Medicinal & Aromatic Plants
Open Access
ISSN: 2167-0412
ISSN: 2167-0412
Research Article - (2016) Volume 5, Issue 6
Two Verbena species (Verbena officinalis L. and Aloysia citrodora L.) were investigated to analyze their total phenolic and flavonoids content, DPPH free radical scavenging activity, allelopathic activity and antimicrobial activity. The total phenolic content (Folin-Ciocalteu assay) was shown to be between 160 μg GAE/mg dry weight basis (V. officinalis) and 360.03 μg GAE/mg dry weight basis (A. citrodora). The total flavonoids content (aluminum chloride method) was shown that, total flavonoids content varied from 7.91 to 10.62 μg QE/g DW in V. officinalis and from 11.70 to 12.93 μg QE/g DW in A. citrodora. The antioxidant activity was determined by using a DPPH radical scavenging assay and their antimicrobial activity was determined by utilizing an agar disc diffusion assay. Methanol extracts of the two species analyzed showed high antioxidant activity and among them A. citrodora possessed the highest quantity (249.31 mM ACE/g DW). The aqueous extracts of the species were found to inhibit the germination and radicle elongation of canary grass (P. canariensis L.) and lettuce (L. sativa L.). The methanol extracts showed antibacterial activities against a number of microorganisms. These findings suggest that the aqueous and methanol extracts of the plants tested contain compounds with allelopathic and antimicrobial properties. These exhibited properties propose that such plant extracts can possibly be used as natural preservatives in the food and pharmaceutical industries.
Keywords: Verbena officinalis L.; Aloysia citrodora L.; Polyphenols; Antibacterial activity; Allelopathy
Verbena is a vegetal genus in the family Verbenaceae. The genus Verbena contains about 250 species of annual and perennial herbaceous or semi-woody flowering plants [1]. The majority of the species are native to the Americas and Asia. Verbena has longstanding use in herbalism and folk medicine, usually as an herbal tea. In fact, phytochemical investigations of Verbena species have revealed the presence of polyphenols, flavonoids, verbascosides, triterpenoids, phenylpropanoids, caffeoyl derivatives, volatile oils, and iridoids [2-4].
Verbena officinalis L. (common Verbena) is a perennial herb growing by roadsides and sunny pastures of semiarid environments throughout most of Europe and North Africa, as well as in China and Japan [4,5]. As a member of the Verbena genus, the aqueous extracts V. officinalis represent a good source of antioxidants [6], and exhibit various biological activities including sleep-promoting, diuretic, expectorant, anti-inflammatory and antibacterial activities [7,8].
Aloysia citrodora L. (Lemon Verbena) is a perennial medicinal shrub in the same family Verbenaceae. It can grow up to 3 m and is characterized by the presence of fragrant, lemon-like scented narrow leaves [9,10]. The importance of lemon Verbena can be inferred from the number of commercial crops present in different European, African and South American countries [11]. The fresh leaves and flowering tops are utilized for the preparation of herbal tea and beverage flavor. The dried tissues and their extracts are included in different food and medicinal preparations [11]. As any Verbena species, A. citriodora contains several flavonoid compounds, phenolic acids as well as essential oils [9,10,12]. These compounds have applications in the pharmaceutical industry as well as perfumery and cosmetics, and may show interesting antibacterial and antifungal properties [9]. As a medicinal plant, lemon Verbena is known to have antispasmodic, antipyretic, sedative, digestive, antimicrobial, local analgesic, and antioxidant activities [13,14]. Due to these properties, lemon Verbena represents a huge market potential for herbal preparation and of essential oil extraction [15].
Although plant secondary metabolites are generally associated with plant defense against herbivores and pathogens, these unique compounds can be involved in a broad array of ecological functions [16]. Phenolic compounds are one of the largest groups of secondary metabolites, consisting of four main groups divided according to the number of phenol rings and the structural elements that bind those rings, including flavonoids, phenolic acids, tannins, stilbenes and lignans [17]. Indeed, some secondary metabolites such as benzoxazinoids are regarded as natural pesticides against pathogens, microorganisms, fungi, insects, and weeds [18].
The ubiquitous use of agrochemicals has resulted in negative impacts on human health and the environment. The use of the biological activity of plant extracts against certain pests of crops remains an environmental friendly alternative. Allelopathy is a phenomenon whereby secondary metabolites synthesized by microorganisms or plants may positively influence biological and agricultural systems [19,20]. Marichali et al. reported that allelopathy is the inhibitory or stimulatory effect of one plant or microorganism on other plants through the release of chemical compounds into their surrounding environment [21]. However, these stimulatory and inhibitory effects depend on the concentration of the released compounds [22]. For instance, allelopathy offers an important tool for selective biological weed management through the production and release of natural allelochemicals from leaves, flowers, seeds, stems and roots of certain plants [23,24].
Phytotoxicity bioassays such as germination and seedling growth inhibition are primary tools for determining the positive or negative allelopathic potential of secondary metabolites on plants [25]. Thus, the allelopathic and antifungal activities of aqueous extracts of plants have been studied. Indeed, Gupta and Chabbi showed that water extracts from 23 common weeds and crop residues inhibited the germination and growth of wheat [26]. Shafique et al. indicated that aqueous extracts of the following plants, Allium sativum, Cymbopogon proxims, Carum carvi, Azadirachta indica, and Eugenia caryophyllus had strong antifungal activity against three potential phytopathogens namely, Fusarium oxysporum, Botrytis cinerea and Rhizoctonia solani [27].
The aims of this study were: (i) to assess the polyphenols and flavonoids contents of extracts from aerial part (leaves and stems) of Verbena officinalis L. and Aloysia citrodora L.; and (ii) to evaluate their antioxidant, allelopathic and antimicrobial activities.
Plant material
Fresh aerial tissues of two Verbena species (Figure 1) namely, Verbena officinalis L. (a) and Aloysia citrodora L. (b) were collected in their vegetative stage during the fall season of 2014 from three different regions in the northern part of Tunisia.
Sampling sites were Sejnane (latitude 37° 03’ 31. 20” (N); longitude 9°13’ 28.35” (E); altitude 137 m), Teskraya (latitude 37° 12’ 21. 30” (N); longitude 9° 32’ 20. 10” (E); altitude 23 m), and Mograne (latitude 36° 25’ 34. 53” (N); longitude 10° 05’ 41. 51” (E); altitude 150 m). The harvested tissues were air-dried at room temperature (20 ± 2°C) for one week, ground in Retsch blender mill (Normandie-Labo, France), then sieved through 0.5 mm mesh to obtain a uniform particle size. The samples were stored in the dark at 4°C until used.
Chemicals
Folin-Ciocalteu reagent, gallic acid, quercetin, sodium hypochlorite, aluminum chloride, methanol, ascorbic acid and 2,2-diphenyl-1- picrylhydrazyl (DPPH) were purchased from Sigma-Aldrich (St. Louis, MO, USA). All solvents and reagents were of the highest purity grade.
Extraction techniques
Preparation of methanol extract: Methanol extracts of V. officinalis and A. citrodora were prepared as described by Dallali et al. [4] with slight modification. Briefly, 1 g of each plant sample was mixed with 20 mL of methanol (80%) into 100 mL flasks. After 48 h of stirring at room temperature, the supernatant was filtered and dried at 50°C using a rotavap. The dried sample was then weighed, re-dissolved in 3 mL methanol and stored at 4°C until analysis [28].
Preparation of aqueous extracts: Aqueous extracts were prepared by mixing 10 g of powdered aerial tissues in 100 mL of sterilized distilled water and shaking for 24 h. Then, the mixture was filtered through Whatman # 1 filter paper (Bärenstein, Germany) and the resulting filtrate was centrifuged at 10,000 rpm for 15 min at 10°C (Eppendorf 5810 R, France). The supernatant was then filtered and diluted with distilled water to prepare a five dilution series. Solutions were stored at 4°C in the dark until analysis.
Total phenolic content (TPC)
Total phenolic contents in Verbena officinalis L. and Aloysia citrodora L. were determined with the Folin-Ciocalteu method according to Singleton and Rossi [29] slightly modified by Dallali et al. [30]. Briefly, 500 μL of diluted methanol extract was added to 5 mL of freshly 10-fold diluted Folin-Ciocalteu reagent, and the mixture was neutralized with 4 mL of sodium carbonate solution (7%). The mixture was kept reacting in the dark for 15 min then absorbance was measured at 765 nm using a UV/Vis spectrophotometer (Jenway 6300, Jenway Ltd., UK). A blank was prepared according to the procedure described above except that the sample was substituted by distilled water. Gallic acid was used as the calibration standard and TPC was expressed as μg gallic acid equivalent per g dry weight (μg GAE g-1).
Total flavonoid content (TFC)
Total Flavonoid content in methanol extracts was determined using aluminum chloride colorimetric method [4]. One milliliter of diluted methanol extract was mixed with 1 mL of 2% AlCl3 methanol solution. After incubation at room temperature for 15 min, the absorbance was measured at 430 nm using spectrophotometry. As with gallic acid, TFC was expressed as μg quercetin equivalent per g dry weight (μg QE g-1).
Antioxidant activity
The antioxidant potential of plant extracts was highlighted by estimating the DPPH radical scavenging activity. Ascorbic acid was used as the reference antioxidant [31]. The reaction solution was prepared by mixing 50 μL of extract sample with 2 mL of 0.004% (w/v) stock solution of DPPH in methanol (80%). After 30-min incubation at room temperature, the absorbance was read against a blank sample at 517 nm in a UV/Vis spectrophotometer. The DPPH radical scavenging activity in terms of percentage was calculated according to the following equation:
I (%)=[(A0-A1)/A0] × 100
where, I is DPPH inhibition (%), A0 the absorbance of the control, and A1 the absorbance of the extract/standard.
Allelopathic activity
The allelopathic activity of both plant species was determined by monitoring the phytotoxicity of aqueous extracts using the 5-d seed germination/root elongation inhibition test [32]. Two test plant species were chosen for this assay: Phalaris canariensis L. (weed) and Lactuca sativa L. (cultivated) Seeds were first disinfected with sodium hypochlorite solution (15%) for 10 min then thoroughly washed with distilled water. Twenty seeds of each plant species were evenly placed on filter papers (Whatman, number 2) soaked with 2 mL of the diluted aqueous extracts (100%, 50%, 25%, 12.5%, and 6.25%) in sterile glass Petri dishes (Ø=90 mm). Distilled water was used as the control medium for germination. Petri dishes were hermetically sealed with parafilm (Neenah, Wisconsin, USA) and placed randomly in a growth chamber for 5 d at 22°C in the dark.
At the end of the incubation period, the number of germinated seeds was recorded, and the radicle length was measured to the closest millimeter. Germination was considered when a radicle of least 3 mm protruded beyond the seed coat. Final germination percent (FGP), index of germination (IG) and inhibition of radicle elongation (IRE) were calculated as follows:
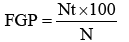
where, Nt: Final number of germinated seeds in respective treatments. N: number of seeds used in bioassay.
IG=(Gt × Lt)/(Gc × Lc)
where, Gt: number of germinated seeds in respective treatments, Lt: respective value radicle length in treatment, Gc: number of germinated seeds in control treatment and Lc: respective value radicle length in control treatment.
The percent reduction in germination, index of germination and radicle length were determined as under [33]:
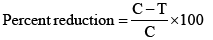
where, C: Respective value in control treatment and T: Respective value in treatment.
Antimicrobial activities
The antibacterial and antifungal potential of V. officinalis and A. citrodora was studied using the crude methanol extract dissolved in DMSO (dimethyl sulphoxide). Four pathogenic bacteria and two pathogenic fungi were selected for this test namely, Listeria monocytogenes and Salmonella DMS 560 (Gram+), Escherichia coli and Pseudomonas aeruginosa (Gram -ve), and Fusarium oxysporum and Penicillium chrysogenum, respectively. Test microorganisms were initially grown in nutrient broth at 37°C for bacteria and in potato dextrose agar (PDA) at 25°C for fungi.
The antibacterial activity of methanol extracts was assessed by the disk diffusion method [34]. For each pathogenic bacterium, a suspension of the test microorganism was first spread on solid Mueller-Hinton agar (MHA) plates. Filter paper disks (Ø=6 mm) were afterwards soaked with 100 μL of DMSO-dissolved extract and placed on the inoculated plates. After incubation at 37°C for 24 h, the diameter of the inhibition clear zones or halos around the disks was measured in millimeters when observed. The evaluation of antifungal activity consisted of measuring mycelial growth inhibition as described above except that plates were incubated for 72 h at 35°C.
Statistical analysis
Data were processed using STATISTICA 5.0 software package (Tulsa…). For each plant species, all assays were performed in triplicate and the results were expressed as mean ± standard deviation (SD). One way analysis of variance (ANOVA) followed by Duncan’s Multiple Range Test was applied to compare means at P ≤ 0.05.
Extraction yield
Methanol and aqueous extractions were used to estimate the capacity and selectivity of recovering phenolic compounds from the aerial parts of V. officinalis and A. citrodora as well as their influence on antioxidant activities. As illustrated in Table 1, a statistically significant variation was noticed for the extraction yield between both plant species for all sampling regions. Similarly, the difference in yield was significant between extraction methods (around 10-fold) for V. officinalis and A. citrodora (Table 1). V. officinalis had consistently a higher extraction yield compared to A. citrodora. On the other, plants collected from the Mograne region possess the highest extraction yield for both species (Table 1).
| Sampling region | Extraction yield (%) | |||
|---|---|---|---|---|
| Aqueous extract | Methanol extract | |||
| V. officinalis | A.citrodora | V.officinalis | A.citrodora | |
| Mograne | 57.40±3.9a | 45.07±5.3b | 5.40±1.7a | 4.26±0.8b |
| Teskraya | 41.37±3.7a | 29.40±8.7b | 3.74±0.8a | 2.12±0.4b |
| Sejnane | 51.10±5.1a | 40.83±5.3b | 5.08±0.7a | 3.97±1.1b |
Values with different superscripts (a-b) are significantly different at P<0.05
Table 1: Extraction yield of aqueous and methanol extract of V. officinalis and A. citrodora.
Among the steps to obtain phytochemicals from plant, extraction is the main step for recovering and isolating phytochemicals from plant materials. Indeed, extraction efficiency is affected by the chemical nature of phytochemicals, the extraction method used, sample particle size, as well as the presence of interfering substances [35,36]. The variation on extract yields might be explained by physical properties of plant samples, other polar compounds besides phenolics, namely polysaccharides and plant debris [37,38]. The yield of extraction depends on the pH, temperature, extraction time, and composition of the sample [36,39].
Total phenol content
The total phenol content in methanol extracts of V. officinalis and A. citrodora is given in Figure 1. The first observations indicate that regardless to sampling region, A. citrodora showed consistently higher phenol content than V. officinalis. The total phenolcontent varied in the different extracts and ranged from 108.59 to 160 μg GAE/gDW and from 264.23 to 360.03 μg GAE/gDW respectively for V. officinalis and A. citrodora. The extracts with the highest total phenol content in all provenances were observed in A. citrodora and the extract samples from Teskreya shows the highestvalue (360.03 μg GAE/gDW) (Figure 2).
Our results showed that the total phenol content varies from one plant to another. This can be attributed to several factors climatic and environment, geographical area, drought, diseases [40,41], harvest time and stage of plant development [42]. The method of extraction and quantification also influences the estimation of total phenols content [43]. In fact, an increase in the biosynthesis and accumulation of phenolic compounds occurs frequently in plant tissue in response to biotic and abiotic stresses. These compounds may prevent the oxidative modification by neutralizing free radicals, oxygen scavenging or decomposition of peroxides through their antioxidant activities [44]. The increase in the production of phenolics was attributed to the increased activities of enzymes of secondary pathway namely polyphenols oxidase (PPO), shikimate dehydrogenase (SKD) and phenylalanine ammonialyase (PAL) [45,46]. Additionally, considering that the carbon skeletons for phenols synthesis are provided either by the Calvin cycle or by the oxidation of pentose phosphate pathway (OPP), it appears that there has been increased activity of OPP leading to an increased substrate supply for the synthesis of phenolic compounds [46]. Phenolic substances have been shown to be responsible for the antioxidant activity of plant materials [47]. Indeed, phenols are very important plant constituents because of their scavenging ability on free radicals due to their hydroxyl groups. Therefore, the phenolic content of plants may contribute directly to their antioxidant action [48].
Total flavonoids content
The contents of flavonoids in methanol extracts of V. officinalis and A. citrodora were estimated using spectrophotometric method with aluminum chloride. The résultats of total flavonoids analysis of both species extracts are presented in Figure 2. Total flavonoids content varied from 7.91 to 10.62 μg QE/g DW in V. officinalis and from 11.70 to 12.93 μg QE/g DW in A. citrodora. The extract of A. citrodora from Teskreya has the highest content of flavonoids (12.93 μg QE/g DW) compared with V. officinalis (10.62 μg QE/g DW) (Figure 3).
These results agree with Casanova et al. [49], who found that V. officinalis contains polyphénols, flavonoids, phenolic compounds [derivatives of phenolic acids (verbascoside and isoverbascoside)], tannins and caffeic derivatives. Flavonoids are class of secondary plant metabolites with significant antioxidant and chelating properties. Antioxidant activity of flavonoids depends on the structure and substitution pattern of hydroxyl groups [50]. Flavonoids are polyphenolic compounds with low molecular mass, found in leguminous, fruits, flowers, and leaves [51]. They are as one of the most diverse and widespread group of natural compounds are probably the most important natural phenolics. These compounds possess a broad spectrum of chemical and biological activities including radical scavenging properties [52]. Alongside the phenolic compounds; the presence of flavonoids may also affect the antioxidant capacity. Thereby, antioxidant activity of flavonoids depends on the structure and substitution pattern of hydroxyl groups [53]. The phenolic content of the plant can directly contribute to their antioxidant and it is likely that extracts of the activity is due to these compounds [48].
DPPH radical scavenging activity
The antioxidant activity of different extracts from V. officinalis and A. citrodora was determined using a methanol solution of DPPH reagent. A number of methods are available for determining the free radical scavenging activity, but the radical-scavenging DPPH test has received the most attention [54]. Because it can accommodate many samples in a short period and is sensitive enough to detect active ingredients at low concentrations, it has been extensively used for screening antiradical activities of extracts [55]. Results in Figure 4 demonstrated that all extracts were found to be effective scavengers against DPPH radical. The methanol extractsfrom Teskreya has the highest content of antiradical activityfor both species. It is the order of 249.31 mM ACE/g DW for A. citrodora and 219.80 mM ACE/g DW for V. officinalis.
The methanolic extracts from A. citrodora have high concentration of total phenols (Figure 1) and flavonoids (Figure 2), which is in correlation with intense antioxidant activity of these extracts. It is well known that phenolic compounds are potential antioxidants and free radical-scavengers; hence, there should be a close correlation between the content of phenolic compounds and antioxidant activity. The methanol extract of the areal parts of V. officinalis and A. citrodora exhibits strong anti-radical activity that may be related to the abundance of phenols and flavonoids [56]. The mechanism of the reaction between the antioxidant and DPPH radical depends on the structural conformation of the antioxidant [57]. The antioxidant effect of various extracts may also be due to synergism between polyphenols and other minor components. The DPPH scavenging activity of the extract depends on various biochemicals further that the polyphenol content [52]. Indeed, Babbar et al. [58] showed that phenolic compounds alone are responsible for antioxidant activity in plant organs. However, other components such as ascorbate, tocopherols, carotenoids, terpenes and pigments as well as the synergistic effect between them could possibly contribute to the total antioxidant activity. Fallah et al. [59] showed that antioxidant activity does not only depend on the concentration of polyphenols, but also on the nature and structure of the antioxidants in the extract. The extracts that perform the highest antioxidant activity (Figure 3) have the highest concentration of polyphenols (Figure 1 and 2). Numerous studies have shown a correlation between radical scavenging activity and phenolic compounds [60,61]. A significant linear correlation was found between the values for the concentration of phenolic compounds and the antioxidant activity of extracts from Marrubium peregrinum [53]. Phytochemical investigation revealed the presence of flavonoids, phenolic compounds, and tannins in some of the plant extracts, and it is well established that flavonoids are responsible for antioxidant properties [62]. The role of natural antioxidants attracting more and more interest in the prevention and treatment of various diseases (cancer, diabetes, hypertension, inflammatory and cardiovascular diseases); they are also used as additives in food, pharmaceutical and cosmetics [63,64].
Allelopathic activity
Extracts from various species can exhibit negative allelopathic effect on plant germination and growth. To evaluate the methanolic extract of V. officinalis and A. citrodora possible allelopathic effects, they were assayed in-vitro on germination and radicle elongation of canary grass (Phalaris canariensis L.) and lettuce (Lactuca sativa L.) seeds. For the three provenances, the inhibitory effect of aqueous extracts of V. officinalis on the germination of L. sativa seed is always more important than A. citrodora (Table 2). This effect is significant (P<0.05) at the plant extracts from Teskreya and Sejnen. However, the inhibitory effect of A. citrodora from Mograne on radicle elongation of L. sativa is higher (12.5%) than recorded from Teskreya and Sejnen.
| Species | Phalaris canariensis | Lactuca sativa | ||||
|---|---|---|---|---|---|---|
| Mograne | Teskreya | Sejnen | Mograne | Teskreya | Sejnen | |
| V.officinalis | 11.1± 0.6a | 10.94± 0.79a | 10.6± 1.63a | 24.02 ± 4.07b | 15.99± 3.22a | 16.01± 2.5b |
| A.citrodora | 12.15 ± 2.73a | 30.94 ± 8.66b | 26.91 ± 7.16b | 16.01 ± 1.62a | 26.75± 3.62b | 6.79a± 0.25a |
Values with different superscripts (a-b) are significantly different at P<0.05
Table 2: Inhibitory effects (IC50) of V. officinalis and A. citrodora aqueous extract on radicle elongation of test plant spp.
The inhibitory effect of A. citrodora aqueous extracts on radicle elongation of Phalaris canariensis is less important than that recorded in Lactuca sativa. However, extracts of V. officinalis plants from Teskreya show a higher inhibitory effect (15.99%) compared with that of A. citrodora (26.75%) (Table 2).
Seedling growth performance of canary grass and lettuce in response to V. officinalis and A. citrodora extract treatment was found different as compared to control treatment. The seed germination and radicle length was inhibited in all concentrations (Table 2). Radicle length was strongly inhibited by the aqueous extract of V. officinalis and A. citrodora in all the tested crops. It was also noticed that the decrease in radicle length seemed to increase with increase in concentrations of the extract. The results showed extracts had greater effect on seedling growth rather than on germination which agrees with findings of Konstantinović et al. [65] who concluded that effect of allelochemicals is more pronounced on the growth of seedlings [66]. Several investigations indicating the allelopathic or phytotoxic determine of aqueous extracts of plants contain Sorghum bicolor [67], Argemone mexicana [68], Carum carvi [21], Euphorbia thiamifolia [69], Chrysanthemum coronarium [70] and Vicia faba [71]. All these investigations indicated the discharge of phototoxic chemicals during the preparation of aqueous extracts. The result of the present work also supported the finding of above workers who found a significant decrease of radicle growth in many crop and garden plants. This showed that germination was less sensitive than seedling growth in canary grass and lettuce. Similar conclusion was also obtained by Verma et al. [72], Emeterio et al. [73] in different other plants. Also, the effect of plant extract on radicle growth was studied [24,74]. The inhibitory effect of weed extracts on the germination and radicle growth of the test plants can be attributed to the presence of allelochemicals. These results agree with other studies reporting that water extracts of allelopathic plants had more pronounced effects on radicle growth [75]. This is likely because those roots are the first to absorb the allelochemicals from the environment [76]. The results confirm the findings of Fateh et al. [77] and Shang and Xu [78], showing that allelochemicals have inhibitory and/or lethal effects on seed germination, growth and development of crops. Reduction in seedling growth might have been caused by some of allelochemicals. All these researches have reported inhibitory effect in seed germination, root length and other primary growth parameters caused by allelochemicals present in aqueous extracts [76]. In fact, allelochemicals, of many plant have been reported to effect the growth of the other plants, a wide range of injurious effect on crop growth has been reported as being due to phytotoxic decomposing products, release from leaves, stem, roots, fruit and seeds. On the other hand, Hosni et al. [70] reported that the species-dependent response to aqueous extracts confirmed that the susceptibility of target species depends on the physiological and biochemical characteristics of each species. Fateh et al. [77] reported that, those most commonly identified as allelopathic agents are phenolic compounds, which include simple phenols, phenolic acids, cinnamic acid derivatives, coumarins, flavonoids, quinones, and tannins. Also, many researchers have found that inhibitory substances involved in allelopathy are terpenoids and phenolic substances [79]. Very small seeds make contact with the aqueous extract easily, so that even low concentrations can cause an immediate negative effect [78]. However, as allelopathic effect can be both stimulatory and inhibitory it could be utilized both in weed control and in promotion of crops growth [80].
Antibacterial activity
The methanolic extract of V. officinalis and A. citrodora was evaluated for antibacterial activity against pathogenic strains of Grampositive (Listeria monocytogenes, Salmonella DMS 560) and Gramnegative (Escherichia coli, Pseudomonas aeruginosa) bacteria. It was found to be active against all of the bacterial strains. The activity of the extract varies with its concentration and kind of bacteria.
The antibacterial activity of the methanolic extract against Escherichia coli is shown in Figure 4. E. coli bacterial strain is sensitive to the methanol extracts of both species. However, it appears significantly more sensitive to methanol extracts of V. officinalis (11.33 ± 2.08 to 14 ± 2.33 mm). On other hand, we notice a significant effect and inhibition zone variation from one region to another. Indeed, the zone of inhibition extracts from Sejnen is the most important (14 ± 2.33 mm) (Figure 5).
The antibacterial activity of the methanolic extract against Listeria monocytogenesis was illustrated in Figure 5. From the figure, we see that the effect of antibacterial activity is more important for extracts V. officinalis except for the extracts from Teskreya region where the extracts of A. citrodora showed a remarkable effect (8.33 ± 3.51 mm). In fact, the highest activity is reported in the methanol extracts from Mograne region for V. officinalis (9.66 ± 1.11 mm). So for A. citrodora, the highest activity is noticed in the extracts from Teskreya region (8.33 ± 0.51 mm) (Figure 5).
Figure 6 represents the antibacterial activity of the methanolic extract against Pseudomonas aeruginosa. As for L. monocytogenes, the zone of inhibition of antibacterial activity is higher in the methanol extracts of V. officinalis (7.00 ± 2.00 to 10.33 ± 2.08 mm) except the extracts from Teskreya region where the extracted from A. citrodora showed a stronger effect (7.33 ± 3.05 mm) (Figure 7).
The results of antibacterial activity of the methanolic extract against Salmonella DMS 560 were presented in Figure 7. The data of this figure show that antibacterial effect of Salmonella DMS 560 varies from one plant to another and depending on its origin. Thus, the zone of inhibition extracts of V. officinalis was significantly greater for Teskreya (9.33 ± 1.53 mm) followed by extracts of plants from the Mograne region (6.33 ± 0.57 mm). For extracts of Sejnen region, the effect is more important for A. citrodora (8.33 ± 2.08 mm) and the variation is significant (Figure 8).
The antimicrobial activities of the investigated extract were evaluated by determining the zone of inhibition against two grampositive and two gram-negative bacteria using a disc diffusion method. The effects of the areal parts extract bacteria of V. officinalis and A. citrodora on the tested microorganisms are shown. This antimicrobial activity against bacteria has also been demonstrated in the methanol extracts from Artemisia absinthum and Artemisia santonicum aerial parts [81]. The pronounced antioxidant activity of V. officinalis and A. citrodora areal parts extract was possibly due to its high phenolic content. Thereby, further work is needed to identify bioactive molecules. It is demonstrated that this antibacterial activity may be related to the presence of hydrolysable tannins and polyphenolics in the pomegranate extract [82,83]. Tannins may act on the cell wall and across the cell membrane because they can precipitate proteins [83]. They may also suppress many enzymes such as glycosyltransferases [84]. Hence, the antibacterial activity of V. officinalis and A. citrodora may be related to polyphenol structures because polyphenols may affect the bacterial cell wall, inhibit enzymes by oxidized agents, interact with proteins and disturb co-aggregation of microorganisms [84,85]. In fact, plant polyphenols are considered to possess antibacterial activity [86,87]. The antimicrobial activity of berry phenolics has been studied previously [86] with the ethanol extracts of berry and berry skins showing inhibitory effects on the growing of gram [88,89]. Antibacterial activity against another genus of Gram-negative bacterium (Escherichia coli) was previously described for the ethanolic extract of A. triphylla [90]. The relatively higher antibacterial activity of the areal parts extract of V. officinali sand A. citrodora may be indicative for the presence of some active metabolites such as alkaloids, sulfur compounds, terpenes and saponines, among others [55]. These authors reported that the extraction procedure, the origin of plants, the tested micro-organisms and the inoculum size are the main source of variation of the Antibacterial activity. Najjaa et al. [91] reported that strong antibacterial activity against E. coli, Pseudomonas aeruginosa, Salmonella typhimurium, Staphylococcus epidermis, micrococcus luteus and Staphylococcus aureus, of the methanolic extract from A. roseum collected in the south of the country. It was found that the methanol extracts of the plant were significantly active against the bacteria Gram (+) and Gram (-) and fungi studied [55,92].
Antifungal activity
The evaluation of the antifungal activity consists of measuring the mycelial growth of Fusarium oxysporum and Penicillium chrysogenum in the presence of methanolic extracts. The antibacterial activity of the methanolic extract against F. oxysporum and P. notatum is shown in Table 3. Indeed, the antifungal activity is more pronounced among A. citrodora than V. officinalis. On the other hand, extracts of Sejnen region have the largest effect on F. oxysporum for A. citrodora (18.33 ± 0.32 mm), by cons for V. officinalis; extracts of this region have the largest mycelial growth (28.3 ± 0.4 mm). No activity was found against P. notatum was relatively sensitive to all extracts of V. officinalis (Table 3). Mycelial growth varies with the origin of the extracts. In fact, the extract from the Sejnen region have the largest effect (16.8 ± 0.15 mm) on P. notatum (Table 3).
| Species | Sample region | Mycelial growth (cm) | ||
|---|---|---|---|---|
| F. oxysporum | P. notatum | Control | ||
| V.officinalis | Mograne | 26 ±0.15a | - | 34 |
| Teskreya | 25±0.08a | - | 34 | |
| Sejnen | 28.3±0.4a | - | 34 | |
| A.citrodora | Mograne | 25.1±0.05a | 17.7±0.05 | 34 |
| Teskreya | 23±0.15a | 25.7±0.15 | 34 | |
| Sejnen | 18.33±0.32a | 16.8±0.32 | 34 | |
Values with different superscripts (a-b) are significantly different at P<0.05
Table 3: Effect of antifungal activity of methanolic extracts of V. officinalis and A. citrodora on Mycelial growth of F. oxysporum and P. notatum.
Natural products, including plants, may be a source of compounds with antifungal effects and therefore possible candidates for the development of new antifungal agents [93]. Consequently, there is an increasing need for new compounds with antifungal activity.
Owoyale et al. evaluated the antifungal and antibacterial activities of alcoholic extracts of Senna alata leaves [94]. The antimicrobial activity of ethanolic and aqueous extracts of Sida acuta on microorganisms from skin infections has been documented by Ekpo and Etim [95]. Ethanolic extracts of Picralima nitida seeds were tested for their antifungal activities using Aspergillus flavus, Candida albicans and Microsporum canis as test organisms [96]. Some metabolites in plants have been reported to elicit inhibitory effect on microorganisms [97]. Thus, Baba- Moussa et al. [98] reported that tannins present in some plant species possess antifungal property. It has also been shown that saponins are active antifungal agents [99]. It is hoped that the elucidation of the structure of the active principle(s) and its/their subsequent use in antifungal investigations would give better results. It was observed that the values obtained for the zones of inhibition differed, for each test organism (Table 3). These results corroborate the findings of Ubulom et al. [96] and Rajakaruna et al. [100]. Results of this study also agree with the report of Karou et al. [101]. The difference in susceptibility observed in this study could be attributed to the inherent resistance factor of the test organisms among other factors [95,96].
To conclude, the methanolic extracts of the two plant species were found to possess polyphenols and exhibited antioxidant activity. Based on these results, it is possible to conclude that the aerial parts of V. officinalis and A. citrodora exhibit antibacterial activity against a number of bacteria. The present study provides the evidence of V. officinalis and A. citrodora has allelopathic potential. These results suggested that V. officinalis and A. citrodora areal parts can be regarded as a natural source of antimicrobials and antioxidants and maybe considered for future use in replacing synthetic antioxidants and antimicrobial agents in pharmaceutical products.sihak for her active participation.