaceae Rhamnaceae). Pueraria is the main component of PD, which is traditionally used to promote blood circulation, to reduce dry mouth and to treat cardiovascular diseases [23,24]. One of the main components of Ephedra is ephedrine, which is a sympathomimetic agonist that can act on both α- and β- adrenergic receptors [25]. It also can induce sympathetic activation by enhancing the release of norepinephrine from sympathetic neurons [26]. Another component of PD is Ginger, which shows anti-inflammatory, antiemetic, analgesic, antioxidant, glucose and lipid-lowering, anti-cancer and neuroprotective effects [27-30]. Peaonia lactiflora can inhibit cerebral and peripheral inflammatory responses [31,32]. Additionally, Glycyrrhiza is reported to have the ability to potentiate parasympathetic nerve activity [33].
Considering the roles of the components of PD on autonomic functions, we hypothesized that it might improve the agedependent imbalance of sympathetic and parasympathetic activity. To test this hypothesis, we administered PD to 2 groups of healthy subjects, young at their 20s and middle-aged at their 50s, and compared their autonomic function before and after taking PD using HUTT. We found that PD improves autonomic imbalance, especially in the middle-aged population.
Subjects
This study and the experimental procedures were approved by the ethical committee of Shimane University Graduate School of Medicine. Twenty healthy male subjects without prior history of syncope or without any cardiovascular, neurological, or other major diseases were recruited in the study. The subjects had no history of medicine use due to any chronic diseases. Among them, 10 subjects were between the age range of 20 to 30 years with a mean age of 22.11 ± 1.05 years, and the other 10 subjects were between the age ranges of 50 to 60 years with a mean age of 55.67 ± 2.18 years.
Experimental plan
To evaluate the effects of PD, the autonomic functions of every subject was tested twice using HUTT. First HUTT was performed to get an idea of the basal autonomic function of the subject. Then, 5 g of PD (TSUMURA company, Tokyo, Japan) dissolved in 100 ml of warm water was given to the subject. After taking PD, the subject rested in a quiet room for 30 min. Then, HUTT was performed again. The procedure of HUTT was described in the following section.
HUTT procedure
All subjects were tested in a quiet room at a controlled temperature of 24°C. The test was done at least 2 hours after their last meal. The subject was laid on the tilt table with both feet touching the footrest, and the body was supported by two belts at the levels of thigh and waist. When the heart rate and blood pressure attained a stable condition after taking rest for 15 min at supine position, the subject was passively tilted up 60° and kept at the position for 5 minutes. Before starting the test, the subject was instructed not to talk, and breathe spontaneously during the entire test period. The schematic diagram of the test procedure is depicted in Figure 1. Arterial Blood Pressure (BP) and Heart Rate (HR) were continuously monitored at the right wrist using ANS 508 (OMRON Korin Corporation, Kyoto, Japan).
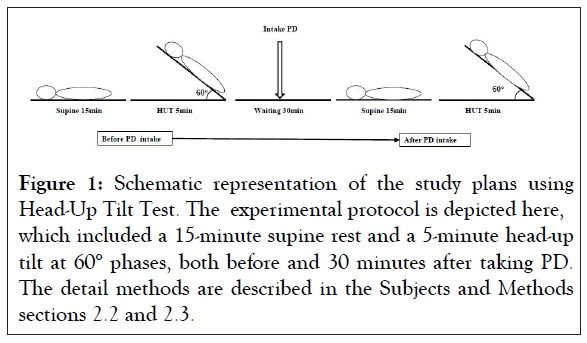
Figure 1: Schematic representation of the study plans using Head-Up Tilt Test. The experimental protocol is depicted here, which included a 15-minute supine rest and a 5-minute head-up tilt at 60° phases, both before and 30 minutes after taking PD. The detail methods are described in the Subjects and Methods sections 2.2 and 2.3.
Autonomic functions were analyzed by assessing the variation of BP and HR (RR-interval in electrocardiogram), and by the spectral analysis of blood pressure and RR-interval variability. The autoregressive spectral analysis provides low-frequency (LF, from 0.04 to 0.15 Hz) and high-frequency components (HF>0.15 Hz) of RR interval and SBP over a series of 128 consecutive points. These oscillatory components were expressed in absolute units (ms2 or mmHg2). After extraction from the RR variability, the spectral indexes are expressed as RR-LF, RR-HF, or the ratio of RR-LF to RR-HF (RR-LF/HF). According to previous studies, RR-HF is considered as a marker of vagal cardiac activity [13,14]. The ratio of RR-LF to RR-HF (RRLF/ HF) is referred as a measure of cardiac sympathetic activity [34,35]. SBP-LF is used as an index of sympathetic vasomotor control [15,17].
Statistical analysis
All data are presented as the mean ± Standard Deviation (SD). The statistical analysis was done using paired t test. The significance level was set at p value <0.05 (corrected value in the Bonferroni method). The analysis was conducted with SPSS 22.0 (IBM SPSS, New York, NY, USA).
Changes of heart rate and blood pressure of the study participants before and after taking PD were analyzed. Twenty healthy malesubjects were recruited for the study. Among them, 2 participants (1 from each group) withdrew from the study. The study was done with the remaining 18 participants (9 subjects of each group), and the data were collected for statistical analysis. At first, Heart Rate (HR), Systolic Blood Pressure (SBP), Mean Blood Pressure (MBP) and Diastolic Blood Pressure (DBP) of the study participants were evaluated to understand the effects of PD on autonomic nerve functions. The results showed that HR, SBP, MBP and DBP were not different between young and middle-aged groups at supine position both before and after taking PD. By tilt, HR was increased in all study both before and after taking PD. When compared according to the age, HR was increased by tilt in the young group both before and after taking PD. But in the middle-aged group, tilt increased HR only after taking PD. In the case of SBP, tilt decreased it after taking PD in the middle-aged group (Table 1).
| Before intake PD | After intake PD | ||||
|---|---|---|---|---|---|
| Supine | Tilt | Supine | Tilt | ||
| HR | All | 68.76 ± 10.32 | 82.24 ± 13.96** | 66.29 ± 9.84 | 85.06 ± 12.39** |
| Young | 67.22 ± 10.76 | 82.23 ± 12.71* | 65.22 ± 10.96 | 86.44 ± 10.96** | |
| Middle | 70.50 ± 10.23 | 82.13 ± 16.15 | 67.50 ± 9.04 | 83.50 ± 14.43* | |
| SBP | All | 118.82 ± 12.93 | 112.47 ± 14.15 | 125.18 ± 13.72 | 114.53 ± 12.28 |
| Young | 117.00 ± 11.43 | 114.22 ± 13.58 | 126.89 ± 14.52 | 113.44 ± 9.15* | |
| Middle | 120.88 ± 14.96 | 110.50 ± 15.45 | 123.25 ± 13.47 | 115.75 ± 15.67 | |
| MBP | All | 85.35 ± 12.84 | 81.12 ± 10.64 | 86.82 ± 10.62 | 83.24 ± 10.93 |
| Young | 79.89 ± 10.08 | 79.22 ± 9.30 | 83.78 ± 10.06 | 79.33 ± 6.96 | |
| Middle | 91.50 ± 13.38 | 83.25 ± 12.26 | 90.25 ± 10.81 | 87.63 ± 13.28 | |
| DBP | All | 67.82 ± 12.02 | 66.35 ± 10.57 | 66.71 ± 9.67 | 68.82 ± 10.38 |
| Young | 63.00 ± 9.80 | 63.89 ± 10.30 | 64.11 ± 9.88 | 65.11 ± 7.56 | |
| Middle | 73.25 ± 12.52 | 69.13 ± 10.84 | 69.63 ± 9.01 | 73.00 ± 11.96 | |
Note: Heart rate and blood pressure data of study population during HUTT are shown in Table 1.
HR: Heart Rate; SBP: Systolic Blood Pressure; MBP: Mean Blood Pressure; DBP: Diastolic Blood Pressure; All: All subjects; Young: Young group (20-30 years old); Middle: Middle-aged group (50-60 years old). Statistical analysis was done with paired t test, where p<0.05 was considered as significant. Data are presented here as mean ± standard deviation. Statistical significance is denoted as follows: *p<0.05, **p<0.01. *: tilt position vs. supine position significant difference.
Table 1: Changes in the heart rate and blood pressure at supine and tilt position during HUTT.
Spectral analysis of RR intervals and systolic blood pressure of the study participants before and after taking PD
Spectral analysis of RR intervals and SBP of all study participants was done for the assessment of autonomic functions, and the results are presented in Figure 2. Before taking PD, SBP-LF was significantly increased at tilt position compared to supine position (supine: 0.040 ± 0.025 mmHg2, tilt: 0.102 ± 0.084 mmHg2, **p<0.01) (Figure 2A and Table S1). A similar change of SBP-LF by tilt was observed after taking PD, where the power value at the supine position was 0.055 ± 0.050 mmHg2, and that of at the tilt was 0.112 ± 0.096 mmHg2 (*p<0.05) (Figure 2A and Table S1). When SBP-LF of all study participants at the supine position was compared between before and after taking PD, the difference was not statistically significant (Table S1). Similarly, when SBP-LF of all study participants at the tilt position was compared between before and after taking PD, the difference was not statistically significant (Table S1).
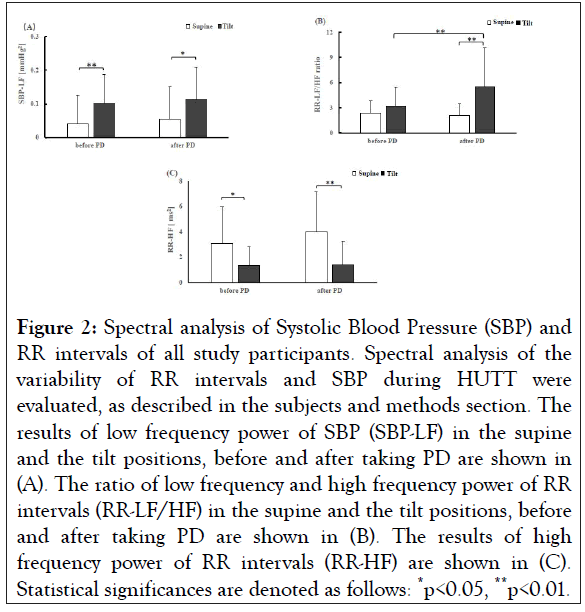
Figure 2: Spectral analysis of Systolic Blood Pressure (SBP) and RR intervals of all study participants. Spectral analysis of the variability of RR intervals and SBP during HUTT were evaluated, as described in the subjects and methods section. The results of low frequency power of SBP (SBP-LF) in the supine and the tilt positions, before and after taking PD are shown in (A). The ratio of low frequency and high frequency power of RR intervals (RR-LF/HF) in the supine and the tilt positions, before and after taking PD are shown in (B). The results of high frequency power of RR intervals (RR-HF) are shown in (C). Statistical significances are denoted as follows: *p<0.05, **p<0.01.
Next, the ratio of RR-LF and RR-HF (RR-LF/HF), which represents the activity of cardiac sympathetic nerve function, was evaluated in all study participants. The results showed that before taking PD, RR-LF/HF was not statistically different between the supine and the tilt positions (supine: 2.383 ± 1.463mmHg2, tilt: 3.189 ± 2.218 mmHg2, p=0.182) (Table S1). However, after taking PD, it was significantly increased at the tilt position compared to the supine position (supine: 2.112 ± 1.374, tilt: 5.280 ± 4.661, **p<0.01) (Figure 2B and Table S1). Importantly, RR-LF/HF was increased significantly after taking PD at the tilt position (before taking PD-tilt: 3.189 ± 2.218, after taking PD-tilt: 5.280 ± 4.661, **p<0.01), whereas such tendency was not observed at the supine position (Figure 2B and Table S1).
Vagal parasympathetic nerve activity was evaluated by analyzing RR-HF. Before taking PD, RR-HF values at the tilt position were lower compared to the supine position when all study participants were considered (supine: 3.053 ± 2.900 ms2, tilt: 1.350 ± 1.497 ms2 *p<0.05). A similar change of RR-HF was observed after taking PD, the power value at the tilt position was lower than at the supine position (supine: 3.992 ± 3.183 ms2; tilt: 1.411 ± 1.837 ms2, **p<0.01). However, there was no significant difference in RR-HF values before taking PD and after taking PD at the supine position. Neither was there a significant difference of RR-HF at the tilt position between before and after taking PD.
Comparison of spectral analysis of RR intervals and systolic blood pressure between the age groups
Next, we evaluated and compared the autonomic functions between the young and the middle-aged groups. The results were shown in Figure 3. Before taking PD, SBP-LF was higher at the tilt position compared to that at the supine position in both the young and the middle-aged groups (young group supine: 0.047 ± 0.027 mmHg2, young group tilt: 0.125 ± 0.092 mmHg2, *p<0.05, middle-aged group supine: 0.032 ± 0.022 mmHg2, middle-aged group tilt: 0.079 ± 0.075 mmHg2, *p<0.05) (Figure 3A). A similar change of SBP-LF was observed in the middle-aged group after taking PD, the values at the tilt position were higher than the supine position (supine: 0.036 ± 0.019 mmHg2, tilt: 0.095 ± 0.069 mmHg2, *p<0.05). However, in the case of the young group, SBP-LF values were not statistically different between the supine and the tilt positions after taking PD (Figure 3A and Table S1). In both age groups, when SBP-LF at the supine position was compared between before and after taking PD, the difference was not statistically significant (Table S2). Similarly, SBP-LF at the tilt position was not statistically different between before and after taking PD (Table S2).
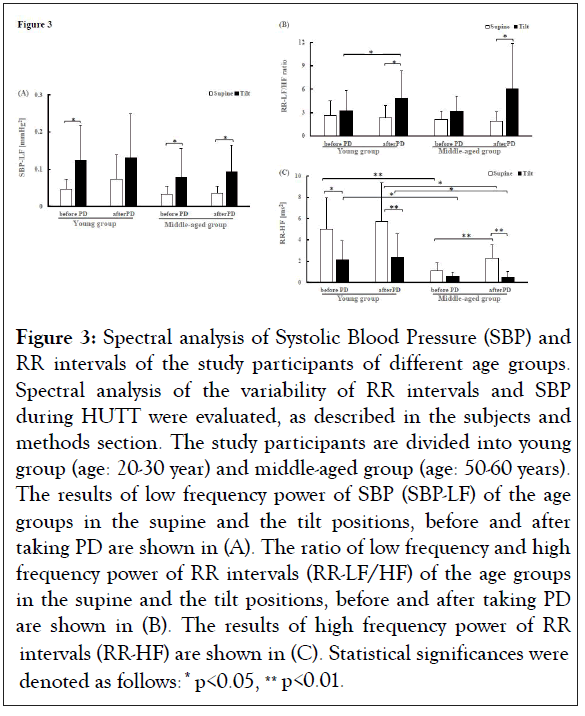
Figure 3: Spectral analysis of Systolic Blood Pressure (SBP) and RR intervals of the study participants of different age groups. Spectral analysis of the variability of RR intervals and SBP during HUTT were evaluated, as described in the subjects and methods section. The study participants are divided into young group (age: 20-30 year) and middle-aged group (age: 50-60 years). The results of low frequency power of SBP (SBP-LF) of the age groups in the supine and the tilt positions, before and after taking PD are shown in (A). The ratio of low frequency and high frequency power of RR intervals (RR-LF/HF) of the age groups in the supine and the tilt positions, before and after taking PD are shown in (B). The results of high frequency power of RR intervals (RR-HF) are shown in (C). Statistical significances were denoted as follows: * p<0.05, ** p<0.01.
Compared to the supine position, there were no significant differences observed in the values of RR-LF/HF ratio between the supine and the tilt positions in the young and the middleaged groups before taking PD (Figure 3B). However, after taking PD, RR-LF/HF ratio of both the young and the middle-aged groups at the tilt position was significantly increased compared with that at the supine position (young supine: 2.317 ± 1.571, young tilt: 4.885 ± 3.499, *p<0.05, middle-aged supine: 1.907 ± 1.203, middle-aged tilt: 6.076 ± 5.756, *p<0.05) (Figure 3B and Table S1). In addition, RR-LF/HF of the young group at the tilt position was significantly increased after taking PD compared to before taking PD (before taking PD tilt: 3.248 ± 2.573, after taking PD tilt: 4.885± 3.499, *p<0.05). However, such a difference was not observed in middle-aged group (Figure 3B and Table S1). At supine position, RR-LF/HF was not significantly different before and after taking PD in any age group. Furthermore, when the RR-LF/HF of corresponding positions between the young and middle-aged groups were compared, the differences were not statistically significant either before or after taking PD (Figure 3B).
The RR-HF value at the tilt position was lower than that at the supine position in the young group before taking PD (supine: 5.728 ± 3.623 ms2, tilt: 2.321 ± 2.245 ms2, *p<0.05), which was not different in the case of middle-aged group (supine: 1.074 ± 0.782 ms2, tilt: 0.601 ± 0.360 ms2, p= 0.098) (Figure 3C and Table S1). After taking PD, RR-HF values were decreased at the tilt position compared to that at the supine position in both the young and the middle-aged groups (young supine: 5.728 ± 3.623 ms2, young tilt: 2.321 ± 2.245 ms2, **p<0.01, middle-aged supine: 2.255 ± 1.272 ms2, middle-aged tilt: 0.501 ± 0.520 ms2, **p<0.01). For the middle-aged group, PD intake significantly increased the RR-HF values at the supine position, but not at the tilt position (before taking PD supine: 1.074 ± 0.782 ms2, after taking PD supine: 2.255 ± 1.272 ms2, **p<0.01; and before taking PD tilt: 0.601 ± 0.360 ms2, after taking PD tilt: 0.501 ± 0.520 ms2, p=0.408) (Figure 3C and Table S1). However, in the case of the young group, PD intake did not change RR-HF significantly either at the supine or at the tilt position (supinebefore taking PD: 5.033 ± 2.904 ms2, after taking PD: 5.728 ± 3.623 ms2, p=0.659; tilt-before taking: 2.099 ± 1.835 ms2, after taking: 2.321 ± 2.245 ms2, p=0.542) (Figure 3C and Table S1). Before taking PD, RR-HF values of the middle-aged group at both the supine and the tilt positions were significantly lower than that of the young group (supine-middle-aged group: 1.074 ± 0.782 ms2, young group: 5.033 ± 2.904 ms2, **p<0.01; and tiltmiddle- aged group: 0.601 ± 0.360 ms2, young group: 2.099 ± 1.835 ms2, *p<0.05) (Figure 3C and Table S2). After intake of PD, RR-HF value of the middle-aged group at supine and tilt positions were significantly lower than that of the young group at corresponding positions (middle-aged supine-after 2.255 ± 1.272 ms2 vs. young supine-after 5.728 ± 3.623 ms2, *p<0.05 and middle-aged tilt-after 0.501 ± 0.520 ms2 vs. young tilt-after 2.321 ± 2.245 ms2, *p<0.05) (Figure 3B and Table S2).
In the present study, we investigated the effects of PD on the autonomic nervous activity in healthy subjects using power spectral analysis of heart rate variability and systolic blood pressure. We have done this study on the young and the middleaged subjects because autonomic functions are reported to deteriorate with increasing age. Our study demonstrated that PD intake increased RR-LF/HF at the tilt position in all study participants, suggesting its effectiveness in the regulation of autonomic activity. In the young group, RR-HF was significantly decreased at the tilt position, whereas such changes were not observed in the middle-aged group. Moreover, the power values were significantly decreased in this group at the supine position compared to their young counterpart, suggesting an attenuation of vagal regulation. Attenuation of vagal activity and alteration of autonomic balance is related to increased mortality of cardiovascular diseases like myocardial infarction or heart failure [5,6]. Intake of PD is demonstrated to restore such decreased vagal activity (RR-HF) and autonomic balance (RR-LF/HF) towards the young age group. Hence, PD could provide some protection in the age-dependent risk of cardiac diseases by influencing autonomic functions.
Cardiovascular health and prognosis rely on sympathovagal balance, which is indicated by RR-LF/HF and RR-HF. At the tilt position, RR-LF/HF was increased after taking PD in all study populations, suggesting an increased effect of sympathetic activity. SBP-LF, which is referred to as an index of sympathetic vasomotor control, also showed an increasing tendency. But RRHF values, a measure of parasympathetic activity, were not significantly altered after taking PD, suggesting that the sympathetic part of the sympathovagal balance was mainly regulated by it. Ephedra is a major component of PD. Ephedra contains ephedrine, which shows direct agonistic effects on adrenergic receptors, and also augments the release of norepinephrine from the presynaptic nerve [25]. A previous report demonstrated that the administration of ephedra can increase sympathetic nerve response and RR-LF/HF [36,37]. Simultaneously, it decreases parasympathetic nerve response as evidenced by decreased RR-HF [36-38]. Since RR-HF response was not affected by PD, its effects on the autonomic system might not be solely due to Ephedra, but other compounds also have some effects that keep RR-HF stable. These different effects might be due to the combined actions of different major components of PD eg. Pueraria, Cinnamon Twig, and Glycyrrhiza which has an effect on increased parasympathetic activity [20,39]. Another previous study also showed that Pueraria, one of the PD compounds can lower blood pressure and increase blood flow by the reduction of vascular resistance [40]. These combined effects of PD are interesting and may play protective role for the cardiovascular system.
Previously, it was reported that autonomic nerve activity, both sympathetic and parasympathetic functions decrease with age [7,8,41], confirmed not only by heart rate variability analysis, but also by general tests for autonomic nerve functions [42,43]. Reports on anatomical analysis of the autonomic nerve also showed the decrease of sympathetic and parasympathetic nerve fibers [44,45]. Then, it is important to evaluate the autonomic nerve response to PD in different age groups. Previous research reported that both values of RR-LF and RR-HF were decreased with age, which was especially obvious in the case of RR-LF [46]. As a reason why cardiac sympathetic function dominantly decreases compared with parasympathetic one, it speculated sympathetic disturbance might be due to a decrease in cardiac sympathetic input and/or the adrenoceptor desensitization. In our study, the RR-LF/HF at tilt position was not changed significantly by intake of PD in the middle-aged group, which was significantly increased in the young group. These findings might suggest that sympathetic response to PD is weaker in the middle-aged group. Nevertheless, it is confirmed that PD did increase the sympathetic response to tilt position in this age group. On the other hand, RR-HF values demonstrated an obvious decrease of parasympathetic nerve response in the middle-aged group and did not respond to tilt. However, PD intake recovered RR-HF in the supine position, which indicates that it might have a role to increase parasympathetic response in addition to the sympathetic response.
The data of peripheral sympathetic regulation, which was evaluated by SBP-LF, showed that in the young group, demonstrated that tilt increased the activity before taking PD. Such change was not seen after taking PD, which could be its effects on the peripheral autonomic system. However, in the middle-aged group, SBP-LF still increased in the tilt position compared to the supine position after taking PD, suggesting a different status of the peripheral autonomic system in this group and its response to PD. Nevertheless, PD did not change SBP-LF at either supine or tilt position in any age group, suggesting its complex interaction with the peripheral autonomic system, which demands a detailed study in this matter. Since, the peripheral autonomic system is related to peripheral vascular resistance, and increase in peripheral arterial resistance is an important matter for elderly patients with arteriosclerosis and related cardiovascular diseases, PD could be used in that population in the merit of no effect of SBP-LF.
There were some limitations to this study. First, the number of participants was small because the procedures are complex and long examination time was necessary for one test. Also, we selected men as study participants to exclude the effect of autonomic nerve change influenced by hormonal changes. Secondly, the subjects in the present study were all healthy and therefore it is plausible that the observed effects would be different in the patients that potentially have cardiovascular or other diseases or risk factors. Despite these limitations, our study could clarify the effects of PD and its potential benefits on age-dependent changes of the autonomic nerve system.
Our study includes important aspects because the spectrum analysis enables us to understand the effect of Kampo medicine, objectively. It is also found that PD has a function of stimulating sympathetic nerve and simultaneously restoring the decrease parasympathetic functions. These findings suggest that PD might have combined effects to protect the whole autonomic nervous system, harmoniously by several mixed compounds in one medicine.
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Citation: Garu A, Shiota Y, Shibly AZ, Sheikh MA, Yano S, Araki T, et al. (2021) Age-Dependent Analysis of the Effects of Pueraria Decoction on Autonomic Nerve Activities using Head-Up Tilt Test. J Clin Trials. 11:453.
Received: 18-Jan-2021 Accepted: 01-Feb-2021 Published: 08-Feb-2021 , DOI: 10.35248/2167-0870.21.11.453
Copyright: © 2021 Garu A, et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.