Journal of Chemical Engineering & Process Technology
Open Access
ISSN: 2157-7048
ISSN: 2157-7048
Review Article - (2024)Volume 15, Issue 2
As the world shifts away from fossil fuels and toward renewable energy, electrical energy is becoming more significant. Aluminum-Ion Batteries (AIBs) are highly appealing possibilities for electrochemical energy storage. While Lithium- Ion Batteries (LIBs) have long dominated the market due to their high energy density and durability, sustainability concerns arise from the environmental impact of raw material extraction and manufacturing processes, and performance-related drawbacks include a limited lifespan, safety hazards such as thermal runaway, and recycling challenges. AIBs excel in sustainability and theoretical capacity by utilizing trivalent aluminum ions (Al3+), which are abundant in the Earth's crust and have a widely accepted recycling infrastructure. Despite the benefits of AIBs in terms of sustainability and theoretical capacity, their widespread commercial application has been hampered by electrochemical limitations, such as difficulties in achieving competitive energy density and addressing issues related to the efficient cycling of trivalent aluminum ions. The study delves into the advantages of AIBs, examining their potential to outperform LIBs and become the premier battery technology of the future.
Aluminum; Battery; Energy; Lithium; Trivalent
How lithium and aluminum ion batteries work
Lithium-Ion Batteries (LIBs) dominate the battery market with their high energy density and long cyclability, which means they can withstand countless charge and discharge cycles while preserving capacity and performance, allowing for a more electrified world. Lithium-Ion Batteries (LIBs) dominate the battery market because to their high energy density and long cyclability, which means they can withstand several charge and discharge cycles while preserving capacity and performance, allowing for a more electrified world. However, when it comes to overall sustainability and performance, they fall well short of other electrochemical systems. Because of its natural abundance and trivalent nature, Aluminum-Ion Batteries (AIBs) exhibit intriguing properties that suggest they may outperform lithium-ion batteries in terms of sustainability and theoretical capacity.
It's important to comprehend how LIBs and AIBs work in order to compare them correctly. An aluminum anode, a cathode (often composed of graphite or similar materials), an electrolyte, a separator, and two current collectors make up a standard AIB. The reversible electrochemical reaction of aluminum with oxygen to create aluminum oxide is the basis for the operation of AIB batteries. Similar to the lithium ions in lithium-ion batteries, the aluminum in the anode performs the function of charge carrier. The positively charged aluminum ions go through the electrolyte and separator from the anode to the cathode. This migration process creates free electrons in the anode, culminating in a charge at the positive current collector. Aluminum at the anode undergoes electron loss during discharging, or oxidation, to produce aluminum ions. As seen in Figure 1a, the electrons go via the external circuit to perform useful tasks, while the ions migrate through an electrolyte to the cathode where they are received. The process is reversed during charging: Aluminum ions move from the cathode to the anode, where they pick up electrons to transform back into metallic aluminum [1,2].
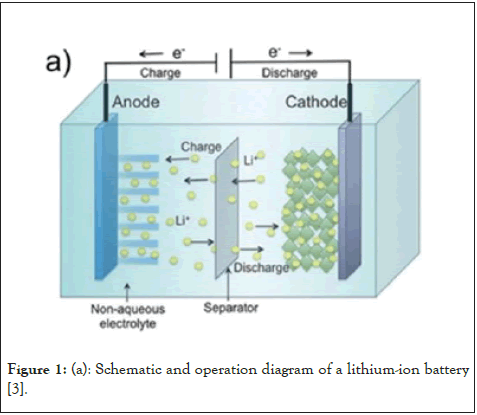
Figure 1: (a): Schematic and operation diagram of a lithium-ion battery [3].
As seen in Figure 1b, the flow of lithium ions between the anode and the cathode is the basis for how a Lithium-ion (Li-ion) battery operates. The most typical configuration uses an electrolyte of lithium salt between a metal oxide cathode and graphite anode. Lithium ions travel from the graphite anode via the electrolyte and intercalate into the metal oxide cathode during discharge. Concurrently, electrons discharged from the anode traverse the external circuit, supplying energy to devices, prior to reuniting with the lithium ions at the cathode. The charging process reverses this movement, with lithium ions deintercalating from the cathode and returning to the anode [3].
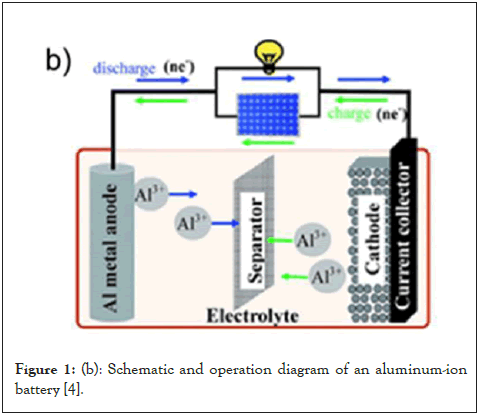
Figure 1: (b): Schematic and operation diagram of an aluminum-ion battery [4].
Unlike the monovalent lithium ions in LIBs, which have a+1 charge, trivalent aluminum ions, or AIBs, have a+3 charge. This difference in charge magnitude has a significant impact on the corresponding electrolytes' conductivity, ion mobility, and energy storage. Trivalent ions, due to their capacity to convey more charge per ion than monovalent ions, may exhibit elevated charge/discharge potentials [4]. Structurally, LIBs intercalate (insert between crystal layers) between anode and cathode materials without significant structural alterations, AIBs predominantly witness the plating of aluminum ions onto, or their stripping from, the metal anode, bypassing the typical intercalation [5]. Furthermore, on average aluminum costs $2.55 per kilogram while lithium costs $18.75 per kilogram [6]. The cost of Li is >7x higher than aluminum, making this cost difference compelling at large scale and because of this, news articles praise aluminum batteries as "dirt cheap" compared to li-ion batteries [7]. Lastly, while LIBs incorporate various metal oxide cathodes, AIBs frequently adopt carbon-based materials like graphite, underscoring the distinct material requirements and possibilities each battery system presents [4,5].
The efficiency with which chemical energy is converted to electrical energy through redox reactions is known as electrochemical operation, and this is a major consideration when selecting the best battery for a given application. However, other considerations like cost, safety, and sustainability should also be taken into account. Although the metals aluminum and lithium used in the batteries are both found naturally in the crust of the Earth, there are a number of factors that make one metal significantly more palatable to consumers and the environment than the other, such as the huge disparity in abundance. Furthermore, an extensive amount of research is being done right now to match aluminum ion battery electrochemical performance to the industry standard. In short, the advent of aluminum-ion batteries has the potential to completely reshape the financial aspects of energy storage. This invention could provide access to cutting-edge energy solutions and have an influence on entire industries in addition to consumers by providing performance comparable to lithium-ion batteries at a substantial cost savings. It can be easier to compare these two metals properly if the extraction technique and life cycles of each are examined.
Comparison of resource extraction
The quantity of lithium and aluminum in the Earth's crust is one of the primary causes of their high and low respective prices. After silicon and oxygen, aluminum is the third most plentiful element. Aluminum comprises around 8% of the Earth's crust by mass, while lithium metal barely makes up approximately 0.002% of the crust overall [8]. This significant discrepancy highlights how scarce lithium supplies are on Earth and provides more evidence that this is not a long-term fix. A more reliable and perhaps less expensive supply chain from miner to producer to customer could result from using a more plentiful material. Moreover, because lithiumion batteries have not undergone the same level of refinement over the centuries as aluminum mining, aluminum-ion batteries may be more affordable due to the well-established extraction procedures.
Other than abundance, mining metals takes a toll on our planet. Lithium extraction calls for a large amount of water, which could cause environmental problems in areas with limited water supplies. Liu W et al. claim that hard rock mining in places like Australia and brine extraction in places like the lithium triangle cause water stress, which has an effect on nearby ecosystems [9]. Estimates for water usage in hard rock mining suggest that it can range from about 1 to 2.5 cubic meters per ton of lithium produced. Brine extraction can use anywhere from approximately 500 to 5,000 cubic meters of water per ton of lithium produced, depending on factors like brine concentration, extraction efficiency, and local operational practice [9]. Moreover, the environmental benefits of aluminum battery adoption do not stop at mining. Moreover, adopting aluminum batteries has environmental advantages that extend beyond their mining. Lithium can only be recycled once, whereas aluminum metal can be recycled 50-70 times [10]. The money saved by mining a more plentiful metal can be used to finance recycling facilities that would otherwise dispose of used aluminum batteries.
Hubertus Bardt’s research states that aluminum is not categorized as vital when taking supply concerns, political ramifications, and the reserves-to-production ratio into account [11]. The different sources of aluminum are one of the possible explanation for this. Aluminum silicates rather than pure aluminum are the most common forms of the metal in nature. Aluminum must be mined from minerals like bauxite or recycled from scrap in order to generate the pure form needed for a variety of uses. A research work done by Ostojic et al. states that 4 kg of bauxite can provide 1 kg of pure aluminum [12]. Furthermore, raw material concentrations of aluminum are twice as high as those of lithium. This estimate can be used to calculate the amount of soil that has to be shifted in order to mine aluminum compared to mining the equivalent quantity of lithium. Moreover, aluminum is more efficiently used in batteries than lithium since one kilogram of raw material may yield more than twice as many aluminum atoms as lithium can.
Despite the fact that aluminum is widely distributed throughout the Earth's crust, the material's life cycle and overall sustainability are determined by the recycling process. Aluminum has long had a developed worldwide infrastructure, and each continent has its own resources for mining, producing, and recycling the metal [13,14]. Significant progress has been made in lowering the energy consumption of the aluminum production process by up to 95%, according to a 2003 study by Fathi Habashi. This indicates that, in contrast to lithium batteries, which supply 5% of the world's aluminum consumption, recycled aluminum accounts for 35% of it today [1,10].
The production and recycling processes used to make aluminum have an effect on the environment. About 1% of greenhouse gas emissions are attributable to the aluminum industry, which is divided into two categories. Of the total emissions, 40% come directly from the process of producing aluminum, and the remaining 60% come indirectly from the production of power [15]. A kilogram of raw aluminum has a carbon footprint of 5 to 40 kg CO2. As aluminum production increases, it is crucial to take renewable energy processes into account in order to offset these carbon emissions. The fact that most aluminum production facilities are positioned strategically near to hydroelectric power stations because of the high energy consumption is an example of ongoing efforts to offset [16].
A study on the extractive metallurgy of aluminum states that temperatures of approximately 1,000°C and energy inputs of 9-12 kWh are needed to produce 1 kg of aluminum, with process efficiencies ranging from 85%-95%. On the other hand, temperatures as high as 1,150°C [10] are required for the synthesis of lithium. The fused-salt electrolysis process is utilized to manufacture both metals, but aluminum requires a lot less electrical energy to produce than lithium does-especially when other parameters like each metal's gravimetric or volumetric capacity are taken into account. The manufacturing parameters and criteria discussed are compiled in Table 1.
| Parameter | Aluminum production | Lithium production |
|---|---|---|
| Energy source | Primarily hydro-electric power stations | Primarily non-renewable electricity sources |
| Production temperature | -1000°C | Up to 1150°C |
| Energy input per kilogram | 9-12 Wh | 12+ Wh |
| Process efficiency | 95% | 97% |
| Production method | Fused-salt electrolysis | Fused-salt electrolysis |
| Energy consumption comparison | Aluminum strategically located near hydro-electric sources and consumes significantly less electrical energy than lithium. | While specific figures vary, lithium extraction generally consumes more electrical energy than aluminum due to its higher production temperature and geographic location. |
| Additional considerations | Efforts to counterbalance energy demands, considering gravimetric or volumetric capacity of each metal. | Sustainable lithium production efforts focus on improving energy efficiency in high-temperature processes through advanced electrolysis methods, optimized reaction conditions, and innovative materials. |
Table 1: Comparative analysis of aluminum and lithium production processes for battery manufacturing. Highlighting energy sources, production temperatures, energy input, process efficiencies, and additional considerations for sustainable production [10].
Electrochemical disadvantages of aluminum
The primary obstacle to the widespread use of aluminum-ion batteries in substitution of lithium-ion batteries, despite the fact that aluminum is a significantly more sustainable metal than lithium, is their superior electrochemical performance. Before AIBs to completely replace LIBs and gain long-term adoption, they must overcome a few obstacles. Energy density and voltage are important factors to take into account. The electrochemical potential difference between a battery's anode and cathode is the source of the battery's potential difference. The typical AIB design is notably less voltage-sensitive than the LIB equivalent, which results in a lower energy density-an important measure for applications such as electric vehicles, where balancing weight and range is critical. Aluminum is a trivalent metal, meaning that when it ionizes, it releases three electrons, making it more difficult for the ion to intercalate into cathode materials [1]. As opposed to this, LIBs make use of a monovalent lithium ion and carefully studied and refined cathode materials such as lithium iron phosphate or lithium cobalt oxide. Finding an appropriate electrolyte that can effectively manage Al3+ ions while maintaining stability throughout the battery's operating voltage is a formidable task. An additional constraint pertains to the AIB discharge rate. As a result of the intrinsic difficulties in moving trivalent Al3+ ions as opposed to monovalent Li+ ions, AIBs are still unable to attain the high rates of energy release observed in LIBs, which makes them less appropriate for high-demand applications. Furthermore, AIBs' cycle life and long-term stability are inferior to LIBs', particularly in situations where usage and temperature fluctuate [17].
The drive for Al-ion innovation is not without justification, nevertheless, as it offers advantages in terms of sustainability and safety in addition to specific electrochemical performance measures. Furthermore, safer energy storage options are made possible by the Al-ion's resistance to damage and decreased possibility of dangers like thermal runaway. Al-ion batteries are less likely to develop dendrites, which are undesired growths that can result in battery short circuits. Furthermore, because of aluminum's higher thermal conductivity, heat can be dissipated more effectively, lowering the possibility of overheating and thermal runaway-a major safety problem in battery technology. By reducing the possibility of chemical reactions that could jeopardize the integrity of the battery, Al-ion batteries' strong compatibility with stable electrolytes further improves their safety. Furthermore, the general toughness and resilience of these batteries are enhanced by the inherent stability of aluminum as a material. All things considered, even though Al-ion batteries presently lag behind Li-ion in some performance areas, their inherent advantages and potential to overcome existing obstacles make them a strong contender to displace Li-ion batteries.
Improvements to electrochemical performance
Scientists are aware of the aspects of AIBs that require improvement. Specifically, scientists have developed the “Molten AlCl3/urea electrolyte” AIB, the “Super long-life CMK-3” AIB, the “High coulombic efficiency aluminum-graphite” AIB, and the “Graphene film 3H3C ultrafast quarter-million life cycle” AIB [18-21]. As shown in Figure 2, the performance of general AIBs is improved to meet or even surpass current state-of-the-art LIBs by the following four proprietary AIB systems, each of which has a distinct feature.
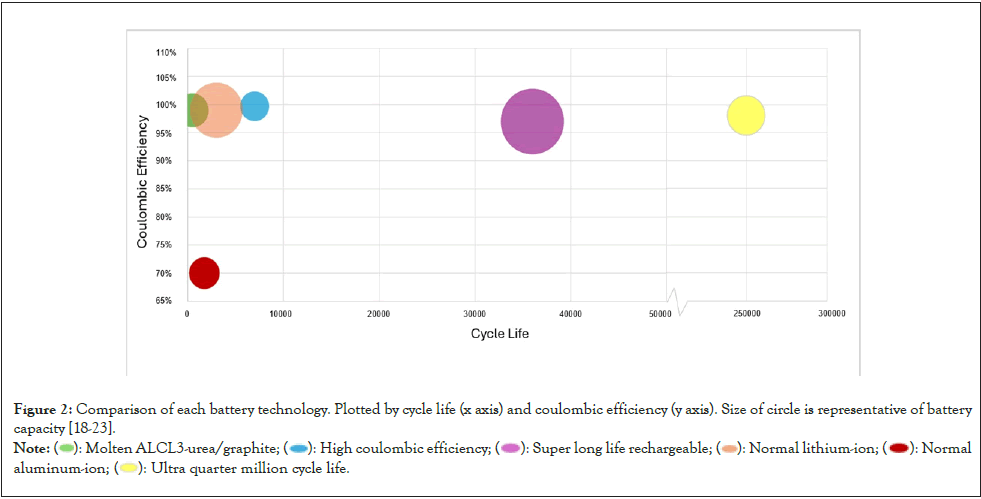
Figure 2: Comparison of each battery technology. Plotted by cycle life (x axis) and coulombic efficiency (y axis). Size of circle is representative of battery
capacity [18-23].
Note: ( ): Molten ALCL3-urea/graphite; (
): Molten ALCL3-urea/graphite; ( ): High coulombic efficiency; (
): High coulombic efficiency; ( ): Super long life rechargeable; (
): Super long life rechargeable; ( ): Normal lithium-ion; (
): Normal lithium-ion; ( ): Normal aluminum-ion; (
): Normal aluminum-ion; ( ): Ultra quarter million cycle life.
): Ultra quarter million cycle life.
Cycle life and discharge rate are the two primary electrochemical performance factors of Al-ion batteries that need to be enhanced. A recently proposed innovation aimed at enhancing cyclability is a graphite battery made of molten aluminum chloride and urea. Using a special electrolyte composed of melted AlCl3/urea, this battery enhances the mechanism of aluminum ions migrating between their anode and cathode during the charging and discharging phases. Trivalent aluminum ions (Al3+) are released during the oxidation process of the aluminum anode during charging. These ions mix with chloride ions to generate AlCl4- ions as they travel through the electrolyte to the graphite cathode. These ions then insert themselves between the graphite layers of the cathode. When the discharge occurs, the AlCl4-ions leave the graphite layers and release an aluminum ion as they get closer to the aluminum anode. After assimilating three electrons, this ion returns to its metallic state. At an operating temperature of roughly 120°C, the performance gain is attained with a specific AlCl3 to urea ratio of 1.5 in the electrolyte, which permits the cationic species AlCl2(urea)+ to move with fewer losses. One possible downside of this development is that high temperature batteries can require an additional power source, which presents management issues. These parameters prevent unwanted side effects in addition to ensuring effective ionic transport. The effectiveness of the battery system depends on these conditions, thus it's important to apply the proper safety precautions and thermal management techniques, even though this elevated temperature can raise questions about the safety of battery operation. The battery shows that it can store a significant amount of energy in relation to its weight by boasting a high specific capacity. This, along with its remarkable endurance and rate capability (notable capacity retention even after 500 cycles), makes it a viable option for large-scale energy storage, particularly when compared to more expensive electrolyte-based classical Al-ion batteries [18].
A potential replacement for conventional lithium-ion batteries is the "Super long life aluminum battery," which is intended to increase the cyclability of aluminum-ion batteries even further. The volumetric capacity of this battery can reach up to 8046 mAh cm^−3. This measurement shows how much energy these batteries can hold in a small amount of space, which makes them perfect for small electronic gadgets. Additionally, large levels of cyclability are achieved by using CMK-3 (mesoporous carbon), an ordered mesoporous carbon, as an effective and commercially available cathode. This chemistry exhibits over 36,000 charge/discharge cycles with minimal degradation. One of the main features of CMK-3 is its architecture; the large surface area and well-organized pores facilitate quick ion flow, which improves the battery's rate capacity. Al-ion intercalation on the anode side is possible with CMK-3, which is comparable to the chemistry of molten aluminum chloride. have validated this process, exposing the ways in which these anions interact with the structure of CMK-3 to guarantee reliable and steady battery performance. With an energy density close to 45 Wh kg^−1, the Al/CMK-3 battery packs a powerful punch and is competitive with numerous widely used battery technologies. Better yet, this Al-ion battery has a high safety profile, in contrast to their lithium-ion counterparts, which are infamous for safety problems, including fire dangers [19].
Coulombic efficiency and discharge rate go hand in hand. For this reason, the development of the high CE aluminum-ion battery is a viable solution to the Al-ion batteries' discharge rate issue. The unique electrolyte used in this battery technology is a mixture of urea and AlCl3 with a 1.3:1 molar ratio. Graphite serves as the cathode and aluminum serves as the anode in this battery. The battery shows clear voltage plateaus electrochemically between 1.9 V and 1.5 V, with an average discharge of 1.73 V. These distinct voltage plateaus simplify battery management and provide more steady performance during operation by providing constant and predictable energy supply stages. This battery stands out for having an exceptional CE of about 99.7% and a respectable cathode capacity of roughly 73 mAh g-1 at a current density of 100 mA g−1. This aluminum-ion prototype has a number of benefits over LIBs. Moreover, in-situ Raman spectroscopy clarified the intercalation and deintercalation of the chloroaluminate anion in the graphite throughout the charge and discharge cycles of the battery [17]. This suggests that, when completely charged, a stage 2 graphite intercalation compound will occur. Crucially, this battery solves one of the main issues with LIBs by providing an enhanced safety profile due to its nonflammable electrolyte. These qualities make aluminum-ion battery technology a viable option for future highperformance, reasonably priced energy storage requirements [20].
Historically, aluminum-ion batteries have proven problematic due to their lower voltage when compared to lithium-ion batteries. Nonetheless, the difficulties associated with lower voltage might be somewhat mitigated by concentrating on increasing the discharge rate. The aluminum-graphene ultrafast all-climate battery is a possible fix for this issue. Graphene sheet cathode with "trihigh tricontinuous (3H3C) design" is the central component of the design. The flow of ions between the anode and the cathode is essential to the electrochemical process. Because of its continuous electronconducting matrix, ion-diffusion highway, and electroactive mass (3C), the graphene cathode, orientation, and channeling (3H) in this battery allows for effective ion movement. The battery may discharge at significantly greater current rates by taking use of this effective ion transport, making up for its lower voltage by providing the necessary power in a shorter amount of time. Because of its design, the battery may maintain its outstanding retention even after a quarter-million cycles and reach a high specific capacity of about 120 mAh g−1.
This aluminum-graphene battery has several advantages over lithium-ion batteries. Its high capacity is attributed to the threeelectron redox feature of the aluminum anode; safety is improved by the materials' non-flammability; and its unique graphene structure allows for fast charging and stable cycling. Moreover, the battery exhibits exceptional flexibility and operates effectively in a broad temperature range of -40°C to 120°C, both of which are critical characteristics for wearable technology that is suitable for all climates. The remarkable rate capability and cycle life of the battery are made possible by the high crystalline nature of graphene and the linked channels that are designed to promote rapid ion diffusion. The aluminum-graphene battery is a formidable rival for the lithium-ion battery because it can efficiently solve the restrictions associated with lower voltage by deliberately boosting the discharge rate [21,24].
Applications
Electrochemical energy storage has been made universally possible with the usage of lithium-ion batteries. Nonetheless, many Alion battery chemistries have certain benefits that might be appropriate for particular uses. Table 2 presents important metrics that compare Al-ion batteries to their lithium-ion counterparts, providing information on how well the former performs in terms of important parameters for particular applications Electric Vehicles (EVs), Portable Devices (PDs), and Energy Storage Systems (ESS) are the top three applications for rechargeable batteries. Each of these applications has specific needs, and Al-ion batteries can adapt their strengths to meet these needs, which makes them a compelling competitor in the rapidly changing field of electrochemical energy storage solutions [24]. As technology develops, a more sophisticated approach to choosing the best power source for a given application is encouraged by the wide range of battery options, which promotes sustainability, safety, and efficiency.
| Energy density? | 270 Wh/kg | 15 Wh/kg | 250 Wh/kg |
|---|---|---|---|
| Life cycle (times) | >3000 (10 yrs) | >3000 (10 yrs) | >3000 (10 yrs) |
| Efficiency (%) | >95% | >95% | >95% |
| Discharging C-rate | 9 hours (C/9) | Portable Devices: 18 hours (C/18) | ESSs: 12 hours (C/12) |
| Charging C-rate | 10min-8 hours | <1 hour | 12 hours |
| Operating temperature © | -20 °C-50 °C | 0 °C-35 °C | 20 °C-30 °C |
| Battery cost | $137 per kWh | $50-$200 | $10,000 |
Table 2: Consists of the current electrochemical performance metrics required by a battery for electric vehicles, personal devices, and energy storage systems [25-28].
Performance and safety metrics combine to make the rechargeable aluminum batteries with CMK-3 Cathode the best option for Energy Storage Systems (ESS). These batteries are perfect for large-scale storage systems that frequently experience charge and discharge cycles because to their exceptional cycle life, which reaches over 36,000 charge/discharge cycles. This assures that these batteries can give sustained performance for years. Together with its inherent high safety profile, this endurance helps to lower the inherent risks, such as fire threats, that come with many traditional battery systems. This is especially important for installations close to inhabited areas.
Performance and safety metrics combine to make the rechargeable aluminum batteries with CMK-3 Cathode the best option for Energy Storage Systems (ESS). These batteries are perfect for large-scale storage systems that frequently experience charge and discharge cycles because to their exceptional cycle life, which reaches over 36,000 charge/discharge cycles. This assures that these batteries can give sustained performance for years. Together with its inherent high safety profile, this endurance helps to lower the inherent risks, such as fire threats, that come with many traditional battery systems. This is especially important for installations close to inhabited areas.
One of the most important requirements for Electric Vehicles (EVs) is a high-performing and dependable battery. The cutting- edge aluminum-graphene battery, renowned for its remarkable rate capabilities and safety profile, would be the best option for EVs. Because of its high specific capacity, which is around 120 mAh g-¹, EVs are guaranteed to travel long distances on a single charge. Longevity is further ensured by its strong cycle life, which exhibits minimal deterioration even after a quarter of a million cycles. This makes it ideal for the demanding use typical of EVs.
Because it is made of non-flammable materials, it is safer and addresses fire threats, which is a major worry with high-capacity batteries. Moreover, its capacity to function effectively in a broad temperature range of -40°C to 120°C guarantees that it can accommodate the many situations that electric vehicles may face. When taking into account the specific needs of Electric Vehicles (EVs) and the necessity for quick charging, the aluminum-graphene battery appears to be a viable option for the future of electric transportation [21].
When it comes to Portable Devices (PDs), battery technology that is safe, long-lasting, and lightweight is essential. For these applications, the aluminum-ion battery with high CE is a particularly good option. Even though they might not need to have the same energy density as Electric Vehicles (EVs), portable electronics nevertheless need to be effective and have a long battery life. With a high CE of almost 99.7%, this battery guarantees that a large percentage of the energy is used efficiently, allowing for longer gadget usage furthermore, PDs can operate for lengthy periods of time without frequent recharging thanks to their remarkable cathode capacity of roughly 73 mAh g⁻¹ at a current density of 100 mA g⁻¹. Safety problems are addressed by the non-flammable electrolyte; They are especially important for devices that people carry around on a regular basis. All things considered, the aluminum-ion battery with high CE is a versatile option for supplying energy to the upcoming generation of portable electronics [20].
The comparison between aluminum-ion batteries and lithium- ion batteries highlights an interesting relationship of advantages and challenges. AIBs, powered by trivalent aluminum ions and sustainable sourcing, hold immense promise for transforming the landscape of electrochemical energy storage. These batteries address critical environmental concerns and resource limitations associated with LIBs. The diverse array of AIB chemistries-from molten aluminum chloride-urea graphite batteries to aluminum- graphene variants-demonstrates ongoing innovation to overcome electrochemical limitations.
While LIBs have long reigned supreme due to their high energy density and proven reliability, the scarcity of lithium and its environmentally impactful extraction processes necessitate alternative solutions.
AIBs, abundant in the Earth’s crust and reinforced by robust recycling infrastructure, present a compelling case for sustainability. Their safety advantages, including resilience against damage and reduced risk of hazards like thermal runaway, further enhance their appeal as safer energy storage options.
Looking ahead, the future of battery technology lies in the hands of AIBs. As research continues, we anticipate breakthroughs in cycle life, discharge rates, and overall performance. Innovations in materials, manufacturing, and design will shape the next generation of energy storage devices. Perhaps we’ll witness AIB- powered electric vehicles dominating the roads, grid-scale AIB installations revolutionizing energy distribution, and portable AIB- based gadgets seamlessly integrating into our lives. Imagine a world where aluminum-ion batteries power our devices, homes, and cities. The journey has just begun, and the outlook is electrifying.
[Crossref] [Google scholar] [Pubmed]
[Crossref] [Google scholar] [Pubmed]
[Crossref] [Google scholar] [Pubmed]
[Crossref] [Google scholar] [Pubmed]
[Crossref] [Google scholar] [Pubmed]
Citation: Arora AS (2024) Aluminum: The Future of Battery Technology. J Chem Eng Process Technol. 15:498.
Received: 30-Apr-2024, Manuscript No. JCEPT-24-31011; Editor assigned: 03-May-2024, Pre QC No. JCEPT-24-31011 (PQ); Reviewed: 23-May-2024, QC No. JCEPT-24-31011; Revised: 30-May-2024, Manuscript No. JCEPT-24-31011 (R); Published: 06-Jun-2024 , DOI: 10.35248/2157-7048.24.15.498
Copyright: © 2024 Arora AS. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.