Journal of Alcoholism & Drug Dependence
Open Access
ISSN: 2329-6488
ISSN: 2329-6488
Research Article - (2014) Volume 2, Issue 2
Background: Liver cirrhosis is a major cause of death in many developed countries. Binge drinking has been shown to increase the risk of liver cirrhosis.
Objective: The aim of this study was to examine the aggregate-level relation between the alcohol consumption and liver cirrhosis mortality rates in Russia.
Method: Age-standardized sex-specific male and female liver cirrhosis mortality data for the period 1970- 2005 and data on overall alcohol consumption were analyzed by means ARIMA (Autoregressive Integrated Moving Average) time series analysis.
Results: Alcohol consumption was significantly associated with both male and female liver cirrhosis mortality rates: a 1 liter increase in overall alcohol consumption would result in a 7.0% increase in the male liver cirrhosis mortality rate and in 6.2% increase in the female mortality rate. The results of the analysis suggest that 61.4% of all male liver cirrhosis deaths and 56.4% female deaths in Russia could be attributed to alcohol.
Conclusions: The outcomes of this study provide support for the hypothesis that alcohol is an important contributor to the liver cirrhosis mortality rate in Russian Federation. The findings from the present study have important implications in regards alcohol-related mortality prevention indicating that a restrictive alcohol policy can be considered as an effective measure of prevention in countries with higher rate of alcohol consumption.
Keywords: Liver cirrhosis mortality; Alcohol consumption; ARIMA time series analysis; Russia; 1970-2005
Liver cirrhosis is a major cause of deaths in many developed countries. Over the last three decades liver cirrhosis mortality rates have gradually declined in the European Union [1]. This overall decline, however, masks large differences in trends between member states. In the wine drinking countries of Southern Europe (France and Italy), a reduction in liver cirrhosis mortality rates is being observed, mainly related to the decline in overall alcohol consumption. Conversely, liver cirrhosis mortality rates have increased across the spirits/beer binge drinking countries of Northern Europe and Britain [2]. An increase in liver cirrhosis mortality rates also appeared in Eastern Europe, particularly in the former Soviet republics [3]. The reasons for geographic differences in liver cirrhosis mortality rates are still poorly understood and most likely are because of differences in the risk factors, including alcohol consumption [2,3].
There is general consensus about causal role of alcohol for the risk of liver cirrhosis [4-7]. Liver cirrhosis mortality rate is a reliable indicator of the alcohol-related harm at the population level since there is a strong relationship between mortality rate and alcohol consumption per capita [8,9]. The outcome of the time series analysis showed a positive and statistically significant relationship between liver cirrhosis mortality and population drinking in 13 of 14 Western European countries for males and in nine countries for females [10]. It appears that the effect of alcohol on liver cirrhosis mortality rate was stronger in the northern European spirits-consuming countries characterized by a binge drinking pattern, than in the southern European wine countries, in which alcohol consumption has high averages but is more evenly distributed throughout the week. A positive and statistically significant relationship between per capita alcohol consumption and liver cirrhosis mortality was found in eight of nine Eastern European countries [11]. Furthermore, estimates were stronger in spirits binge drinking Eastern European countries than in ‘non-spirits’ countries. Taken together, these findings suggest that binge drinking is at least in part responsible for the geographic variations in liver cirrhosis mortality rates across the European region.
The role of binge drinking pattern in modifying the effect of alcohol on the risk of liver cirrhosis has been emphasized in epidemiological and experimental studies [12-15]. In a cohort study of the prevalence of chronic liver disease in the general population, Belentani et al. [16] found that drinking alcohol outside meal increase the risk of developing alcohol induced liver damage. Additional results showed that women who drank primarily on weekend were at higher risk of liver damage than women who drink moderately throughout the week. Another study which evaluated the drinking patterns of patients with cirrhosis reported that 32% of them were binge drinkers [17]. Emerging evidence indicate that binge drinking is more hepatotoxic than consumption of a lower volume of drinks per drinking occasion [18]. Binge drinking has profound effects on biochemical, immunological, and bioenergetics processes in the liver and amplifies the risk of liver damage [19-21].
Russia ranks among the world’s heaviest drinking countries with an annual official consumption rate about 10 litres of pure alcohol per capita, while independent estimates show a figure as high as 17 liters [22,23]. The distinctive trait of Russian drinking culture is a high overall level of alcohol consumption and the heavy episodic (binge) drinking pattern of strong spirits [24-26]. A world wide assessment of drinking pattern showed that Russia and former Soviet republics had the most hazardous pattern of drinking. The liver cirrhosis mortality rate in Russia is among the highest in the world [3]. In 2002, Russia also had the highest rate of alcohol-attributable liver cirrhosis mortality for both genders: 9680 and 5572 person-years per 10, 000 for men and women respectively [27]. Against this background, the aim of this paper is to estimate the relationship between alcohol consumption per capita and liver cirrhosis mortality rate in Russia from 1970 to 2005.
Data
The data on age-adjusted sex-specific liver cirrhosis mortality rates per 1000.000 of the population are taken from the Russian State Statistical Committee (Rosstat). The Rosstat’s cause of death classification has undergone several changes in recent decades. Until 1988 the cause of death classification was based upon the Soviet nomenclature which had a limited number of causes of death in comparison with the International Classification of Diseases (ICD) system. From 1989-1998 Rosstat used a coding scheme that was based on ICD-9. From 1999 a new coding system based on ICD-10 was introduced. Rosstat issued a table of correspondence between its classification system and ICD-9 and ICD-10 and it has been claimed that the Russian system of coding is compatible with ICD-9 codes 571.0 and with ICD-10 codes K 70-74.
Realizing the difficulties associated with measuring alcohol consumption at the population level in Russia, we employed an alternative measure of overall alcohol consumption relative to Nemtsov’s estimates [22]. Estimation of alcohol consumption per capita was based on a set of indicator of alcohol-related harm which was adjusted for the effect of recorded alcohol consumption employing ARIMA (autoregressive integrated moving average) model [23]. More specifically, we calculated the level of unrecorded alcohol consumption as the difference between observed changes in the harm indicator and changes that would be expected on the bases of alcohol sales. The harm indicator series used was alcohol psychoses incidence rate because this indicator depends almost entirely on alcohol consumption [8]. We specified the number of persons, witch was admitted for the first time for the treatment as incidence of alcohol psychoses: (ICD–10: F 10).
Statistical analysis
To examine the relation between changes in the alcohol consumption and liver cirrhosis mortality across the study period a time-series analysis was performed using the statistical package “Statistica”. The dependent variables were the annual liver cirrhosis mortality and the independent variable was aggregate overall alcohol consumption. Bivariate correlations between the raw data from two time-series can often be spurious due to common sources in the trends and due to autocorrelation [28]. One way to reduce the risk of obtaining a spurious relation between two variables that have common trends is to remove these trends by means of a ‘differencing’ procedure, as expressed in formula:
Δxt = xt - xt-1
This means that the annual changes ‘Δ’ in variable ‘X’ are analyzed rather than raw data. The process whereby systematic variation within a time series is eliminated before the examination of potential causal relationships is referred to as ‘prewhitening’. This is subsequently followed an inspection of the cross-correlation function in order to estimate the association between the two prewhitened time series. It was Box et al. [29] who first proposed this particular method for undertaking a time series analysis and it is commonly referred to as ARIMA modeling. We used this model specification to estimate the relationship between the time series liver cirrhosis mortality and alcohol consumption rates in this paper. In line with previous aggregate studies [28,30] we estimated semi-logarithmic models with logged output. The following model was estimated:
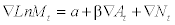
where Δ means that the series is differenced, M is liver cirrhosis mortality rates, a indicates the possible trend in liver cirrhosis mortality due to other factors than those included in the model, A is the alcohol consumption, β is the estimated regression parameter, and N is the noise term. The percentage increase in liver cirrhosis mortality rate rates associated with a 1-litre increase in alcohol consumption is given by the expression: (exp (β1)-1)*100. The temporal structure of the error term was estimated by using Autoregressive (AR) or Moving Average (MA) parameters in the model. A diagnostic test for residual correlation is given by the Box-Ljung Q-test, which indicates whether the model has been adequately fitted.
A change in aggregate level drinking is expected to have an immediate effect on acute forms of alcohol-related problems, as well as a long-term effect on chronic problems [8]. As liver cirrhosis is attributed to the chronic rather from acute alcohol-related problems, we should expect that the mortality response to changes in aggregate level alcohol consumption will be distributed over several years [28]. In order to deal with the time-lag problem we inspected the crosscorrelations between two time series at different lags.
In addition to the estimated effect parameter, the alcohol effect will also be expressed in terms of Alcohol-Attributable Fraction (AAF), which can be calculated from the estimates obtained in ARIMA models according to following formula: AAF=1-exp(-bX), where X is alcohol consumption for the whole study period and b is the estimated effect parameter [31].
According to official statistics, the male liver cirrhosis mortality rate increased 3.5 times (from 134.9 to 469.5 per 1000.000 of population) and female mortality rate increased 3.9 times (from 65.4 to 255.2 per 1.000.000 of population) in Russia from 1970 to 2005. Across the whole period the male liver cirrhosis mortality rate was 2.2 times higher than the female rate (214.8 vs. 98.9 per 1 000.000) with a rate ratio of 2.1 in 1970 decreasing to 1.8 by the 2005 (Table 1). For both sexes the time series liver cirrhosis mortality rates fluctuated greatly over the period: decreased markedly between 1984-1988 (by 31.8% and 19.8% for men and women respectively), than started on an upward trend from 1988-1989, before increasing substantially during 1992 to 1995 (by 84.8% and 74.8% for men and women respectively). From 1995- 1998 there was a fall in the rates before they again jumping dramatically between 1998 and 2005 (by 113.9% and 147.5% for men and women respectively). Although the trends in liver cirrhosis mortality rates are rather similar over time series for both sexes, the male mortality rate tends to fluctuated across time series to a much greater extent than the female rate.
| Mortality males | Mortality females | |||
|---|---|---|---|---|
| Lag | r | SE | r | SE |
| -3 | 0.020 | 0.177 | 0.044 | 0.177 |
| -2 | 0.135 | 0.174 | 0.185 | 0.174 |
| -1 | 0.234 | 0.172 | 0.258 | 0.172 |
| 0 | 0.605 | 0.169 | 0.520 | 0.169 |
| 1 | 0.099 | 0.172 | 0.091 | 0.172 |
| 2 | 0.255 | 0.174 | 0.199 | 0.174 |
| 3 | 0.156 | 0.177 | 0.287 | 0.177 |
Table 1: The results of cross-correlation analysis between prewhitened time series. Effects of alcohol consumption per capita on liver cirrhosis mortality rates
The graphical evidence suggests that the trends in both alcohol consumption per capita and liver cirrhosis mortality for males and females seem to follow each other across the time-series (Figures 1-3). As can be seen, there were sharp trends in the time series data across the study period. These trends were removed by means of a first-order differencing procedure (Figures 2-4). After prewhitening the crosscorrelations between alcohol consumption and the liver cirrhosis mortality time series were inspected. This indicated that there was a statistically significant cross-correlation between alcohol consumption and liver cirrhosis mortality for males and females at lag zero (Table 1). The specification of the bivariate ARIMA model and outcome of the analyses are presented in Table 2. According to the results, alcohol consumption is significantly associated with both male and female liver cirrhosis mortality rates, implying that a 1-litre increase in per capita consumption is associated with an increase in male mortality of 7.0% and female mortality of 6.2%. Table 2 also shows the relative proportion of alcohol-attributable deaths to all liver cirrhosis deaths by gender. The results of the analysis suggest that 61.4% of all male deaths and 56.4% female deaths from liver cirrhosis in Russia could be attributed to alcohol.
| Parameter | Model | Estimation | P | AAF |
|---|---|---|---|---|
| Mortality males | 0.1.1* | 0.070 | 0.000 | 0.614 |
| Mortality females | 0.1.1 | 0.062 | 0.000 | 0.564 |
Table 2: Estimated effects (bivariate ARIMA model) of overall alcohol consumption on liver cirrhosis mortality rates
The outcome of the time-series analysis suggests a positive and statistically significant relationship between liver cirrhosis mortality rate and population drinking in Russia. These results are consistent with the previous findings from other settings that highlighted close temporal association between alcohol consumption and liver cirrhosis mortality at the aggregate level [10]. It is important to point out that the results suggest a fairly quick response of liver cirrhosis mortality rates to changes in alcohol consumption per capita. The instantaneous response in mortality rates from chronic alcohol-related diseases seem quite surprising when considering the long latency period at the individual level [28]. The reasonable explanation from this seeming inconsistency has been suggested by Norström and Skog [28]. They argue that in a population there exists a reservoir of heavy drinkers who are near the critical threshold-value for a dying from chronic alcohol-related consequences. In case when these high risk individuals increase alcohol consumption during a given year, they will exceed the threshold value and die from alcohol-related diseases. This is the reason why the immediate impact on chronic alcohol related mortality can be registered from marked changes in aggregate consumption.
Understanding the reasons the substantial fluctuations in liver cirrhosis mortality rate in Russia over recent decades is very important from a public health perspective. There is evidence that the liver cirrhosis mortality trends in Russia influenced by the three major factors: Gorbachev’s anti-alcohol campaign 1985-88; severe socioeconomic crisis imposed by rapid societal transformation in the early 1990s; financial crisis and worsening economic situation in 1998. A fairly close match between trends in alcohol consumption and liver cirrhosis mortality during the Gorbachev’s anti-alcohol campaigns may be used as evidence for the hypothesis suggesting that alcohol is responsible for a substantial number of liver cirrhosis deaths in Russia. This empirical evidence also indicates that a restrictive alcohol policy can be considered as an effective measure of liver cirrhosis mortality prevention.
It seems plausible that alcohol is a key variable in explaining the increase in the liver cirrhosis mortality rate in the early-1990s. An increase of alcohol consumption in this period was to a great extent due to increase of alcohol availability following the repeal of the state alcohol monopoly in January 1992 [24]. There are several potential factors behind the decrease in alcohol consumption and liver cirrhosis mortality rate between 1994 and 1998. They include better regulation of the alcohol market that may have resulted in a relative increase in prices for vodka compared to those for food products [22]. Another possible factor in the decrease in alcohol consumption was impoverishment and decrease in the purchasing capacity of the population due to unpaid or delayed salaries [24]. The subsequent rise in alcohol consumption and liver cirrhosis mortality rates from 1998 may be associated with the financial crisis which resulted in hyperinflation, increasing poverty, political and economic uncertainty [32].
Before concluding, we should address the potential limitations of this study. A close relationship between per capita alcohol consumption and liver cirrhosis mortality does not rule out the possibility that other factors have also influenced the development liver cirrhosis mortality rate. Although alcohol is regarded as the most important risk factor for liver cirrhosis, a number of other causal factors that influence the risk of development of liver cirrhosis have been indentified, including diet, obesity and concomitant infection with viral hepatitis [6]. The presence of these causal factors is likely to have distorted the estimated alcohol effects. It seems that the role of non-alcohol causes in liver cirrhoses mortality increased during the last decade. In particular, the epidemic of viral hepatitis among drug users might contribute to the dramatic increase in liver cirrhosis mortality in Russia during the past several years [33]. It should be also emphasized that the sharp rise in viral hepatitis prevalence might actually account for the discrepancies between trends in alcohol consumption and liver cirrhosis mortality rate in most recent years. The etiological importance of other risk factors is clearly modest compared to alcohol, thus limiting their potential influence. Therefore, the controlling for the impact of these risk factors would not have modified our results.
Further, the estimates of AAF for women, where heavy drinking is restricted to a relatively small proportion of the population, gives rise to the suspicion of possible measurement error. It should be recognized that ignoring the confounding variables may imply that the alcohol effect is overestimated. Nevertheless, there are some indications that Russian women are drinking more now which are likely to be a factor in the narrowing of the male-female alcohol-related mortality rate ratio [25]. In his recent study, based on the results of RLMS Perlman [34] highlighted that frequent heavy drinking almost doubled among women between 1994 and 2004. An alternative explanation for these findings is that women are more sensitive to an increased risk of liver cirrhosis, even at the relatively low levels of alcohol consumption [20,35]
In conclusion, the findings from present study support the substantial literature which demonstrates a close link between alcohol consumption and liver cirrhosis mortality at aggregate level. The outcomes of this study provide indirect support for the hypothesis that unfavorable mixture of higher overall level of alcohol consumption and binge drinking pattern is a major risk factor for liver cirrhosis mortality in Russia [36]. The findings from the present study have important implications as regards alcohol-related mortality prevention indicating that a restrictive alcohol policy can be considered as an effective measure of prevention in countries where higher rate of alcohol consumption per capita.
None declared
The author would like to thank the anonymous reviewers for their valuable comments and suggestions on an earlier version of this paper.