Poultry, Fisheries & Wildlife Sciences
Open Access
ISSN: 2375-446X
ISSN: 2375-446X
Research Article - (2016) Volume 4, Issue 2
Ammonium, regarded as a major nitrogen pollutant in aquaculture, can be removed by biofilm formation in a biofilter. This research aimed to study ammonium removal on two kinds of substrates: limestone and bioball. Experiments were conducted through three consecutive steps: (1) Biofilter component set up (a trickling filter and substrate) of four groups; limestone substrate (LC), bioball substrate (BC), limestone substrate with nitrifying bacteria (LT) and bioball substrate with nitrifying bacteria (BT); (2) Monitoring biofilters performance for 15 days, where at day-0, 50 ppm ammonium was added into each system and at day-5 the ammonium level in all systems were adjusted to 70 ppm; (3) Enumeration of microbiome making up biofilm during biofilter conditioning period. In this current study, even though ammonium removal was observed in all experimental groups, inoculation of nitrifying bacteria significantly increased the ammonium oxidation rate. After 5 days, both treatment groups inoculated with nitrifying bacteria (LT and BT) degraded ammonium to 2.20-2.25 ppm, while the control groups (LC and BC) degraded ammonium to 12.38-16.59 ppm only. After ammonium adjustment, both treatment groups inoculated with nitrifying bacteria (LT and BT) degraded ammonium to 1.40-2.41 ppm, while the control groups (LC and BC) degraded ammonium to 17.06-23.53 ppm only. The total ammonium removal over 15 days of biofilter monitoring were 33.10% day-1 and 30.95% day-1 for LT and BT group, respectively, and 15.12% day-1 and 11.94% day-1 for LC and BC group, respectively. Biofilm formation on all groups showed the presence of ammonium and nitrite oxidizing bacteria (AOB and NOB) as well as heterotrophic bacteria. In the treatment groups AOB and NOB pioneered the biofilm formation, in contrast with the control groups where heterotrophic bacteria were the pioneer.
<Keywords: Ammonium removal; Biofilter; Limestone; Bioball; AOB; NOB
Ammonium derived from excess feed and organism’s feces is considered as main pollutant in aquaculture. When it goes into the organism’s body in an excessive amount, internal physiological pH increases and causes imbalance in major metabolism. Previous research reported that tolerable ammonium level of cultivated organism in general is below 2.5 ppm [1,2]. Therefore, maintaining ammonium in low concentration level becomes a crucial aspect to be considered. One of the culture systems that started to be used in aquaculture production to maintain low and stable ammonium level is recirculation aquaculture system (RAS) system [3-6].
In RAS system, a biofilter accounts as one important unit in charge of maintaining water quality through nitrification process, which is required to improve the survival and health of cultivated organism [7]. Nitrification in all aquaculture systems involves two main groups of nitrifying bacteria; first is oxidation of ammonium to nitrite by ammonium oxidizing bacteria (AOB), second is oxidation of nitrite to nitrate by nitrite oxidizing bacteria (NOB). This is how highly the toxic nitrogen compound, ammonium, is biologically converted into a less toxic nitrate compound.
An efficient way to achieve this process is by developing a fixed biofilm, it is a bacterial coat (AOB and NOB) on the surface of filter media arranged inside the biofilter [8]. Biofilms obtain dissolved organic nutrients transported by diffusion and therefore recycle ammonium in the water [9]. One type of biofilter to facilitate nitrification is a trickling biofilter, which applies water effluent in dripping mode to biofilm surface on the biofilter media [10].
Biofilter media is commonly called as substrate, which type influences biofilm formation [11]. Limestone and plastic ball are two types of substrates which are commonly used as a carrier in the biofilter reactor. Both substrates are preferred because they are relatively cheap, provides large surface area to volume ratio to support attachment of abundant microorganisms, and easy to obtain as well as to handle [12-14]. With such characteristics, biofilm allows high load and efficient processing of wastewater [15]. Despite its advantages, few studies had been done to assess microbiological parameters regarding biofilm performances on different types of substrates. Therefore, this study evaluates the biofilter performance using bioball and limestone substrates, with or without inoculation of nitrifying bacteria, upon ammonium degradation in a trickling biofilter by means of biofilm formation with addition of AOB and NOB.
Activation and cultivation of nitrifying bacteria
Nitrifying bacteria isolates were obtained from Microbial Biotechnology Research Group in Laboratory of Aquatic Ecology, School of Life Sciences and Technology, Institut Teknologi Bandung (SITH ITB). Activation and cultivation of isolates were conducted using Winogradsky medium supplemented with 10-30 ppm ammonium to provide specific nutrients for isolate’s growth. Scale up production of nitrifying bacteria was done using 10% (v/v) CaCO3 and 5 ppm ammonium [16].
Trickling filter installation and substrate preparation
In this study, limestone and plastic ball (further referred as bioball) were used as biofilter substrates (Figure 1). Limestone pieces were obtained from Padalarang limestone quarry industry, West Java, Indonesia, and further cut into 2 × 2 cm cubical limestone using a grinding wheel. Available surface area to volume ratio in limestone substrate and bioball substrate was 2 cm2 cm-3 and 3cm2 cm-3, respectively. Due to instrument limitation, microporous contained in limestone was not measured and therefore was not taken into calculation. Both limestone and bioball were disinfected by chlorination (60 ppm calcium chloride in tap water) for 24 hours following treatment with sodium thiosulphate (60 ppm) for 24 hours.
The trickling biofilter system consisted of a 20 L aquarium equipped with a water pump and a substrate container to retain the biofilter substrates was used as the system (Figure 2). 35 pieces of limestone with a total surface area of 105 cm2 cm-3 and 52 bioballs with the equivalent surface area of 104 cm2 cm-3 were used as substrate for both the control and treatment groups. In one biofilter unit, culture water containing metabolic wastes was pumped to the substrate container with biofilter substrates for nitrification process at a rate of 2.597 cm3 s-1. Total biofilm area included the measured substrate surface area of 104-105 cm2.
Experimental setup
Four experimental groups were tested in this study: (1) control group with limestone substrates with no bacterial inoculation (LC), (2) control group with bioball substrates with no bacterial inoculation (BC), (3) treatment group with limestone substrates inoculated with nitrifying bacteria inoculation (10% v/v inoculum with initial density of 3 × 106 CFU mL-1) (LT), and (4) treatment group with bioball substrate inoculated with nitrifying bacteria inoculation (10% v/v inoculum with initial density of 3 × 106 CFU mL-1) (BT). All experimental groups were tested in triplicates. The conditioning period was started with the addition of 50 ppm ammonium into each trickling biofilter. Monitoring of the ammonium, nitrite and nitrate level was done daily for 5 days of conditioning period. On the day 5, the ammonium level in all systems was adjusted to 70 ppm and monitored for 10 days. During monitoring period, the temperature level was kept at room temperature of 25 ± 1°C and dissolved oxygen level of 7-8 ppm was maintained by a constant water agitation after passing through the trickling system.
Water Quality Parameters
Ammonium, nitrite and nitrate were measured every day for chemical parameters using Nessler, diazotized and Nitrate HCl method, respectively [9]. pH level was measured every day to see the dynamics of pH on each biofilter substrate as well as the effect of nitrifying bacteria addition to the biofilter, using Mettler Toledo pH meter.
Enumeration of bacteria forming biofilm on substrate (AOB and NOB)
Bacterial enumeration was conducted using total plate count method [17]. Biofilm samples were taken three times, in the beginning, middle, and at the end of monitoring period. Each sample was added to 0.85% NaCl solution in 1:17 v/v ratio and sonicated for 10 minutes using a sonicator (Bransonic 3510-DTH) to detach cells. Then 100 μL of dissolved solution was spread into Winogradsky agar medium for AOB and NOB growth, and nutrient agar for heterotrophic bacteria growth, each in triplicates [18].
Data analysis
Ammonium degradation were calculated by the following formulas [11,19,20]:
Daily ammonium degradation percentage (%):
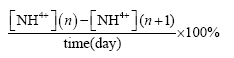
Where [NH4+](n): Ammonium concentration on day n
[NH4+](n+1): Ammonium concentration on day n+1
Ammonium degradation rate (ppm/day):
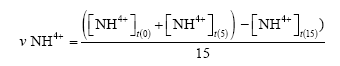
Where υ[NH4+]: average ammonium oxidation rate (ppm/day)
[NH4+]t(0): Ammonium concentration on day 0
[NH4+]t(5): Ammonium concentration on day 5
[NH4+]t(15): Ammonium concentration on day 15
Specific ammonium degradation rate (ppm/cm2/cm3):
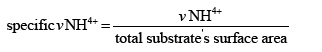
Where υNH4+: Average ammonium oxidation rate (ppm/day)
Data of ammonium degradation were analyzed using One-way Analysis of Variance (ANOVA) with SPSS 17 software. T-test was used to see influence of different substrates for attachment of AOB and NOB in the beginning, middle and at the end of conditioning period. Bivariate Pearson correlation test was used to evaluate the relationship and significance of psychochemical factors.
Nitrogen fluctuation with respect to ammonium removal
Nitrification is the most important biological process concerned in this study, especially the ammonium removal from the biofilter. Total ammonium nitrogen (NH3, NH4 +), nitrite (NO2 -) and nitrate (NO3 -) on all groups are shown in Figure 3. A better nitrification process was observed in the treatment groups compared to the control groups. At the end of the conditioning period (day 5), the ammonium concentration in the treatment groups (2.20 ppm and 2.25 ppm for LT and BT group, respectively) were lower than the control groups (12.38 ppm and 16.59 ppm for LC and BC group, respectively). Following the adjustment of ammonium concentration, lower ammonium concentrations of 1.40 ppm and 2.41 ppm were measured in the LT and BT treatment group, respectively, while the ammonium concentration in the control group cannot be lower than 23.53 ppm and 17.06 ppm for BC and LC control group, respectively. These results suggest that the inoculation of the nitrifying bacteria prior to biofilter operation is critical to obtain higher ammonium degradation. The ammonium removal rate of each experimental group is shown in Table 1. The highest average ammonium removal rate was 33.10% day-1, obtained by the LT group; followed by BT (30.95% day-1), LC (15.12% day-1) and BC (11.94% day-1) groups. Treatment group showed significant results compared to the control groups (p<0.05). Beside the higher ammonium degradation rate, higher nitrite degradation rate was also observed in the treatment groups compared to the control groups (Figure 3b), indicated by the higher accumulation of the less toxic nitrate as the final product of nitrification process at the end of the monitoring period (Figure 3c) [7].
| Daily removal percentage (%) | Ammonium removal rate (ppm day-1) | Specific ammonium removal rate (ppm m-2 m-3) | |
|---|---|---|---|
| LC | 15.12a | 6.83a | 0.065a |
| BC | 11.94a | 6.40a | 0.061a |
| LT | 33.10b | 7.88b | 0.076b |
| BT | 30.95b | 7.81b | 0.075b |
| Different superscript letters within a column denote significant differences (p<0.05). | |||
Table 1: Ammonium removal in all experimental groups.
Even though a high nitrite oxidation was observed, the nitrite concentrations in the treatment groups were still relatively unstable. This might be due to the NOB culture in the biofilm that has not reached its optimum population growth to consistently convert nitrite into nitrate. This slow population growth can be caused by two major factors: (1) NOB culture growth highly depends on the AOB metabolites products, i.e. nitrite, [21] and (2) NOB culture has longer doubling time compared to AOB culture [22].
Physicochemical water quality parameter
The pH levels of the treatment groups during 15 days of monitoring period were more stable with the pH range of 6 to 7, while the control groups shifted to a higher pH on day 6-11 (Figure 4). High pH can caused by an increased proportion of the un-ionized form of ammonium (NH3), which is toxic to fish even at low concentrations (above 2.5 ppm) [23]. The more stable pH level in the treatment groups could be due to the presence of CaCO3 powder in the system that was put in along with the inoculation of nitrifying bacteria. In water, carbonate-bicarbonate ion is a buffer system maintaining stable water pH, where poorly buffered water in the biofilter systems will negatively affect the biofilter performance.
Limestone substrate has higher pH increase than bioball substrate, because 54% mineral that made up limestone can be released as carbonate ion (CO3 2-) [24]. pH fluctuates when ammonium and carbonate ion is released without limits or the buffer system is acidified from organic load. In the treatment group, limestone substrate maintain more stable pH level around 6.7-7.0, close to neutral pH in freshwater. A slight decrease of pH was observed in limestone treatment because the effect of acidification during the conversion of ammonium to nitrite, it can be balanced by the slow-released of carbonate ion from the limestone substrate. This is an advantage for application in aquaculture system because acidification will most likely occur due to organic load from excretion by cultured organism [23]. As limestone can buffer biofilter system rather than bioball substrate, limestone considered as a better media to maintain pH stability in the biofilter.
Population dynamics of microorganism forming biofilm
Biofilm formation on limestone and bioball substrates in each group was evaluated upon nitrifying bacterial inoculation. Ammonium oxidizing bacteria (AOB) community was the pioneer species attached on the surface of substrate, because ammonium reduction was the initial reaction takes place in the biofilter (Figure 3). Ammonium oxidation produces nitrite compound providing growth of nitrite oxidizing bacteria (NOB) to develop the biofilm. Besides AOB and NOB, the presence of organic compounds during biofilm formation triggers heterotrophic bacteria growth on biofilm [9]. Thus, AOB, NOB and heterotrophic bacteria are species made up the biofilm [12].
Biofilm formation also occurred in the control groups, even though these groups were not inoculated with nitrifying bacteria. This suggested that suitable nutrient addition (in this case, NH4Cl) may trigger the growth of particular bacteria species. This bacterial community may play a major role in the ammonium removal in the control groups, as there were no accumulation of nitrite and its oxidation product, nitrate (Figure 3). In aquaculture production system, AOB will grow naturally when sufficient ammonium level presents on water [25]. However, in this study the natural occurrence of AOB did not dominate the bacterial community, indicated by its less abundance than heterotrophic bacteria (Table 2). This may explain the low ammonium removal capacity of the control groups (Figure 3).
| Limestone control (LC) | Bioball control (BC) | |||||
|---|---|---|---|---|---|---|
| Beginning | Middle | End | Beginning | Middle | End | |
| AOB (CFU ml-1) | 9.29×103 | 1.85 × 107 | 4.31 × 1010 | 1.85 × 103 | 2.01 × 107 | 1.35 × 1010 |
| NOB (CFU ml-1) | 1.47 × 103 | 2.58 × 107 | 7.43 × 108 | 1.23 × 102 | 1.10 × 107 | 5.06 × 108 |
| NOB/AOB ratio | 01:06 | 01:01 | 01:58 | 01:15 | 01:02 | 01:27 |
| Heterotroph (CFU ml-1) | 4.99 × 103 | 2.41 × 109 | 1.49 × 1012 | 9.22 × 105 | 5.26 × 109 | 3.10 × 1010 |
| Limestone treatment (LT) | Bioball treatment (BT) | |||||
| Beginning | Middle | End | Beginning | Middle | End | |
| AOB (CFU ml-1) | 4.33 × 104 | 1.12 × 108 | 9.22 × 1010 | 1.35 × 104 | 1.51 × 108 | 4.69 × 1010 |
| NOB (CFU ml-1) | 1.96 × 104 | 1.01 × 108 | 3.30 × 109 | 5.31 × 103 | 1.25 × 107 | 6.13 × 108 |
| NOB/AOB ratio | 01:02 | 01:01 | 01:28 | 01:02 | 01:12 | 0.094444 |
| Heterotroph (CFU ml-1) | 1.04 × 105 | 1.47 × 1010 | 2.61 × 1012 | 4.43 × 105 | 1.60 × 109 | 1.58 × 1011 |
Table 2: Abundance of bacteria in biofilm on all experimental groups.
Compared to the control groups (LC and BC), both treatment groups (LT and BT) had a higher microbial load (AOB, NOB and heterotrophic bacteria) in the beginning of monitoring period (Figure 5a). It is suggested that the higher AOB to almost 10-folds accelerated ammonium conversion to nitrite, and the availability of nitrite further enables NOB in the treatment groups to shift chemical equilibrium of the nitrification reaction by nitrate production resulted in a continuous nitrification process in the water. Incomplete ammonium removal in the control groups were possibly caused by insufficient AOB present in the beginning of monitoring period. After 15 days, all treatment groups contained relatively higher microbial loads (AOB, NOB and heterotrophic bacteria) compared to the control groups, providing a better ammonium and nitrite removal.
When we compare the ratio of NOB to AOB made up the biofilm (Figure 5b), generally AOB always outnumbered NOB. This is a normal phenomenon as ammonium becomes the first substrate consumed in the system and NOB grows dependently on nitrite produced by AOB. NOB population then increased in the middle of monitoring period, which possibly caused by nitrite accumulation after first ammonium addition (50 ppm), but AOB growth was then stimulated and a balanced ratio was recovered upon second ammonium addition (70 ppm). Besides, NOB has longer doubling time compared to AOB [22].
Heterotrophic bacteria are generally present in a higher load than nitrifying bacteria, because AOB and NOB are slowly growing organisms with doubling times from 12 to 32 h [25] while heterotrophs are opportunistic bacteria that can multiply as fast as 20 minutes [24]. Therefore, it is really important to stimulate nitrifying bacteria growth during the biofilter conditioning to ensure minimum nitrifies needed for sufficient and continuous ammonium removal. In application, ignoring nitrifying bacteria inoculation and conditioning in biofilter will result in an incomplete ammonium removal because nitrifying bacteria are easily outnumbered by heterotroph bacteria, especially in aquaculture production that produce large amount of organic substance [14].
Even though there was no significant difference in ammonium removal capacity between limestone and bioball substrates in the treatment groups, limestone substrate showed slightly higher ammonium removal compared to bioball substrate. Basically the main factor affecting biofilter performance is the surface area-to-volume ratio of corresponding substrate. However, there are other factors affecting ammonium removal, such as the material and physical characteristic of substrates. In this study, limestone substrate has rougher texture than plastic bioball. Irregular limestone carry microporous that formed naturally in limestone [24], that takes forms as small holes spreads thorough limestones innards, but due to technology limitation, the total limestones surface area cannot be measured. These microporous may contribute to a larger surface area-to-volume ratio, which provide more space available for bacterial adherence in the biofilter. Therefore, the more AOB adhere to substrate more ammonium degradation will occur, seen from material aspect, limestone is mainly consist of calcium carbonate (CaCO3) [26]. Such material gives at least two advantages than bioball; release of carbonate ion maintains alkaline condition which is favorable in nitrification [23], and calcium cations derived from calcium carbonate carry positive charge. In contrast, Gram negative bacteria cell surface has negative charge due to the presence of negatively-charged lipopolysaccharide on outer phospholipid of cell membrane [27]. Opposite charge attracts bacteria to limestone surface. Compared to bioball that are made from plastic material with no charge [24], attraction between bioball substrate and bacteria can be assumed to be less than that of limestone. Additionally, limestone also consists of oxygen-containing functional groups which turn out affecting ammonium adsorption [28]. Using limestone as a biofilter can adsorb 35% ammonium present, while the plastic ball can only adsorb 29.3% ammonium [29]. However, ammonium percentage removal from this study was 33.10% day-1 and 30.95% day-1 for LT and BT group, respectively, and 15.12% day-1 and 11.94% day-1 for LC and BC group, respectively. Even though limestone has greater theoretical capacity to perform even better ammonium removal [28,29], upon addition ammonium of 50-70 ppm, bioball can perform almost as good as limestone substrate. It is suggested that the differences in the ammonium removal performance of the nitrifiers between limestone and bioball substrate on could have been observed at a higher ammonium level.
Biofilter performance with respect to removal of ammonium differs significantly between inoculated substrates (LT 33.10% day-1 and BT 30.95% day-1) and uninoculated substrates (LC 15.12% day-1 and BC 11.94% day-1). Both substrate inoculated with nitrifying bacteria (LT and BT) degraded ammonium almost completely to 1.40-2.41 ppm, whereas control groups (LC and BC) cannot degrade ammonium after its concentration reached 14-17 ppm. Ammonium removal was mainly carried out by biofilm formed during monitoring period. Characterization of microbial components adhering to this biofilm reveals presence of ammonium and nitrite oxidation bacteria (AOB and NOB) as well as heterotrophic bacteria on all groups, but nitrifying bacteria inoculation on treatment groups result in initial dominancy of AOB and NOB, which explain enhanced ammonium oxidation rate on treatment groups.
A more standardized biofilter conditioning strategy needs to be applied in order to ensure a stable nitritation process before being used in aquaculture production, including prolongation of biofilter conditioning period of up to minimum 14 days, and/or periodic reinoculation of nitrifying bacteria (AOB and NOB) to shorten the biofilter conditioning period. In the near future, we need to formulate the most suitable substrate that match all the criteria’s mentioned above, considering material selection, large surface area by microporous availability and provide buffering advantage as well as nutrition to nitrifying bacteria.
This research was partially supported from Limestone Grant by Padalarang Limestone Processing Plant, Padalarang and West Java, Indonesia.