
Journal of Clinical Trials
Open Access
ISSN: 2167-0870

ISSN: 2167-0870
Research - (2022)Volume 12, Issue 6
Background: Subject diaries are utilized in approximately 25% of all clinical trials and are an essential component for gathering patient reported outcomes. Depending on the study design, various methods are utilized to capture patient reported outcomes either through paper, electronic means, or handheld devices. Regardless of how data is being collected, study participants can easily become overwhelmed with the complexity and tedium of recording daily outcomes, which subsequently can lead to inefficient, non- compliant reporting. Even though Adverse Event (AE) reporting and changes in concomitant medication collection are vital in validating the safety and efficacy of an investigational product, the gold standard for collecting real time data is still lacking. Therefore, a standard, all-encompassing, simple patient safety outcome tool is warranted in clinical trials.
Methods: Twenty subjects who were concurrently enrolled in a clinical trial that included either paper or electronic diaries at Perseverance Research Center (PRC) were consented and enrolled in the RTD-01 study. Diary compliance and satisfaction questionnaires were administered to consented study participants. After registering study participants’ cellular information, a two-way SMS mobile messaging service was deployed regarding changes in health (AEs) and concomitant medications daily to study participants for 6 weeks. Daily text message responses were uploaded to an FDA 21 CFR Par 11 compliant study portal. At the end of their 6 weeks of participation, study participants completed additional diary outcome compliance and satisfaction questionnaires.
Results: All participants completed the full 6 weeks of the study. Study data supported the primary objective of the RTD-01 study, demonstrating on average a 95.7% compliance in daily AE and concomitant medication reporting. A paired t-test was utilized to determine mean changes in responses for satisfaction and compliance diary questionnaires at Baseline and at Week 6. Both compliance diary responses and satisfaction diary responses demonstrated statistically significant mean changes from Baseline to Week 6 (p=0.012, p=0.001). In addition, study participants reported the SMS two-way texting tool was more convenient (M = 1.1, SD=0.5), p=0.00001, and less time consuming (M=1.6, SD=0.9) than their current study diary (M=3, SD=1.3), p=0.002.
Conclusion: Inaccurate data or failure to report health and medication changes over the course of a clinical study could affect an investigational product’s safety and efficacy results. Data from the RTD-01 study demonstrated that capturing real time data through implementation of a two-way SMS texting tool not only improves participant compliance but also eliminates inaccurate reporting, recall bias and enhances the quality of data being collected. Overall, satisfaction in utilizing a real time text messaging tool improves engagement and compliance, providing future clinical trials with a non- antiquated patient reported outcome measuring tool that can replace paper or electronic diaries.
Safety; Adverse events; Concomitant medications; Patient reported outcomes; Compliance; Text messaging; Study tool technician
Clinical trials have the capability of providing beneficial clinical treatment to study participants as well as allowing clinical researchers to discover additional and more effective treatments for others in the future. Data collected from each study participant aids pharmaceutical and medical device companies to gather knowledge, determine side effects (adverse events), evaluate risks, discover added benefits, and provide advancements in their investigational product. Unfortunately, concomitant medications or adverse events that could possibly or probably be related to an investigational product may be collected, and/or improperly reported to a Sponsor. Validated and standardized surveys and diaries such as paper patient reported outcomes (PROs), or electronic clinical outcome assessments (eCOA) have been implemented in clinical trials to improve study treatment compliance and drug related event reporting [1]. Although recording health changes in either paper or electronic diaries have been advantageous to research, continuous apprehension regarding inaccurate reporting still occurs due to lack of real time data collection [2]. Reliability, quality of data, misplacing diaries, lack of completion, or forgetting to return diaries to the clinical site for verification are all shortcomings of paper diary reporting. Electronic diary collection can also be problematic since data collected is contingent on the function of the electronic device, accessibility, or WIFI connectivity. In addition, electronic diary devices can be costly and more cumbersome to use than paper reported diaries. Although both methods are valuable ways of collecting subject data, such assessments vary from trial to trial and continuously struggle with capturing real time data entry, such as medication changes and failure to report adverse events in a timely manner. While PRO’s and eCOA’s have been getting the attention of regulatory agencies more recently, the need to regulate and utilize a standard PRO in clinical trials has become a priority for regulators [3]. In fact, during a recent public Federal Drug Administration (FDA) panel discussion, agency panelists asserted that the FDA aims to ensure that a patient-centric approach to drug development becomes a standard practice in clinical trials [4].
Therefore, a patient-centric way of capturing real time study data in a more universal, simplistic, minimal time restraining, and less costly way is needed and warranted. In the present research, it was hypothesized that subjects could easily comply and collect real time safety data without spending a considerate amount of time as required in clinical trials utilizing current PROs and eCOAs. Since it is reported that 98% of the national population has access to a mobile device with text messaging services [5], and 90% of phone users prefer text messages over direct phone calls [6], a text messaging diary study tool was created, deployed, and studied in the RTD-01 study. The RTD-01 study was an observational, non-therapeutic pilot trial that was established to determine if such a two-way SMS text mobile messaging platform could collect real time safety data from subjects and improve subject reporting compliance to ensure better study outcomes. The primary objective of the study was to determine if a daily SMS text message study tool could improve overall compliance in adverse event and concomitant medication reporting through real time reporting. Data from the RTD- 01 study demonstrated collecting real time reported outcome measures not only improves compliance but also is less time consuming and more convenient to use. In addition, data collected from this study allows for a more sophisticated safety study tool to be implemented into other clinical trial sites and can ultimately be used to enhance delivery of medical care for standard of care patients in a variety of ways.
Subjects
Men and women 18-85 years of age inclusivity who were concurrently enrolled in a clinical trial that included either paper or electronic diaries at Perseverance Research Center (PRC), were consented and enrolled into the RTD-01 study. Additional inclusion criteria included the ability to receive and respond to SMS text messages through a phone provider. Study participant compliance and satisfaction questionnaires were administered at Baseline and again at the Week 6 visit. Subjects were required to report daily changes in their health (i.e., adverse events) and medication via text messages. All text messages were sent and stored into a de-identified study portal.
Study design
This single-centre, non-interventional pilot trial was designed to determine the effectiveness of a SMS text messaging study tool that can be utilized in clinical trials to collect real time health and concomitant medication changes. The study consisted of a HIPAA and 21 CRF Part 11 compliant SMS texting tool that sent daily text messages from a protected phone number to study participants daily for 6 weeks. The study protocol, informed consent and study questionnaires were approved by the Western Institutional Review Board (WIRB). All study conduct adhered to Good Clinical Practice guidelines and applicable regulatory requirements and ethical principles, including the Declaration of Helsinki. The study was registered with ClinicalTrials.gov (NCT05553288).
Study procedures
After study participants were consented, a subject study ID was assigned and linked to a subject’s cellular number. Subjects received their first text message in clinic to ensure they understood what their participation entailed, as well as confirm the functionality of the SMS text messaging tool and study portal. A brief questionnaire evaluating study diary compliance (Table 1) was provided to study participants at Baseline along with a study diary satisfaction questionnaire (Table 2). Study participants were instructed to answer the questions pertaining to their current clinical trial diary. At Week 6 (approximately 42 days) after Baseline, study participants returned to PRC for their final visit to complete the same compliance and study diary satisfaction questionnaires. At the Week 6 visit, study participants were instructed to answer questions pertaining to their experience utilizing the SMS two-way texting diary tool. Upon completion, subject study ID and phone numbers were removed from the SMS text messaging tool and the study concluded.
| Questions | Response options |
|---|---|
| How often have you forgotten to fill out your study diary? | 0=N/A |
| How often have you forgotten to report health changes (side effects, adverse reactions, colds, etc.)? | 1=Never 2=Often (about once a week) |
| How often have you forgotten to report medication changes? | 3=Occasionally (less than once a week) |
| *How often have you forgotten to return study supplies such as diaries? | 4=Frequently (several times a week) |
| Note: *was omitted from 6 week data analysis since it did not pertain to this study | |
Table 1: Diary compliance questionnaire.
| Questions | Response options |
|---|---|
| It is convenient and easy to report daily changes in your health in a diary/study tool | 1=Strongly Agree |
| Is it convenient and easy to record daily changes to medications in a diary/study tool | 2=Agree |
| You spend a lot of time filling out/reporting changes in a diary/study tool | 3=Neutral |
| You are more aware of your health by filling out information in a diary/study tool | 4=Disagree |
| You are more aware of medication changes by filling out information in a diary/study tool | 5=Strongly Disagree |
| You are satisfied using a diary/study tool |
Table 2: Diary satisfaction questionnaire.
Daily SMS text messaging tool and study portal
The SMS text messaging safety tool was created and developed by PRC and clinically experienced software engineers. A Business Agreement (BAA) was executed with Twilio, a single SMS platform with built-in intelligence [7]. Using advanced automation technology, Twilio complied with Protected Health Information (PHI) and HIPAA to ensure that study participants’ information was safe and secure. The SMS text response from each study participant was automatically uploaded to an Application Programming Interface (API) where the study was managed and stored. Daily questions were deployed at the same time daily for 6 weeks (Figure 1).
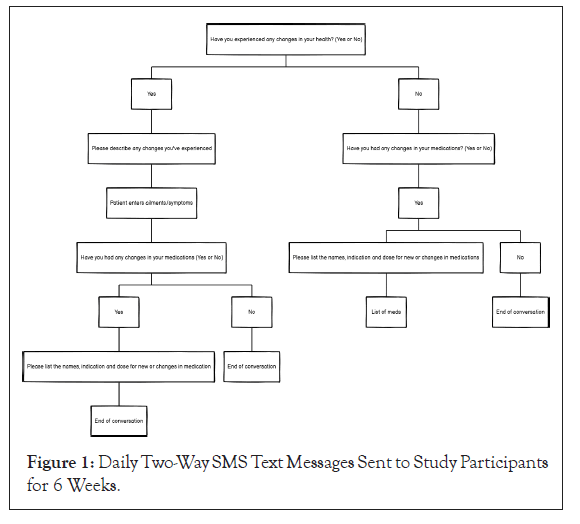
Figure 1: Daily Two-Way SMS Text Messages Sent to Study Participants for 6 Weeks.
Study participant responses were immediately uploaded to a study portal that only delegated PRC personnel had access to. Delegated study personnel at PRC reviewed the study portal daily to assess and record any AEs or changes in concomitant medication. Any changes were immediately assessed by the appropriate clinical trial’s Principal Investigator (PI), then reported and documented in the study subject’s current clinical trial chart.
The study was conducted between August 10th, 2022, and October 25th, 2022. Twenty subjects who were currently enrolled in a clinical trial that required diary reporting at Perseverance Research Center were enrolled. Ten men and ten women from different races and different concurrent clinical trials completed the full 6 weeks of the study (Table 3).
| Demographics | |
|---|---|
| Age (Mean) | 50 (18-77) |
| Sex | |
| Male % | 50% (10) |
| Female % | 50% (10) |
| Race | |
| White | 60% (12) |
| Black or African American | 10% (2) |
| Asian American | 10% (2) |
| Hispanic or Latino | 20% (4) |
| Concurrent Clinical Trial | |
| Obesity Trial | 20% (4) |
| Diabetes | 25% (5) |
| Traumatic Brain Injury | 15% (3) |
| Atopic Dermatitis | 25% (5) |
| Pediatric Migraine | 15% (3) |
| Study Diary Form | |
| Paper Diary | 60% (12) |
| Electronic Diary | 25% (5) |
| Hand-Held Device | 15% (3) |
Table 3: RTD-01 study participant demographics and concurrent clinical trial and diary form.
Raw data in the RTD-01 study showed that participants were on average 95.7% compliant to daily AE and concomitant medication reporting (Figure 2). Compliance diary responses demonstrated that ninety percent (N=18) of study participants were 100% in diary compliance responses, in addition, study participants indicated an improvement in AE and concomitant medication reporting (Figure 3). Study participant diary compliance responses were compared and measured by a paired t-test (Table 4) to determine whether there was a mean difference at Baseline compared to end of study visit (Week 6). Data illustrated there was a statistical significance in mean responses from Baseline to Week 6.
Figure 2: Raw Data SMS Text Messaging Responses for All Study Participants in 6 Weeks. Note: ( ) Total Questions, (
) Total Questions, ( ) Unanswered Questions.
) Unanswered Questions.
Figure 3: Stacked bar graph depicting individual patient diary compliance responses at Baseline and Week 6. Note: ( ) N/A (
) N/A ( ) Never (
) Never ( ) Occasionally (
) Occasionally ( ) Often (
) Often ( ) / (
) / ( ) Frequently.
) Frequently.
| t-Test (Paired Two Sample for Means) | ||
|---|---|---|
| Avg Baseline | Avg 6 Week | |
| Mean | 2.35 | 1.066667 |
| Variance | 0.0775 | 0.000833 |
| Standard Deviation | 0.278388218 | 0.028868 |
| Observations | 3 | 3 |
| P(T ≤ t) two-tail | 0.012577035 | |
Table 4: Paired t-test (p=0.012) and standard deviation of compliance diary responses at Baseline and Week 6.
The mean responses for diary satisfaction were also statistical significance (p=0.0001) from Baseline to Week 6 (Table 5) indicating satisfaction in convenience, improved cognizance of personal health and medication changes and all-around contentment utilizing the SMS texting tool over their current paper, electronic or hand-held diary devices (Figure 4).
| t-Test (Paired Two Sample for Means) | ||
|---|---|---|
| Baseline | 6 Weeks | |
| Mean | 3.033333333 | 1.375 |
| Variance | 0.033666667 | 0.07775 |
| Standard Deviation | 0.183484786 | 0.278837 |
| Observations | 6 | 6 |
| P(T ≤ t) two-tail | 0.000150365 | |
Table 5: Paired t-test (p=0.0001) and standard deviation of satisfaction diary responses at Baseline and Week 6.
Figure 4: Cluster bar graph illustrating individual patient diary satisfaction responses at Baseline and Week 6. Note: ( ) Strongly Disagree, (
) Strongly Disagree, ( ) Disagree, (
) Disagree, ( ) Neutral, (
) Neutral, ( ) Agree, (
) Agree, ( ) Strongly Agree.
) Strongly Agree.
While Adverse Event (AE) reporting and concomitant medication collection during clinical trials is a vital component in validating the safety and efficacy of an investigational product, a standard way of accurately and adequately recording or tracking such data is lacking. Study diaries are utilized in more than 25% of all phase II-IV clinical trials [8] to assess patient study specific information regarding safety, efficacy, and drug compliance. Although such Patient Reported Outcomes (PROs) and electronic clinical outcome assessments (eCOAs) have become an integral part of research, there is continuous apprehension regarding inaccurate reporting, recall bias and noncompliance which, unfortunately, can invalidate a clinical trial [9]. Moreover, a gold standard in collecting and recording real time patient reported outcomes is warranted. In this non-interventional pilot trial, it was demonstrated that study participants were more compliant and more apt to comply to daily health and concomitant medication reporting via a SMS two-way texting tool (M=1.1, SD=0.3) than reporting health or medication changes in their current clinical trial diary (M=2.6, SD=1), p=0.000002.
Based on raw data, study participants on average were 95.7% compliant with daily responses during the 6 weeks (42 days) of reporting. In addition to AE and concomitant medication reporting, subjects reported the SMS two-way texting tool was more convenient (M=1.1, SD=0.5) p=0.00001, and less time consuming (M=1.6, SD=0.9) than their current study diary (M=3, SD=1.3), p=0.002. In addition, all study participants reported improvement in all around satisfaction in utilizing a SMS two-way texting tool (M=1.3, SD=0.7), then their current diary (M=3.4, SD=1.3) p=000002. Study participants also reported they were more cognizant of their health and more aware of their medication changes through the SMS two-way text messaging tool then they were on their current study (M=2.9, SD=1.2) by filling out either paper or electronic diaries (M=1.6, SD=0.7), p=0.00035. Although the data was extremely promising and statistically significant across individual questions and average questionnaire responses, one limitation of the study is the small number of subjects at a single site.
Depending on the clinical trial, participation may last years, with several months between onsite visits. Even though study drug efficacy and safety are still being obtained between onsite study visits, interaction between site personnel and study participants typically start to decline. During this time, study participants may still experience AEs and changes in concomitant medications; however, reporting changes in health or medications seem to be underreported due to study subject complacency, forgetfulness or may be too time consuming. By not reporting real time changes, study participants may not receive appropriate medical attention that may be needed. In addition, data that can be detrimental to determining the safety of a clinical trial may not be collected, possibly creating unwarranted “failures” in study treatment or even erroneous treatment approval due to lack of reporting of safety and efficacy data. In all phases of clinical trials, especially early phase trials, when safety and dose toxicity of an investigational device or drug is contingent on adverse events, it is imperative that real time safety data is collected appropriately and adequately. With the adoption of today’s digital world and usage of cell phones, an opportunity to revolutionize clinical trials through text messaging should be indispensable. Through utilizing such a tool, significant improvements in time, satisfaction and compliance in AE and medication reporting were demonstrated in the RTD- 01 study. If such a tool can enhance safety, data accuracy, patient compliance and decrease time and effort, it should be implemented in future clinical trials in lieu of paper and electronic diaries for the collection of real time safety and patient outcome data. In addition, such a tool can be applied to standard of care practice for providers to deliver quality oversight to their patients by offering a more efficient form of communication.
[Google Scholar] [PubMed]
[Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
Citation: Hank NC, Christians L, McCravey B, (2022). An All-in-One Convenient Patient Safety Outcome: Assessment Tool Improving Study Patient Reported Outcome Compliance through Real Time Data Collection. J Clin Trials 12: 514
Received: 08-Nov-2022, Manuscript No. JCTR-22-20002; Editor assigned: 11-Nov-2022, Pre QC No. JCTR-22-20002 (PQ); Reviewed: 25-Nov-2022, QC No. JCTR-22-20002; Revised: 02-Dec-2022, Manuscript No. JCTR-22-20002 (R); Accepted: 09-Dec-2022 Published: 14-Dec-2022 , DOI: 10.35248/2471-9870.14.12.514
Copyright: © 2022 Hank NC, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.