
Biochemistry & Pharmacology: Open Access
Open Access
ISSN: 2167-0501

ISSN: 2167-0501
Research Article - (2017) Volume 6, Issue 3
The study investigated the protective effect of the flavonoid rich fraction (FRF) of M. myristica on carbon tetrachloride induced hepatotoxicity and oxidative stress in experimental animals. Wistar albino rats of both sexes were maintained on 1.0 ml/kg b.wt of carbon tetrachloride to induce hepatotoxicity and oxidative stress. Added to this, the animals received FRF at a dose of 250 or 500 and 100 mg/kg b.wt of silymarin for 8 days. At the end of the experiment, levels of serum hepatic enzyme biomarkers (SGOT, SGPT and SALP) as well as lipid peroxidation product (MDA) and total bilirubin were significantly raised in the CCl4 treated groups. Conversely, CCl4 administration elicited obvious decline in enzymatic antioxidants activities and non-enzymatic antioxidants (GSH, CAT, SOD, vitamin C, vitamin E) co-administration with FRF of M. myristica at varying doses as well as silymarin substantially decreased the elevated levels of the serum hepatic enzyme biomarkers as well as MDA in the CCl4 treated groups in a dose dependent manner. Additionally, FRF of M. myristica exhibited reversal potential on CCl4 toxicity at the tested doses as its administration resulted to a pronounced increase in GSH, SOD and CAT levels. Histopathological results demonstrate that FRF of M. myristica is effectual in the amelioration of hepatic damage and oxidative stress arising from CCl4 induced hepatotoxicity. These results establish the effectiveness of the flavonoids of M. Myristica in the amelioration of hepatic damages arising from CCl4-induced toxicity which is manifest by the bioaccumulation of free radicals in our animal models.
<Keywords: Flavonoids; Monodora myristica; Hepatotoxicity; Oxidative stress; Rats
SGOT: Serum Glutamate Oxaloacetate Transaminase; SGPT: Serum Glutamate Pyruvate Transaminase; SALP: Serum Alkaline Phosphatase; MDA: Malondialdehyde; SOD: Superoxide Dismutase; CAT: Catalase; GSH: Reduced Glutathione
When there is unevenness between reactive oxygen species and cellular antioxidant defences, oxidative stress results. Reactive oxygen species have been implicated in the study of the causes of many disease conditions including cardiovascular diseases, neurodegenerative disorders, autoimmune disorders, rheumatoid arthritis, cataract, cancer and aging [1-3]. It has been reported that some of the major sources of free radicals in vivo include autoxidation of flavin thiols, activity of electron transport chain, oxidases, cyclooxygenases and peroxidases [4]. Environmental sources of oxidative stress include xenobiotics, organic solvents, pesticides, tobacco smoke, anaesthetics, drugs and radiation [5]. Crude extracts of some medicinal plants, their fractions and bioactive compounds are known to play essential role in detoxification of such toxins and mop up free radicals [6,7].
Flavonoids represent an astonishing group of plant secondary metabolites and have long been used as traditional medicine with scientifically established pharmacological benefits. Flavonoids are found abundantly among the plant kingdom and constitute an essential portion of our daily diet such as vegetables, fruits, nuts, seeds, stem, flowers, tea and wine [8]. They serve enormous ranging medicinal activities that may lead to drug discovery with new and conceivable therapeutic proof. Principally, antioxidants act by interrupting, averting or eliminating oxidative damage to a target molecule. Flavonoids are known to act by quenching free radical elements, chelating key metal, suppressing the enzymes associated with free radical generation and stimulation of internal antioxidant enzymes [9].
Monodora myristica (African nutmeg) belongs to the family of flowering plants called Annonaceae [10]. The plant grows well in African evergreen forests where it is widely used as seasoning for different delicacies. In traditional system of medicine, it is used to cure sores from guinea worm infections, stomach-ache, headache, constipation as well as to stop intra-uterine bleeding in women after child birth [11-14]. Additionally, the root is chewed to alleviate toothaches and arthritis and is also utilized in the management of anaemia, haemorrhoids as well as sexual weakness [15]. Previous studies have reported the in vitro antioxidant properties of the different extracts of M. myristica seeds [10,15,16]. The seeds have also been investigated to possess cholesterol lowering activity [17], antisickling activity [18], antimicrobial activity [19] as well as anthelmintic activity [20]. Akinwunmi and Oyedapo [10] reported that M. myristica is a good source of Phenolics, suggesting that its inclusion in human diet could contribute to potential health benefit. Feyisayo and Oluokun [21] in their report estimated that the total Phenolic content of seeds of M. myristica as 1478.32 mg/100 g and used GC-FID (Gas Chromatography-Flame Ionization Detector) analysis to identify about fifty three different types of Phenolics (Table 1). Phytochemical studies of M. myristica seeds showed that it is rich in alkaloids, glycosides, flavonoids, tannins, saponin and steroids [13,22,23]. The Flavonoid rich fraction of M. myristica has also been reported to possess in vitro anti-inflammatory potentials [10] and in vitro antioxidant activity [21]. Akinwunmi et al. [24] in their work on the acute and sub-lethal toxicological evaluation of flavonoid fraction of M. myristica seeds concluded that the spice is relatively safe for oral consumption.
| Pk | Retention time | Area (pA*s) | Amount (mg/100g) | Names |
|---|---|---|---|---|
| 1 | 3.63 | 207.97 | 72.21 | Catechin |
| 2 | 6.78 | 18.53 | 1.48e-4 | Phenol |
| 3 | 7.35 | 9.52 | 7.59e-5 | Phenylacetic acid |
| 4 | 7.65 | 21.18 | 1.69e-4 | Salicylic acid |
| 5 | 7.96 | 8.61 | 6.86 | Myrcene |
| 6 | 8.78 | 4.00 | 3.19e-5 | Cinnamic acid |
| 7 | 9.69 | 48.59 | 13.21 | Protocatechuic acid |
| 8 | 10.01 | 1.91 | 1.28e-2 | Carvacrol |
| 9 | 10.11 | 2.97 | 2.36e-5 | Gentisic acid |
| 10 | 10.79 | 1.64 | 1.98e-2 | ρ-coumaric acid |
| 11 | 11.17 | 3.38 | 4.00e-2 | Vanilic acid |
| 12 | 11.37 | 101.88 | 165.39 | Safrole |
| 13 | 11.70 | 3.75 | 15.08 | Eugenol |
| 14 | 11.96 | 4.11 | 3.27e-5 | Isoeuenol |
| 15 | 12.32 | 4.28 | 79.76 | Methyleugenol |
| 16 | 12.69 | 3.44 | 20.49 | Methyl isoeugenol |
| 17 | 13.21 | 10.02 | 7.98e-5 | Gallic acid |
| 18 | 13.69 | 23.50 | 56.50 | Elemicin |
| 19 | 13.78 | 5.24 | 629.72 | Myristicin |
| 20 | 14.16 | 42.60 | 345.79 | Caffeic acid |
| 21 | 14.94 | 15.48 | 5.69e-2 | Ferulic acid |
| 22 | 15.39 | 35.18 | 2.80e-4 | Syringic aci |
| 23 | 15.50 | 4.87 | 7.87e-3 | Piperic acid |
| 24 | 16.14 | 16.35 | 1.12e-2 | Sinapinic acid |
| 25 | 16.55 | 24.36 | 9.82e-3 | Daldzein |
| 26 | 17.52 | 4.06 | 1.10e-3 | Coumestrol |
| 27 | 18.39 | 3.33 | 3.25e-3 | Genistein |
| 28 | 18.77 | 5.36 | 1.10e-2 | Apigenin |
| 29 | 19.00 | 6.35 | 5.06e-5 | Naringenin chalcome |
| 30 | 19.31 | 3.77 | 3.77e-5 | Naringenin |
| 31 | 19.67 | 9.39 | 2.88e-2 | Shogaol |
| 32 | 20.61 | 2.34 | 1.92e-3 | Gycitein |
| 33 | 21.52 | 3.40 | 32.95 | Kaempferol |
| 34 | 21.83 | 1.03 | 1.11e-3 | Luteolin |
| 35 | 22.39 | 1.92 | 2.98e-3 | Capsaicin |
| 36 | 22.70 | 2.06 | 1.64e-5 | Epicatechin |
| 37 | 23.22 | 3.37 | 2.92e-5 | Epigallocatechin |
| 38 | 23.34 | 9.30e-1 | 9.01e-4 | Gingerol |
| 39 | 24.14 | 9.74e-1 | 9.44e-3 | Myricetin |
| 40 | 24.50 | 8.44e-1 | 2.50e-1 | Isorhamnetin |
| 41 | 25.05 | 49.42 | 40.11 | Quecertin |
| 42 | 25.26 | 3.63 | 2.96e-4 | 3-o-caffeoylquinic |
| 43 | 25.53 | 3.90 | 6.25e-3 | Chlorogenic acid |
| 44 | 26.24 | 4.46 | 7.01e-3 | Rosmarinic acid |
| 45 | 26.93 | 3.49 | 2.83e-3 | Curcumin |
| 46 | 27.20 | 1.72 | 2.76e-3 | Miqueltanin |
| 47 | 27.48 | 9.09e-1 | 1.44e-3 | Eriocitrin |
| 48 | 28.25 | 2.48 | 4.02e-3 | Rutin |
| 49 | 29.00 | 5.27 | 4.29e-6 | Papain |
| 50 | 29.31 | 4.08 | 3.25e-5 | Phenyl-6-o-maonyl-beta-D-glucoside |
| 51 | 29.48 | 9.21 | 7.33e-5 | 4-o-methyl-epl-gallocatechin |
| 52 | 30.06 | 48.07 | 3.83e-4 | Epl-gallocatechin-3-o-gallate |
| 53 | 30.26 | 19.03 | 1.52e-4 | Lupeol |
Table 1: Phenolic compounds identified in Monodora myristica seed by GC-FID (Source: Feyisayo and Oluokun [21]).
Despite the weighty medicinal benefits possessed by M. myristica, there is paucity of information on the effect of flavonoid fraction of the spice on hepatic toxicity and carbon tetrachloride induced oxidative stress in experimental animals. The study was therefore designed to investigate the defensive effect of M. myristica flavonoid fraction on carbon tetrachloride induced hepatic toxicity and oxidative stress in experimental animals.
Ethics statement
All experimental animals will be handled in accordance with the study procedures approved by the animal ethics committee of the Department of Biochemistry, Faculty of Science, Imo State University Owerri.
Plant materials
For the investigations, botanically authenticated sample (seeds of Monodora myristica (Gaertn) Dunel) was locally procured and shade dried as previously indicated [13]. The seeds were decocted to release the kernels which were later milled to fine powder with blending machine.
Drugs and chemicals
Silymarin (Micro labs Limited, India) was used as the standard hepatoprotective drug. All chemicals were of analytical grade and were purchased from Sigma Chemical Company (Taufkirchen, Germany).
Preparation of the flavonoid rich fraction (FRF)
The flavonoid rich fraction (FRF) of the pulverized air-dried plant material was obtained by solvent-solvent extraction as previously reported by Ekeanyanwu and Njoku [25] with slight modification. Briefly, 1 kg of the ground plant material was macerated with 1.3 litres of 70% ethanol for 72 hours at room temperature. The macerate was passed through Whatman No.1 filter paper and the solvent removed by rotatory evaporator under reduced pressure. The flavonoid rich fraction was obtained by liquid-liquid partition of the extract. Thirty grams of the extract was dissolved in methanol: water proportion (9:1) and the liquid-liquid extraction was carried out using n-hexane (500 ml) as solvent extractor. After, to obtain the FRF, the aqueous residue was partitioned by liquid-liquid extraction process with ethyl acetate. The ethyl acetate fraction was subsequently concentrated and utilized as the FRF. The total flavonoid content of the fractional extracts and standard was estimated using the method of Chang et al. [26]. A weighed quantity of the fractional extract (0.5 g) was dissolved with 1.5 ml of 95% ethanol, 0.1 ml of 10% Aluminium chloride hexahydrate, 0.1 ml of 1 M potassium acetate and 2.8 ml of Bidest water. Forty minutes after incubation at temperature 250°C, the absorbance of the reaction mixture was measured at 415 nm against a blank. Based on the measured absorbance, the concentration of flavonoids was read (μg/ml) on the calibration curve; then concentrations of flavonoids in fractional extract was presented in terms of rutin equivalent (mg of RU/g of extract) as follows.
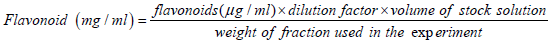
The flavonoid rich ethyl acetate fraction which has a total flavonoid content of 63.66 ± 1.02 mg/g RU/g was dissolved in 1% Tween 20 as a neutral solvent to make final concentration for biochemical analysis and antioxidant assay.
Animals
Thirty six (30) Male and Female Wister albino rats weighing 120 g-150 g were used for this study. They were acquired from the animal house of the Department of Biochemistry, Federal University of Technology Owerri, Nigeria. The animals were fed with standard pellet feed and water Ad libitum and were acclimatised to laboratory conditions for 1 week before experiment: temperature (200°C-250°C), relative humidity (40%-60%) and a light/dark cycle of 12 hours. All procedures were carried out in accordance with the National Institute of Health Care Guide for the Care and Use of Laboratory Animals and as approved by the Ethics committee of the National Institute of Council (US). Various efforts were made by the researchers to lessen animals suffering and to trim down the number of animals used in the experiment.
Experimental protocol
A total number of 30 Wistar rats (male and female) were distributed randomly into six groups of five rats per group based on similarity of their weight. The experiment lasted for a period of seven days during which;
1. Group I (negative control group) received a single dose of olive oil (1 ml/kg, p.o) daily for 7 days.
2. Group II (CCl4 control group) received a single dose of CCl4/olive oil (1:1 v/v, 1.0 ml/kg b.wt, p.o), the dose at which hepatotoxicity occurs [27] on alternate days.
3. Group III (standard control group) received 100 mg/kg of silymarin and a single dose of CCl4/olive oil (1:1 v/v, 1.0 ml/kg b.wt, p.o) on alternate days.
4. Group IV (extract control group) received a single dose of the FRF (500 mg/kg, p.o) on alternate days.
5. Group V (prophylactic treatment group) received a single dose of the FRF (500 mg/kg, p.o) followed by a single dose of CCl4/ olive oil (1:1 v/v, 1.0 ml/kg b.w, p.o) on alternate days.
6. Group VI (prophylactic treatment group) received a single dose of the FRF (250 mg/kg, p.o) followed by a single dose of CCl4/olive oil (1:1 v/v, 1.0 ml/kg b.w, p.o) on alternate days.
The animals were singly housed under laboratory conditions, maintained on natural light and dark cycle and will have free access to food and water. A standard hepatoprotective antioxidant (silymarin) was administered at 100 mg/kg body weight on every other day. The fraction and silymarin were administered p.o. (oral gavage) for 8 days. After the treatment for 8 days, weights of animals in grams were taken at the start of the experiment, Day (0) and on the final day of the experiment (Day 8). Blood was collected from the animals on the 8th day by orbital technique and the blood sample was kept at room temperature for 30 minutes to clot. Afterwards, the test tubes containing the clotted blood sample was centrifuged at 3000 rpm for 10 minutes using a table centrifuge to enable a complete separation of the serum from clotted blood. The clear serum supernatant was then carefully aspirated with syringe and needle and stored in a clean sample bottle for biochemical tests
Biochemical analysis
Estimation of SGOT and SGPT activities for cellular liver integrity were estimated by the method described by Reitman and Frankel [28] with the Randox reagent kit using 2, 4-dinitrophenylhydrazine substrate. ALP activity for biliary tract integrity was determined with the Randox reagent kit using the p-nitrophenylphosphate substrate as described by Bessay et al. [29]. The quantitative assay method of small amount of protein in biological membrane as described by Schacterk and Pollack [30] with bovine albumin as standard was used for the stimation of total protein concentrations in our sample. Albumin concentration was estimated by modified procedure of Pinnell and Northam [31] using bromocresol green (BCG).
Antioxidant assay
The antioxidant enzymes activity was determined spectrophotometrically as follows: Superoxide dismutase activity was determined by its ability to inhibit the phenazine methosulphatemediated reduction of Nitroblue tetrazolium dye determined in absorbance at 560 nm as described by Mishra and Fridovich [32]. The volume of the sample required to scavenge superoxide anion was expressed in U/mg protein (1 unit of enzyme activity). Serum catalase activity was assayed according to methods previously described [33] by monitoring the decomposition of H2O2 in ultra violet (UV) measured by decrease in absorbance at 240 nm in a spectrophotometer and the activity of the catalase (in Units/mg protein) was calculated using the μmole extinction coefficient of 40 cm-1. The modified method of Ellman [34] using the Ellman’s reagent was used for the estimation of reduced glutathione (GSH) content in our sample and the results presented in Units/mg protein. The method of Niehius and Samuelson [35] was used to measure the extent of lipid peroxidation (Malondialdehyde content) in the form of thiobarbituric acid-reactive substances and the results were presented as mmol of MDA/mg protein. The serum level of vitamin C in our samples was assessed by the 2, 4 dinitro phenylhydrazine method [36]. The micro-method [37] was used for analysing serum levels of vitamin E.
Histopathological analysis
Histological study was carried out (Figure 1) after a midline incision (laparotomy) to remove the liver [38]. The liver tissues were immediately fixed in 10% buffered formalin and subsequently embedded in paraffin. Liver sections were processed for routine histological staining applying the Haematoxylin and Eosin (H and E) technique for the general hepatic architecture, Gordon and Sweet (G and S) stain for reticular fibres using standard techniques and sections were viewed under the light microscope. Photomicrographs were obtained using a digital microscope (Leitz-Witzlar) with its monitor.
Figure 1: Histological examination of rat livers stained with haematoxylin and eosin (H and E × 100). G1: Showing no remarkable histologic change; G2: Showing mild vacuolation and necrosis of hepatocytes (arrows); G3: Showing no remarkable histologic change; G4: Showing no remarkable histologic change; G5: Showing no remarkable histologic change; G6: Showing no remarkable histologic change. G1: Control; G2: CCl4; G3: CCl4+silymarin; G4: FRF; G5: CCl4+500mg/kg b.wt. FRF; G6: CCl4+250mg/kg b.wt FRF; CV: Cytoplasmic Vacuolation; H: Hepatocytes; BD: Bile Ducts.
Statistical analysis
The results were expressed as Mean ± SD and tests of statistical significance were carried out using one way analysis of variance (ANOVA). The Statistical Package for the Social Sciences (SPSS) version 20.0 was used. P values<0.05 was considered significant.
Results
Effect of the FRF of M. myristica on serum liver marker enzymes and biochemical parameters: In the present study, a significant elevation in the levels of serum marker enzymes like SGOT, SGPT and SALP content of CCl4 treated group (group II) was observed. In contrast, pre-treatment with the FRF of M. myristica (250 mg/kg b.wt. and 500 mg/kg b.wt.) as well as silymarin (100 mg/kg b.wt.) revealed an ability to neutralize the hepatotoxicity by decreasing serum marker enzymes in a dose dependent manner (p<0.05), (Table 1). A significant increase in the total bilirubin and significant reduction in total protein and serum albumin was observed in the CCl4 treated group (group II). Whereas, pre-treatment with FRF of M. myristica (250 mg/kg b.wt. and 500 mg/kg b.wt.) as well as silymarin (100 mg/kg b.wt.) resulted in a significant reduction in total bilirubin and significant increase in activities of total protein content and albumin in a dose dependent manner (Table 2).
| Parameters | Group I Negative control (Olive oil) |
Group II CCl4 control |
Group III CCl4 + Silymarin (100 mg) |
Group IV 500 mg/kg FRF |
Group V CCl4 + 500 mg/kg FRF |
Group VI CCl4 + 250 mg/kg FRF |
|---|---|---|---|---|---|---|
| AST U/L | 15.00 ± 4.58 | 64.00 ± 7.94b | 26.67 ± 4.61b | 24.33 ± 1.15a | 27.00 ± 5.29b | 28.00 ± 8.54b |
| ALT U/L | 18.00 ± 5.19 | 58.00 ± 21.79a | 20.33 ± 4.16 | 24.00 ± 4.58b | 29.00 ± 8.00a | 26.33 ± 1.52a |
| ALP U/L | 23.00 ± 4.58 | 72.00 ± 14.79a | 20.67 ± 5.69b | 27.67 ± 4.72b | 30.67 ± 5.50a,b | 33.00 ± 2.64b |
| Bilirubin (mg/dl) | 0.31 ± 0.05 | 0.68 ± 0.03a | 0.34 ± 0.03b | 0.41 ± 0.04a,b | 0.55 ± 0.03a,b,c | 0.62 ± 0.09c |
| Protein (mg/dl) | 6.53 ± 0.25 | 3.63 ± 0.45a | 5.83 ± 0.25b | 5.67 ± 0.20a | 5.27 ± 0.31a,b,c | 4.80 ± 0.70 |
| Albumin (mg/dl) | 4.40 ± 0.20 | 2.43 ± 0.42a | 4.16 ± 0.23a,b | 3.83 ± 0.21b | 4.00 ± 0.20b | 3.73 ± 0.21b |
Table 2: Effect of different doses of FRF of Monodora myristica seed extract on serum liver enzymes and biochemical parameters (Values are mean ± standard deviation, (where n=5).
Effect of the FRF of M. myristica on serum lipid peroxidation levels, antioxidant and non-antioxidant enzymes: In the study, a significant increase (p<0.05) in the levels of MDA in the carbon tetrachloride treated group (group II) when compared with the negative control (group I) was observed; however, treatment with the different doses (250 mg/kg b.wt and 500 mg/kg b.wt.) of the flavonoid rich fraction stimulated a significant reduction (p<0.05). The observed decline is comparable to the results obtained for the standard drug group (group III), (Table 2). A significant reduction (p<0.05) was noticed in the hepatic enzymatic and non-enzymatic antioxidants (GSH, CAT, SOD, Vitamin C and Vitamin E) in the carbon tetrachloride treated group (group II) when put side by side with the negative control (group I). GSH level, Vitamin C and Vitamin E concentrations as well as SOD and CAT activities were significantly enhanced in the treatment groups (group V and group VI). The result of the present study showed that the flavonoid rich fraction treated group is comparable with that of the silymarin treated group (group III) (Table 3).
| Parameters | Group I Negative control (Olive oil) |
Group II CCl4 control |
Group III CCl4 + Silymarin (100 mg) |
Group IV 500 mg/kg FRF |
Group V CCl4 + 500 mg/kg FRF |
Group VI CCl4 + 250 mg/kg FRF |
|---|---|---|---|---|---|---|
| SOD (U/mg protein) | 7.03 ± 1.06 | 3.60 ± 0.97a | 6.22 ± 0.56b | 6.20 ± 0.48b | 5.51 ± 0.30b | 4.76 ± 0.33b,c |
| CAT (U/mg protein) | 6.30 ± 0.33 | 4.20 ± 0.87a | 5.49 ± 0.90 | 5.26 ± 0.94b | 5.10 ± 1.45 | 5.24 ± 1.41b |
| GSH (U/mg protein) | 6.02 ± 0.89 | 3.76 ± 0.36a | 5.88 ± 0.47b | 5.03 ± 0.72 | 4.05 ± 0.20a,c | 4.02 ± 1.15 |
| MDA (mmol/mg protein) | 2.92 ± 0.19 | 6.75 ± 0.44a | 2.75 ± 0.41b | 2.94 ± 0.14b | 3.92 ± 0.95b | 3.70 ± 1.03 |
| Vitamin C (mg/dl) | 2.42 ± 0.03 | 1.63 ± 0.15a | 2.38 ± 0.02b | 2.82 ± 0.46b | 2.69 ± 0.33b | 2.03 ± 0.17a,b,c |
| Vitamin E (mg/dl) | 0.87 ± 0.06 | 0.59 ± 0.12a | 0.89 ± 0.02b | 0.93 ± 0.02b,c | 0.83 ± 0.28b | 0.72 ± 0.87c |
Table 3: Effect of different doses of FRF of Monodora myristica seed extract on lipid peroxidation measured as malondialdehyde (MDA) and serum antioxidants (Values are mean ± standard deviation, (where n=5).
Liver histopathology: The result of histopathological studies also provided a supportive evidence for biochemical analysis. The liver sections of the rats treated with CCl4 showed hepatic cells with minor vacuolation and necrosis of hepatocytes in some areas. Pre-treatment with FRF and silymarin exhibited significant liver protection against CCl4 induced liver damage, which is evident by presence of more normal hepatocytes and reduced vacuolation and necrosis.
The result of the present study reveals important data regarding the in vivo antioxidant and hepatoprotective potentials of the flavonoid fraction of M. myristica seed extract. Previous inquiry on the acute and sub-lethal toxicological assessment of the flavonoid rich fraction of M. myristica seeds by Akinwunmi et al. [24] showed that the LD50 value was above 5000 mg/kg b.wt and therefore relatively safe for oral consumption.
It is well known that Carbon tetrachloride, an effective hepatotoxic agent which is widely used in animal models for induction of acute and chronic hepatic injury of oxidative stress is known to cause hepatic necrosis as well as nephrosis [39]. It is well documented that CCl4 are biologically altered under the action of microsomal cytochrome P450 of liver to reactive metabolites [40,41]. The metabolism of CCl4 comprises the production of highly fatal trichloromethyl radical (CCl3*) and peroxytrichloromethyl (*OOCCl3) free radicals through P450 bioactivation [42]. These free radicals have been reported to be infamous for binding covalently to unsaturated lipid peroxides which goes along with pathological changes such as raised levels of serum marker enzymes such as SGOT, SGPT and SALP, diminution of GSH, SOD and CAT, reduction in protein synthesis, triglyceride accumulation, increased lipid peroxidation, destruction of Ca2+ homeostasis which ultimately results to hepatocytes damage [43].
In the assessment of liver damage by CCl4, the determination of enzyme levels such as SGOT, SGPT is mainly used. It is well known that necrosis or membrane damage releases these enzymes into the blood and therefore it can be measured in the serum. High levels of SGOT indicates hepatic damage such as that caused by cardiac infarction as well as viral hepatitis and muscle injury, SGPT catalyses the conversion of alanine to pyruvate and is released in a similar manner. Consequently, SGPT is more specific to the liver and is thus better parameter for detecting hepatic injury. Elevated levels of serum enzymes are indicative of cellular leakage and loss of functional integrity of cell membrane in liver [44]. Serum ALP, bilirubin, total protein and albumin levels on the other hand are related to the function of hepatic cells. Increase in serum level of ALP is due to increased synthesis in presence of increasing billiary presence [45]. Hypoalbunemia and decline in total protein content can be deemed as a useful index of severity of hepatocellular damage.
Administration of CCl4 caused a significant (p<0.05) elevation of enzyme such as SGOT, SGPT and SALP and total bilirubin and decrease in total protein and albumin when compared to control. There was a significant (p<0.05) restoration of these enzyme levels on pretreatment with the FRF of M. myristica in a dose dependent manner and also by silymarin at a dose of 100 mg/kg b.wt. The reversal of increased serum enzyme in CCl4-induced liver damage by the fraction may be due to the prevention of the leakage of intracellular enzymes by its membrane stabilizing activity. This is in agreement with the commonly accepted view that serum levels of transaminases return to normal with the healing of hepatic parenchyma and the regeneration of hepatocytes [46]. The lowered levels of total protein and albumin recorded in the liver of CCl4-treated rats reveal the severity of hepatopathy [47]. Effective control of SALP, bilirubin, total protein and albumin levels points towards an early improvement in the secretary mechanism of the hepatic cells.
The efficacy of any hepatoprotective plant extract is dependent on its capacity of either reducing the harmful effect or restoring the normal hepatic physiology that has been distributed by a hepatotoxin. Both silymarin and the flavonoid fraction decreased CCl4 induced elevated enzyme levels in tested groups, indicating the protection of structural integrity of cell membrane or regeneration of damaged liver cells. The increase in lipid peroxidation level in liver induced by CCl4 suggests enhanced lipid peroxidation leading to tissue damage and failure of antioxidant defence mechanism to prevent formation of excessive free radicals. Pre-treatment with FRF of M. myristica significantly reverses these changes. Hence, it is likely that the mechanism of hepatoprotection of FRF of M. myristica is due to its antioxidant effect.
Decrease in enzyme activity of SOD has been reported as a sensitive index in hepatocellular damage and is seen as the most sensitive enzymatic index in live injury [48]. SOD has been reported as one of the most important enzymatic antioxidant defence system and is known to be able to scavenge the superoxide anion to form hydrogen peroxide and thus diminishing the toxic effect caused by the radical. The FRF of M. myristica caused a significant increase in hepatic SOD activity and thus reduces reactive free radical induced oxidative damage to liver. The enzymatic antioxidant, catalase (CAT) is reported to be widely distributed in all animal tissues and the highest activity is normally found in the erythrocytes and the hepatocytes. It decomposes hydrogen peroxide and protects the tissues from highly reactive hydroxyl radicals [49]. However, reduction in the activity of CAT may result in a number of deleterious effects due to the assimilation of superoxide radical and hydrogen peroxide. A higher dose (500 mg/kg b.wt) increased the level of CAT as produced by silymarin, the standard hepatoprotective drug. Glutathione is a non-enzymatic biological antioxidant present in the liver and is also one of the most abundant tripeptide in living organism. It is reported to be able to remove free radical species such as hydrogen peroxide, superoxide radical and also maintains membrane protein thiols. Also, it is substrate for glutathione peroxidase [50]. Decreased level of GSH is connected to an enhanced lipid peroxidation in CCl4 treated rats. Administration of FRF of M. myristica seed extract significantly (p<0.05) increased the level of GSH in a dose dependent manner.
Extensive vascular degenerative changes and centrilobular necrosis in hepatocytes was produced by CCl4. Pre-treatment with different doses of the FRF of M. myristica seed extract caused absence of centrilobular necrosis, indicating its hepatoprotective efficiency.
Free radical mediated processes have been severally implicated in the source and consequences of various diseases. The protective effect of the FRF of M. myristica seed extract on CCl4 induced hepatotoxicity in rat appears to be related to inhibition of lipid peroxidation and enhancement of antioxidant enzyme levels in addition to free radicals scavenging action. Flavonoids have been reported to be responsible for the stimulation of antioxidant enzymes [51]. The present study also showed that the short term administration of FRF of M. myristica seed extract has no deleterious effect on the antioxidant and biochemical parameters investigated as well as the histology. These agree with the investigations of Akinwunmi et al. [24] on the long term effect of the FRF of M. myristica seed extract.
From the results of the present study, it is suggested that the flavonoid rich fraction of M. myristica can improve liver cell damage caused by carbon tetrachloride poisoning in experimental rats in a dose dependent manner by recovering the antioxidant defense systems as well as alleviating lipid peroxidation associated with carbon tetrachloride toxicity. Further study is underway to identify the flavonoid (s) responsible for the reported hepatoprotective and antioxidant effect and hence the exploration of the active substance in drug and neutraceutical formulation for oxidative stress and liver toxicity.
The authors wish to thank the Department of Biochemistry, Imo State University Owerri for availing Laboratories for this research work. This paper is part of a project work submitted by the second author to Imo State University Owerri.
This research did not receive any specific grant from funding agencies in the public, commercial or not-for-profit sectors.
We declare that we have no conflict of interest.