Journal of Clinical and Experimental Ophthalmology
Open Access
ISSN: 2155-9570
ISSN: 2155-9570
Research Article - (2022)Volume 13, Issue 2
Importance: Hemodialysis is one of the most common renal replacement therapies but regularly causes microcirculatory change. Measuring retinal microcirculation may be the way to evaluate the adverse effect of hemodialysis.
Background: To evaluate the effects of hemodialysis on the microcirculation perfusion in End-Stage Renal Disease (ESRD) patients through observation of the alteration of peripapillary and macular perfusion by Optical Coherence Tomography Angiography (OCTA).
Design: A retrospective study.
Participants: A total of 37 patients (19 ESRD patients and 18 age-matched healthy individuals) were enrolled in this study.
Methods: All 19 ESRD patients underwent ocular assessments 1 hour before, 1 hour and 24 hours after hemodialysis, while18 healthy individuals underwent OCTA measurement twice with four hours interval. Vascular vessel density of optic disc and macular region, peripapillary Retinal Nerve Fiber Layer (RNFL) and retinal thickness were measured by OCTA.
Main outcome measures: Retinal vascular vessel density and thickness.
Results: Increase in peripapillary RNFL thickness along with decrease in peripapillary vessel density was found at 1h after hemodialysis. Increase in peripapillary RNFL thickness last for at least 24 hours after hemodialysis, while decrease in peripapillary vessel density diminished after 24 hours. We also found significant increase in deep capillary plexus which recovered after 24 hours. Retinal thickness is also increased after hemodialysis.
Conclusion: Hemodialysis induced transient decrease in peripapillary perfusion and mild RNFL edema, which may cause transient damage of retina. OCTA may be an ideal method to evaluate the alteration of retinal vascular system and a potential parameter for microcirculatory perfusion in hemodialysis patients.
Haemodialysis; Ocular microcirculation; RNFL thickness; Optical coherence tomography angiography
During haemodialysis, patients are exposed to a fluctuation in intravascular volume status, and level of uremic toxins, electrolytes due to controlled ultrafiltration of several litres within hours [1]. It has been shown that patients with End-Stage Renal Disease (ESRD) have increased risk for ocular dialysis disequilibrium which can cause pain or discomfort during haemodialysis, accompanied by increased risk of glaucoma development over time [2,3]. However, the relationship between ocular hemodynamic change and haemodialysis is not fully understood. Although the exact mechanism of hemodynamic instability remains unknown, it is always accompanied by changes in microvascular perfusion, such as increased blood viscosity, increased platelet aggregation, decreased erythrocyte deformability and increased haematocrit due to reduction in blood volume [4]. This unfavourable condition is not only associated with the inability to extract fluid adequately, but also with changes in serum osmolarity and organ perfusion, leading to increased all-cause mortality and hospitalization [5]. To monitor microcirculatory change during haemodialysis, indirect quantification or measurement of the microcirculatory perfusion in human have been described [1,4-9]. Some articles reported decrease in blood flow after haemodialysis, while others reported improvement of blood flow in different organs. Differences in results may be due to variation in microvascular regulatory mechanism of different organs. Besides, direct quantification of retinal vascular parameters of microcirculatory change before and after dialysis also ended up with different conclusion while data of peripapillary perfusion is still lacking [10,11]. Because of no direct quantified data of peripapillary perfusion was analysed and conflicting reports due to differences of organs and machine measurement, the effect of haemodialysis on retina remains unclear.
The introduction of Optical Coherence Tomography Angiography (OCTA) imaging has opened challenging new perspectives in in-vivo research of microcirculatory alterations. It provides non-invasive and rapid evaluation of the retina and choroidal microcirculation. It is based on the principle of decorrelation, in which the signals of red blood cells on B-scan images are seen to fluctuate in each specific are of the retina [12]. This technique can be used to quantitatively express the nerve fibre thickness and vascular density layer by layer, facilitating the separate Superficial Capillary Plexus (SCP), Deep Capillary Plexus (DCP). The application efficacy of OCTA has been shown in several retinal microvascular diseases such as diabetic retinopathy, age-related macular degeneration and sickle cell anaemia [12]. Besides, OCTA has already been successfully applied in neurology as imaging of the retinal vessels might bring insight into brain microcirculation [13]. Therefore, OCTA can be a reliable tool to non-invasively quantify microcirculatory change.
In the present clinical study, to further investigate the peripapillary perfusion and to confirm the macular vascular change during haemodialysis, we measured the changes in macular and peripapillary microcirculation induced by haemodialysis using an optical coherence tomography angiography system to investigate the effect of haemodialysis on retinal microcirculation in patients with End Stage Renal Disease (ESRD).
Subjects
The study included 37 individuals (19 ESRD patients and 18 age-matched healthy individuals). All were outpatients at Guangdong Provincial People’s Hospital (Guangzhou, China). This study was approved by the Research Ethics Committee of Guangdong Provincial People’s Hospital (registration number: gdrec2016232A). Patients are consented to participate in the study. The causes of renal failure in the 19 patients included chronic glomerulitis, diabetes, hypertension and other causes. All patients were non-smoker and had no evidence of cardiac or cerebrovascular disorders. All had been undergoing regular maintenance hemodialysis for 4 hours three times weekly. Patients continued to take their antihypertensive medications, including calcium channel blockers, β blockers and angiotensin converting enzyme inhibitors during the study.
All participants had a corrected visual acuity better than 20/40 and intraocular pressure under 21 mmHg. No ophthalmic surgery or ocular trauma history. Patients with pathology conditions that could alter retinal structure, such as macular degeneration, diabetic retinopathy, ocular trauma, ocular surgery and ocular inflammation, were excluded.
Ocular assessment
Blood reports, best corrected visual acuity and intraocular pressure were collected before hemodialysis. Blood pressure and weight measurement were performed right before and after hemodialysis. Patients were included during single hemodialysis sessions. All patients were on a dialysis schedule of three times weekly using as part of extracorporeal circuit. The composition of the dialysis fluid was constant during treatment. UF was at a constant rate and calculated to reach a previously assessed dry weight or target end-dialytic weight, which was previously determined by the treating dialysis doctor, during treatment hours. The treatment plan was adapted to clinical parameters such as the absence of edema or symptoms of manifest hypovolemia after previous dialysis sessions in each patient. During ophthalmic examination, slit-lamp examination, visual acuity and intraocular pressure measurement were conducted to exclude ocular disease that may potentially affect the result by a single experienced ophthalmic doctor. OCTA measurement were done 1 hour before and after a single hemodialysis, another measurement was taken 1 hour before and 24 hours after another single hemodialysis to investigate the duration of microcirculatory change. For healthy individuals, the second OCTA measurement was finished 4 hours after the first measurement.
Microvascular imaging and quantification
Participants were assessed for retinal microvascular perfusion after pupillary dilation with AngioVue OCTA system (RTVue-XR Avanti; Optovue, Fremont, CA, USA, version 2016.2.035) [14]. Split-spectrum amplitude decorrelation angiography (SSADA) software algorithm was used for evaluation of vessel density and nerve fiber thickness. The 70-kHz spectral OCT system and 200+ A-scans per cross-sectional B-scan enabled SSADA to assess slowest flow rate to capillary level. Each assessment consisted of two set of imaging, including 1 vertical priority and 1 horizontal priority. Motion artifacts are corrected by an orthogonal registration algorithm, which is used to merge 3D OCT angiograms. Each volume was composed of 400 scan lines. Vessel density is defined as the proportion of the area occupied by vessels (white pixels) out of the whole area of the measurement sector. For vessel density, a slab extending from 3 to 15 μm from the internal limiting membrane was generated for detecting the Superficial Capillary Plexus (SCP), a slab extending from 15 to 70 μm below the internal limiting membrane for the Deep Capillary Plexus (DCP). The inner retinal layer thickness was defined by the distance between ILM and the outer boundary of inner plexiform layer.
The optic disc scan covers an area of 4.5 × 4.5 mm. Average vessel density inside optic disc was calculated. The peripapillary region is divided into 2 sectors (superior hemi and inferior hemi) and 6 sectors (nasal, inferior nasal, inferior temporal, superior temporal, superior nasal, and tempo) (Figure 1). Vessel density in each sector was evaluated automatically by the machine. In macular region, HD 6 × 6 mm measurement was performed in the fovea (1mm diameter central zone), parafovea (1mm -3 mm diameter ring) and perifovea (3 mm-6 mm diameter ring). The parafoveal and perifoveal region is divided into 2 sectors (superior hemi and inferior hemi) and 4 sectors of 90 degrees each (nasal, inferior, superior, and temporal sectors) (Figure 1). Vessel density for each sector and whole image of macula scan were evaluated automatically by the machine.
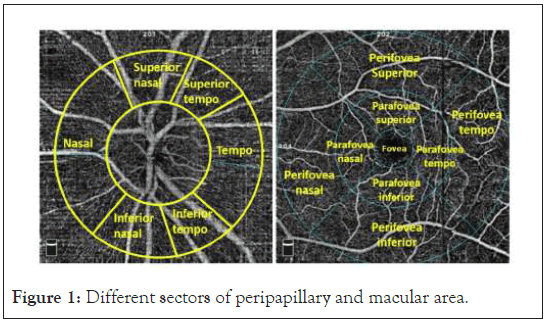
Figure 1: Different sectors of peripapillary and macular area.
In addition, peripapillary Retinal Nerve Fiber Layer (RNFL) was detected by OCTA. The RNFL thickness was defined by the algorithm as the distance between the ILM and the middle of the RPE. The average and regional information of Retinal Thickness (RT) were also demonstrated. The retinal thickness measurement in each area was automatically obtained using the retinal map protocol in Avanti software. The parafoveal and perifoveal thickness was measured using a circular annulus centered on the fovea with an OCT angiogram. Only the images with optimal quality, no motion artifacts, vitreous floaters, or other artifacts were selected. Mean differences were calculated by value of prehemodialysis minus post-hemodialysis.
Statistical analysis
All values were expressed as mean ± SD. The t test for paired samples and Wilcoxon matched-pairs signed-rank test were used to detect the change of vascular parameter pre-hemodialysis and post-hemodialysis according the parametric or nonparametric distribution of the data. P values less than 0.05 were considered as statistically significant.
Basic characteristics of participants
Thirty-seven subjects were enrolled in the study. As shown in Table 1, nineteen were ESRD patients who had been maintenance hemodialysis for 8.6 ± 4.7 years (10 males and 9 females, mean age for 51.8 ± 10.0 years, mean body dry weight 55.3 ± 13.2 kg, including 4 with diabetes, 16 with hypertension, and 3 with coexisting hypertension and diabetes), and eighteen were healthy individuals (6 males and 12 females, mean age for 50.1 ± 12.5 years). ESRD patients with hypertension were treated with antihypertensive medications according to their condition. All dialyzers were made of polysulfone with the dialysate flow rate and temperature stable at 500 mL per minute and 36°C, respectively. Both heparin and low molecular weight heparin was used for anticoagulation.
| Before hemodialysis | After hemodialysis | p value | |
|---|---|---|---|
| Age, years | 51.79 ± 9.98 | / | |
| Gender, M/F | 10/9 | / | |
| Years of HD | 8.63 ± 4.67 | / | |
| DM/Non-DM | 4/15 | / | |
| HTN/Non-HTN | 16/3 | / | |
| Ultrafiltration volume | 2.329 ± 0.83 | / | |
| Body weight (kg) | 57.71 ± 13.82 | 55.36 ± 13.17 | <0.001* |
| SBP | 154.43 ± 19.16 | 152 ± 35.3 | 0.8 |
| DBP | 79.64 ± 16.36 | 87.71 ± 18.7 | 0.157 |
| MABP | 104.57 ± 13.54 | 109.14 ± 21.12 | 0.473 |
Note: Values are expressed as means ± standard deviation PTH: Parathyroid Hormone; LDL: Low Density Lipoprotein; WBC: White Blood Cell; SBP: Systolic Blood Pressure; DBP: Diastolic Blood Pressure; MABP: Mean Arterial Blood Pressure; *Statistical differences, p<0.05.
Table 1: Systemic change after haemodialysis of ESRD patients.
In hemodialysis group, 38 eyes from 19 ESRD patients underwent OCTA measurement 1 h before and 1 h and 24 h after a single dialysis. In normal control group, 18 healthy individuals underwent OCTA measurement twice with time interval of 4 h. Patients on hemodialysis showed non-significant increase in diastolic pressure from 79.64 ± 16.36 mmHg to 87.71 ± 18.7 mmHg and non-significant decrease in systolic pressure from 154.43 ± 19.16 to 152.00 ± 35.30, while no significant change in mean arterial blood pressure was found.
Retinal nerve fiber layer thickness of change participants
As shown in Table 2, peripapillary RNFL thickness increased from 102.65 ± 14.91 µm to 105.71 ± 14.92 µm (p<0.001) while similar increase in peripapillary RNFL were found in all regions except temporal region at 1h after hemodialysis. Most of patients (92.11%) presented an increased peripapillary RNFL in inferior hemi sectors (p<0.01), while only 65.79% patients demonstrated increase in peripapillary RNFL thickness in tempo sectors (p>0.05) (Figure 2). Even 24 h after hemodialysis, increase of peripapillary RNFL thickness from 103.71 ± 14.23 µm to 105.58 ± 13.93 µm was found, and superior and inferior hemi sector of peripapillary RNFL remains increased in 85.19% patients. However, only in 77.78%, 85.19%, 77.78% and 70.37% patients showed significantly increased peripapillary RNFL thickness in superior nasal sector, inferior tempo, nasal and tempo sectors 24 h after hemodialysis respectively (p<0.05).
| Hemodialysis group | Normal control (n=36) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 h (n=38) | 24 h (n=38) | |||||||||||
| RNFL thickness | Pre-hemodialysis | Post-hemodialysis | Mean differences | p value | Pre-hemodialysis | Post-hemodialysis | Mean differences | p value | First measurement | Second measurement | Mean differences | p value |
| Peripapillary | 102.65 ± 14.91 | 105.71 ± 14.92 | -3.06 ± 2.59 | <0.001** | 103.71 ± 14.23 | 105.58 ± 13.93 | -1.87 ± 1.84 | <0.001** | 113.09 ± 15.02 | 113.33 ± 14.51 | -0.24 ± 3.07 | 0.658 |
| Superior hemi | 103.48 ± 17.10 | 106.22 ± 17.10 | -2.74 ± 3.07 | <0.001** | 104.14 ± 15.78 | 106.47 ± 15.98 | -2.33 ± 2.76 | <0.001** | 116.66 ± 21.93 | 116.83 ± 18.43 | -0.17 ± 9.06 | 0.912 |
| Inferior hemi | 101.76 ± 14.48 | 105.17 ± 14.71 | -3.42 ± 3.02 | <0.001** | 103.25 ± 14.71 | 104.62 ± 14.28 | -1.38 ± 1.60 | <0.001** | 111.71 ± 15.19 | 113.56 ± 16.63 | -1.86 ± 8.43 | 0.215 |
| Tempo | 147.35 ± 22.04 | 150.44 ± 26.22 | -3.09 ± 9.56 | 0.053 | 145.49 ± 23.36 | 148.20 ± 22.68 | -2.71 ± 3.73 | 0.001* | 155.61 ± 24.90 | 157.56 ± 28.15 | 1.95 ± 10.90 | 0.298 |
| Superior tempo | 124.37 ± 28.69 | 128.57 ± 29.63 | -4.21 ± 5.49 | <0.001** | 126.01 ± 25.90 | 127.40 ± 24.42 | -1.38 ± 6.66 | 0.291 | 141.72 ± 19.08 | 144.64 ± 28.93 | 2.92 ± 20.98 | 0.423 |
| Superior Nasal | 127.19 ± 31.64 | 130.07 ± 32.80 | -2.87 ± 5.16 | 0.001* | 128.08 ± 29.42 | 130.92 ± 30.17 | -2.84 ± 4.28 | 0.002* | 133.05 ± 30.78 | 131.55 ± 30.12 | 1.50 ± 31.79 | 0.785 |
| Nasal | 171.68 ± 29.35 | 176.86 ± 28.27 | -5.18 ± 5.55 | <0.001** | 175.13 ± 29.97 | 177.61 ± 30.35 | -2.48 ± 4.17 | 0.005* | 196.75 ± 30.87 | 198.68 ± 29.81 | 1.93 ± 13.54 | 0.419 |
| Inferior Nasal | 121.67 ± 21.10 | 127.07 ± 21.73 | -5.41 ± 6.06 | <0.001** | 124.49 ± 18.75 | 125.65 ± 18.01 | -1.16 ± 4.26 | 0.169 | 129.29 ± 34.79 | 132.84 ± 29.52 | 3.55 ± 13.83 | 0.138 |
| Inferior Tempo | 139.76 ± 27.16 | 144.61 ± 28.34 | -4.85 ± 5.81 | <0.001** | 141.04 ± 27.43 | 144.74 ± 27.11 | -3.70 ± 4.35 | <0.001** | 153.42 ± 24.62 | 152.81 ± 23.11 | 0.61 ± 10.38 | 0.733 |
Note: *Statistical differences, p<0.05, **statistical differences, p<0.001
Table 2: The mean ± SD of different sectors of peripapillary RNFL thickness in 3 study groups.
Figure 2: The percentage distribution of patients who demonstrated increased peripapillary RNFL thickness in each sector at 1 hour after haemodialysis.
Retinal vessel density change of participants
Shown in Table 3, total peripapillary vessel density decreased from 48.24 ± 4.41% to 47.43 ± 4.42% (p<0.05). All sectors except superior hemi and tempo sector demonstrated significant decrease in vessel density at 1h after hemodialysis (p<0.05). However, no significant change in optic disc vessel density was found in 24h and normal control group.
| Hemodialysis group | Normal control (n=36) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 h (n=38) | 24 h (n=38) | |||||||||||
| Optic disc vessel density | Pre-hemodialysis | Post-hemodialysis | Mean differences | p- value | Pre-hemodialysis | Post-hemodialysis | Mean differences | p- value | First measurement | Second measurement | Mean differences | p- value |
| Whole Image | 48.24 ± 4.41 | 47.43 ± 4.42 | 0.81 ± 1.88 | 0.012* | 48.82 ± 3.92 | 48.57 ± 3.38 | 0.25 ± 2.16 | 0.551 | 47.33 ± 8.32 | 48.85 ± 2.40 | -1.52 ± 8.32 | 0.281 |
| Inside Disc | 49.09 ± 3.95 | 48.64 ± 5.43 | 0.45 ± 3.56 | 0.439 | 48.96 ± 4.81 | 50.01 ± 3.48 | -1.05 ± 4.58 | 0.243 | 52.02 ± 4.99 | 53.93 ± 6.73 | -1.90 ± 5.69 | 0.056 |
| Peripapillary | 49.96 ± 5.05 | 49.15 ± 4.99 | 0.81 ± 2.35 | 0.040* | 50.41 ± 4.10 | 50.24 ± 3.94 | 0.18 ± 2.37 | 0.703 | 51.30 ± 2.20 | 51.38 ± 2.53 | -0.08 ± 1.95 | 0.815 |
| Superior Hemi | 49.89 ± 5.51 | 49.12 ± 5.45 | 0.77 ± 2.54 | 0.069 | 50.31 ± 4.30 | 50.55 ± 4.47 | -0.24 ± 2.75 | 0.657 | 51.16 ± 3.72 | 51.34 ± 2.99 | -0.18 ± 3.94 | 0.791 |
| Inferior Hemi | 50.04 ± 5.00 | 49.18 ± 4.94 | 0.86 ± 2.59 | 0.048* | 50.51 ± 4.44 | 49.89 ± 3.88 | 0.62 ± 2.67 | 0.235 | 50.60 ± 2.89 | 50.85 ± 3.32 | -0.25 ± 2.08 | 0.492 |
| Nasal | 92.29 ± 10.50 | 90.35 ± 10.18 | 1.94 ± 5.50 | 0.036* | 95.13 ± 9.21 | 93.42 ± 8.48 | 1.70 ± 7.27 | 0.235 | 94.75 ± 5.65 | 93.17 ± 17.32 | 1.58 ± 15.93 | 0.566 |
| Inferior Nasal | 50.05 ± 5.94 | 48.45 ± 5.46 | 1.60 ± 3.52 | 0.009* | 49.75 ± 6.01 | 49.78 ± 5.45 | -0.03 ± 2.78 | 0.955 | 49.63 ± 5.13 | 49.98 ± 4.64 | -0.35 ± 4.43 | 0.652 |
| Inferior Tempo | 55.33 ± 7.34 | 53.96 ± 7.35 | 1.36 ± 3.81 | 0.036* | 55.13 ± 7.47 | 54.40 ± 6.99 | 0.73 ± 3.46 | 0.281 | 56.03 ± 3.68 | 56.15 ± 5.10 | -0.12 ± 3.90 | 0.859 |
| Tempo | 104.59 ± 11.44 | 105.68 ± 9.82 | -1.09 ± 8.05 | 0.411 | 104.71 ± 9.12 | 105.66 ± 8.40 | -0.95 ± 7.86 | 0.538 | 106.24 ± 12.38 | 107.77 ± 10.45 | 1.53 ± 11.20 | 0.424 |
| Superior tempo | 52.85 ± 7.37 | 51.36 ± 7.31 | 1.49 ± 3.59 | 0.015* | 53.00 ± 5.81 | 53.21 ± 5.72 | -0.21 ± 3.55 | 0.763 | 54.57 ± 3.37 | 54.85 ± 4.16 | -0.28 ± 2.80 | 0.557 |
| Superior Nasal | 47.65 ± 7.02 | 46.11 ± 7.91 | 1.53 ± 3.61 | 0.013* | 47.88 ± 7.30 | 47.65 ± 6.60 | 0.23 ± 3.24 | 0.715 | 48.79 ± 3.94 | 47.50 ± 5.23 | 1.29 ± 4.57 | 0.109 |
Note: *Statistic differences, p<0.05, **Statistic differences, p<0.001.
Table 3: The mean ± SD of different sectors of peripapillary vessel density in 3 study groups.
When it comes to macular vessel density, retinal DCP showed significant increase of vessel density in both perifovea and parafovea region at 1h after hemodialysis (p<0.05) (Table 4). Even though similar change was also found in the whole retina, no significant change was found when calculated the SCP in separate region. Except non-specific change of vessel density was detected in several sectors of deep capillary plexus, no significant vascular parameter change was found in both 24 h after hemodialysis and normal individual statistically, which indicates the vascular change is short-lived while exclude the influence of vascular rhythm on OCTA measurement.
| Hemodialysis group | Normal control (n=36) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 h (n=38) | 24 h (n=38) | |||||||||||
| Macular vessel density | Pre-hemodialysis | Post-hemodialysis | Mean differences | p value | Pre-hemodialysis | Post-hemodialysis | Mean differences | p value | First measurement | Second measurement | Mean differences | p value |
| Deep layer | ||||||||||||
| Fovea | 33.81 ± 8.65 | 34.57 ± 7.86 | -0.75 ± 4.84 | 0.343 | 36.24 ± 7.08 | 37.07 ± 6.87 | -0.83 ± 3.20 | 0.182 | 36.48 ± 9.20 | 37.68 ± 8.85 | -1.19 ± 5.19 | 0.176 |
| Parafovea | 51.81 ± 5.74 | 54.61 ± 4.59 | -2.80 ± 4.74 | 0.001* | 53.97 ± 4.94 | 54.86 ± 4.27 | -0.88 ± 4.05 | 0.258 | 53.28 ± 5.85 | 54.58 ± 3.88 | -1.30 ± 6.44 | 0.233 |
| Perifovea | 48.41 ± 5.82 | 51.30 ± 6.08 | -2.88 ± 5.37 | 0.002* | 50.26 ± 5.63 | 51.62 ± 6.01 | -1.36 ± 4.62 | 0.13 | 47.89 ± 7.07 | 50.50 ± 6.06 | -2.60 ± 8.78 | 0.084 |
| Superior hemi | 47.77 ± 5.24 | 50.72 ± 5.73 | -2.95 ± 5.40 | 0.002* | 49.97 ± 5.77 | 51.20 ± 5.65 | -1.22 ± 4.62 | 0.173 | 47.51 ± 6.61 | 50.14 ± 5.42 | -2.63 ± 8.07 | 0.058 |
| Inferior hemi | 47.17 ± 5.49 | 49.64 ± 5.87 | -2.47 ± 5.32 | 0.007* | 48.77 ± 5.04 | 50.43 ± 5.13 | -1.66 ± 4.31 | 0.051 | 46.77 ± 6.78 | 48.62 ± 5.62 | -1.85 ± 8.44 | 0.196 |
| Parafovea | ||||||||||||
| Superior hemi | 52.29 ± 6.38 | 55.24 ± 4.27 | -2.95 ± 5.73 | 0.003* | 54.24 ± 6.11 | 55.29 ± 4.17 | -1.05 ± 5.31 | 0.306 | 53.81 ± 5.57 | 55.18 ± 3.69 | -1.37 ± 6.21 | 0.193 |
| Inferior hemi | 51.33 ± 5.84 | 53.98 ± 5.27 | -2.65 ± 4.37 | 0.001* | 53.71 ± 4.58 | 54.42 ± 4.73 | -0.72 ± 3.95 | 0.344 | 52.74 ± 6.47 | 53.97 ± 4.39 | -1.23 ± 6.84 | 0.289 |
| Tempo | 53.14 ± 6.26 | 55.32 ± 4.72 | -2.18 ± 5.71 | 0.024* | 55.57 ± 4.67 | 55.80 ± 4.09 | -0.23 ± 3.75 | 0.748 | 54.74 ± 6.85 | 55.51 ± 3.98 | -0.77 ± 7.41 | 0.536 |
| Superior | 50.93 ± 8.23 | 54.75 ± 4.68 | -3.82 ± 7.10 | 0.002* | 53.42 ± 7.48 | 54.50 ± 5.06 | -1.08 ± 7.50 | 0.452 | 52.74 ± 6.25 | 54.58 ± 4.24 | -1.84 ± 6.93 | 0.12 |
| Nasal | 53.08 ± 5.96 | 55.39 ± 5.06 | -2.31 ± 5.76 | 0.018* | 54.61 ± 5.01 | 55.49 ± 3.56 | -0.88 ± 3.82 | 0.231 | 54.40 ± 5.14 | 55.96 ± 4.11 | -1.56 ± 6.12 | 0.135 |
| Inferior | 50.07 ± 6.79 | 53.00 ± 6.48 | -2.92 ± 5.37 | 0.002* | 52.29 ± 5.05 | 53.64 ± 6.56 | -1.34 ± 5.22 | 0.184 | 51.22 ± 7.12 | 52.26 ± 5.12 | -1.03 ± 7.73 | 0.428 |
| Perifovea | ||||||||||||
| Superior hemi | 48.77 ± 5.56 | 51.68 ± 6.19 | -2.90±5.51 | 0.002* | 51.16 ± 5.84 | 52.36 ± 6.28 | -1.20 ± 4.71 | 0.198 | 48.34 ± 7.05 | 51.42 ± 5.78 | -3.08 ± 8.32 | 0.033* |
| Inferior hemi | 48.04 ± 6.29 | 50.91 ± 6.31 | -2.87 ± 5.65 | 0.003* | 49.44 ± 5.77 | 51.26 ± 5.85 | -1.82 ± 4.78 | 0.054 | 47.45 ± 7.68 | 49.76 ± 6.73 | -2.31 ± 9.82 | 0.173 |
| Tempo | 51.55 ± 5.56 | 53.57 ± 4.83 | -2.02 ± 5.11 | 0.020* | 52.84 ± 4.96 | 54.39 ± 4.80 | -1.55 ± 3.32 | 0.020* | 50.65 ± 7.32 | 53.29 ± 5.45 | -2.64 ± 8.88 | 0.084 |
| Superior | 47.90 ± 5.58 | 50.58 ± 6.85 | -2.68 ± 6.15 | 0.011* | 49.83 ± 6.28 | 51.34 ± 7.15 | -1.50 ± 5.58 | 0.165 | 47.09 ± 8.06 | 51.08 ± 6.32 | -3.99 ± 9.05 | 0.012* |
| Nasal | 47.30 ± 6.93 | 51.22 ± 7.02 | -3.91 ± 6.06 | <0.001** | 50.70 ± 6.68 | 50.71 ± 6.82 | -0.02 ± 6.26 | 0.99 | 47.64 ± 7.72 | 49.26 ± 6.55 | -1.62 ± 9.45 | 0.31 |
| Inferior | 47.17 ± 6.58 | 49.80 ± 7.09 | -2.63 ± 6.37 | 0.015* | 47.73 ± 6.20 | 50.28 ± 6.52 | -2.55 ± 5.61 | 0.023* | 46.26 ± 8.66 | 48.45 ± 7.88 | 2.20 ± 11.07 | 0.249 |
Note: *Statistical differences, p<0.05, **Statistical differences, p<0.001
Table 4: The mean ± SD of different sectors of retina vessel density in 3 study groups.
Retinal thickness change of participants
Correspondingly, all sections of parafovea and perifovea demonstrated a significant increase in retinal thickness (p<0.05) at 1 h after hemodialysis, probably due to increase in vascular diameter and RNFL. Increased retinal thickness in tempo and inferior hemi sectors of parafovea and all sectors except tempo of perifovea was also found even at 24 h after hemodialysis (p<0.05).
In this study we have investigated the impact of hemodialysis on microcirculatory perfusion by direct observation using OCTA in adult patients on chronic renal replacement therapy. Our findings show that there is an increase in vessel density of DCP in perifovea and parafovea area and peripapillary RNFL thickness 1 h after hemolysis, while decrease in peripapillary vessel density in several sectors. However, both changes diminished 24 h after hemodialysis.
Several studies have investigated the response of hemodialysis on microcirculatory perfusion, using indirect or direct observation methods, ending up with different conclusions. Measuring at 30 minutes prior and every 30 minutes till the end of 180 minutes treatment, significant decrease in blood flow after dialysis in diabetic patients was reported when measuring venous oxygen saturation and relative blood flow using a spectrophotometer [6]. Several reports also showed a substantial reduction in sublingual microvascular flow in patients just after hemodialysis by side stream dark-field imaging [4,5]. Similar results were found when detecting skin blood flow of patients after hemodialysis [7]. These findings are in consistent with our results, which showed decreased vessel density in peripapillary area. In the first period after dialysis, patients suffered from rapid decreased in intravascular volume before interstitial fluid shifting to intravascular compartment [4]. Decrease in intravascular volume is normally compensated by an increase in vascular resistance and cardiac performance. Together, intravascular hypovolemia and increase in vascular resistance may result in decrease blood flow in peripapillary area. Another possible reason for decreased peripapillary perfusion is increased intraocular pressure [2]. In previous study, significant increase in intraocular pressure was found at different time points during hemodialysis [2]. As peripapillary vessel located more superficial than any other retinal vessel, it is more susceptible to be affected by increased intraocular pressure, which can directly increase the resistance of blood flow. The radial peripapillary capillaries form a special vascular bed located in the retinal nerve fiber layer. These superficial radial capillaries originated from deeper layer of retina and steeply to reach the superficial nerve fibers, while rarely anastomosed with each other. Lying superficial to the other retinal capillaries, these individual vessels pursued fairly long straight pathway which appeared to extend outwards from the optic disc in parallel with the retinal nerve fiber [15]. However, the characteristics of lack of anastomosis contribute to higher fragility and higher vascular resistance of peripapillary radial capillaries. Therefore, the reason for decrease in peripapillary vessel density after hemodialysis may probably be due to increased resistance caused by increased intraocular pressure.
Besides change of peripapillary vessel density, we also found significant increase of vessel density in DCP (p<0.05). However, no significant change was detected in separate region of SCP. This phenomenon may be caused by varying degree of vasodilation in retinal vessels. As retinal blood vessels have no neuronal innervation in retinal vascular beds, its regulation mainly rely on the local mechanism, including oxygen, carbon dioxide, angiotensin-II, adenosine, Nitric Oxide (NO), endothelin-1 and so on [16-20]. Previous studies have reported that Asymmetric Dimethylarginine (ADMA), an inhibitor of NO synthase, accumulated in microcirculation of ESRD patients and reduced after hemodialysis, resulting in the enhancement of the NO activity [21,22]. Both lack of neuronal innervation and hyperactivity of NO resulted in vasodilation in retinal vessels. DCP always present with spider-like vortex capillaries which offers the path of least resistance for dilation and collateral formation, serving as primary venous outflow for parafovea area [23]. Thus, the characteristic of least vascular resistance makes vasodilation more easily found in DCP. However, SCP is closer to arteriole with less compliance and higher vascular resistance than DCP, which may explain the reason why no significant change was found in this layer. Our results are in accordance with report from Nagaoka et al. who claimed that averaged values of retinal vessels diameter after hemodialysis significantly increased after hemodialysis using laser Doppler velocimetry system [8]. While Tow et al. came up with similar conclusion which showed shortlived dilatation of retinal venules after hemodialysis through digital retinal photography [1]. The change in retinal vessels diminished 24 h after hemolysis in our findings which is also agreed with finding of Tow et al. indicating the vascular change of retinal vessels induced by hemodialysis is short-lived.
An important finding of our study is that peripapillary RNFL thickness was increased after hemodialysis. Increase in peripapillary RNFL thickness was most profound in inferior hemi sectors, while no significant increase was found in tempo sector. The possible reason for transient increase in peripapillary RNFL is the damage caused by transient diminished peripapillary blood supply. It is important to note that non-myelinated axons in the retina located in the RNFL contributed to high energy commands in this layer, which also makes RNFL a sensitive structure [24,25]. Non-myelinated RGC axon possesses a high density of mitochondria mostly located within regularly spaced bulb-shaped varicosities along the course of the axon. Therefore, ischemic insults resulted from decreased blood supply will place non-myelinated axons in the RNFL in a vulnerable position to injury. Paula et al. has demonstrated the correlation between radial peripapillary capillaries and peripapillary RNFL thickness and indicated that radial peripapillary capillaries are supportive of their functional reliance [26]. The close correlation between radial peripapillary capillaries and RNFL supports the important role of blood supply in maintaining RNFL metabolic balance. During hemodialysis, decrease in peripapillary vessel density caused by increase in intraocular pressure during hemodialysis resulted in decreased oxygen supply to RNFL [2]. Thus, transient peripapillary RNFL edema occurred under hypoxia condition. As shown in Table 2 and Table 3, temporal region without decreased vessel density was corresponding to the region in which peripapillary RNFL was no significant post-hemodialysis 1h, which further indicated the potential causal relationship between peripapillary blood supply and thickness of RNFL increased. Even though we detected a significant reduction in perfusion of peripapillary region, we found no correlation between change of peripapillary vessel density and peripapillary RNFL thickness. Besides small sample size, this discrepancy may be due to mismatched correlation analysis between the optic disc perfusion (including surface nerve fiber layer, prelaminar tissue and lamina cribrosa) and peripapillary RNFL thickness. It is important to notice the alteration of peripapillary RNFL remained after 24 hours even peripapillary vessel density recovered. Repeated transient peripapillary RNFL edema may end up with RNFL damage, which is in accordance with the finding of Hu et al. who claimed that hemodialysis may increase the risk of development of glaucoma [2].
As for change of retinal thickness, all sections of perifovea and parafovea demonstrated a significant increase in retinal thickness at 1h after hemodialysis. This may be due to vasodilation of DCP and peripapillary RNFL edema in the same period.
Hemodialysis resulted in decrease peripapillary perfusion, which is probably due to increase in intraocular pressure, followed by transient edema of RNFL. Retinal microcirculation measured by OCTA is not influenced by neuronal autoregulation. Therefore, OCTA could be helpful in evaluating the hemodialysis as it reflects the alteration of microcirculation in hemodialysis patients.
Statement of ethics
This study was approved by the Research Ethics Committee of Guangdong Provincial People’s Hospital (registration number: gdrec2016232A). Patients are consented to participate in the study.
Disclosure statement
The authors have no conflicts of interest to declare.
Funding sources
The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This research was supported by the National Natural Science Foundation of China (Grant No.81670656 and NO. 81870508).
Author contributions
The coauthors made substantive contributions to the research and the manuscript. All authors have read and approved the content and agreed to submit it for consideration for publication in the journal.
[Crossref] [Google Scholar] [Pubmed]
[Crossref] [Google Scholar] [Pubmed]
[Crossref] [Google Scholar] [Pubmed]
[Crossref] [Google Scholar] [Pubmed]
[Crossref] [Google Scholar] [Pubmed]
[Crossref] [Google Scholar] [Pubmed]
[Crossref] [Google Scholar] [Pubmed]
[Crossref] [Google Scholar] [Pubmed]
[Crossref] [Google Scholar] [Pubmed]
[Crossref] [Google Scholar] [Pubmed]
[Crossref] [Google Scholar] [Pubmed]
[Crossref] [Google Scholar] [Pubmed]
[Crossref] [Google Scholar] [Pubmed]
[Crossref] [Google Scholar] [Pubmed]
[Google Scholar] [Pubmed]
[Google Scholar] [Pubmed]
[Google Scholar] [Pubmed]
[Crossref] [Google Scholar] [Pubmed]
[Crossref] [Google Scholar] [Pubmed]
[Crossref] [Google Scholar] [Pubmed]
[Crossref] [Google Scholar] [Pubmed]
[Crossref] [Google Scholar] [Pubmed]
[Crossref] [Google Scholar] [Pubmed]
Citation: Lin LH, Liu XS, Zhang QY, Lai XC, Xie XJ, Xie JW, et al. (2022) Alteration in Retinal Thickness and Perfusion after Haemodialysis Assessed by Optical Coherence Tomography Angiography. J Clin Exp Ophthalmol. 13:907
Received: 21-Dec-2021, Manuscript No. JCEO-21-15138; Editor assigned: 23-Dec-2021, Pre QC No. JCEO-21-15138 (PQ); Reviewed: 06-Jan-2022, QC No. JCEO-21-15138; Revised: 11-Jan-2022, Manuscript No. JCEO-21-15138 (R); Published: 18-Jan-2022 , DOI: 10.35248/2155-9570.22.13.907
Copyright: © 2022 Lin LH, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Sources of funding : The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This research was supported by the National Natural Science Foundation of China (Grant No.81670656 and NO. 81870508).