Organic Chemistry: Current Research
Open Access
ISSN: 2161-0401
ISSN: 2161-0401
Research Article - (2020)Volume 9, Issue 1
A simple mild, efficient synthetic methodology developed for the preparation of Bis(indolyl)methanes catalyzed by NiI2 in MDC medium at room temperature. Several substituted aldehydes with indoles under this reaction condition is elucidated. The features of this methodology are shorter reaction time, less equivalence of the reagent, clear reaction profile and simple work up procedures.
Transition Metal catalyst; Bis(indolyl)methane; Indole; Aldehydes; NiI2
Indole is one of the privileged structures and featured in many biological and pharmaceutically active compounds [1]. Due to the binding nature to the multiple receptors with high affinity, the applications of indole derivatives covered wide range of therapeutic areas. Recently few of biologically important indoles and their derivatives isolated from marine and terrestrial sources [2-8]. Among several indole derivatives Bis(indolyl)methane and its derivatives are very important sub class and playing a major role in many sectors. Bis(indolyl)methane used as a cancer preventive agents [9], radical scavengers [10], Agro chemicals [11], and many therapeutic areas [12].
The lead indole derivatives reported in this literature are Bis(indolyl)methane, more than 200 exclusive methods available in the literature for the preparation of Bis(indolyl)methane and its substituted derivatives. Many of these methods are conventional type and promoted by either protic acids [13-16] or Lewis acids [17-21]. Few of non-conventional methods are also reported in the literature. The overall preparation of the Bis(indolyl)methane consists the electrophilic bi-substitution reaction of indole with aldehydes or ketones produces azafulvenium salt as an intermediate, which reacts with another molecule of indole forms the Bis(indolyl)methane [22-24]. The strategy of new research for the preparation of Bis(indolyl)methane includes the variation of catalyst, mild reaction conditions, reusability of catalyst, yield of products, reactions rate, simplicity of work-up and green chemistry. However several preparative methods available in the literature, searching the efficient and economic methods are always attractive task to both organic and medicinal chemist.
A mixture of indole, aldehyde and NiI2 (1:2:0.5 mole ratio) was taken in 10 volume of MDC. The resulting heterogeneous reaction mixture stirred for 4-5 hrs at room temperature. 10 volume of water was added to the reaction mass and extracted the product in MDC layer along with additional 5 volumes. Combined the organic layers and dried with sodium sulphate and concentrated under reduced pressure. Obtained residue was further purified with silica gel chromatography with the use of Ethyl acetate and hexane mixture to provide the desired compound.
As a continuation of our research on indole chemistry, herein we are describing the new efficient synthetic methodology for the preparation of Bis(indolyl)methane catalyzed by NiI2 in MDC medium (Scheme 1). The electrophilic bi-substitution reaction of indole and aldehydes shown in the scheme-1 catalysed by NiI2 in MDC medium. During the optimization of other cascade reactions we found that leftover aldehydes were undergoing the addition reaction even with poor nucleophiles. With that idea we have expanded our investigation with well-known indoles and aldehyde combinations. The initial study itself we have chosen the MDC as a solvent and performed the reaction at room temperature. As expected the reaction was successfully completed in short period of time and the reaction profile also highly inspired that no impurity was observed.
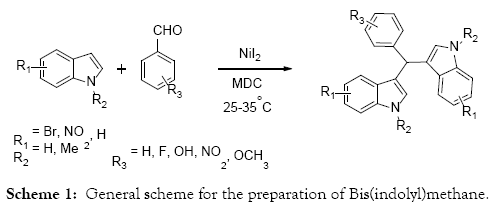
With this above reaction conditions in mind, we have expanded the scope of this work to other aldehydes and indoles. As mentioned above the preliminary experiments was conducted using indole, benzaldehyde and 4-fluorobenzaldehyde in 10 volume of MDC and 1 eq of NiI2. The reaction proceeds smoothly and afforded the Bis(indolyl)methane 97% yield (Table 1, Entry 1, 2). Subsequent to this attempt we examined the solvent effect and temperature on the reactions. Among tried solvents water, MDC, Ethanol and Methanol, MDC shows the neat reaction profile with highest yields. Whereas the reaction progress was slow in the alcoholic solvents and many impurity formation observed in the water medium. With respect to temperature effect we don’t find any difference between reflux and room temperature in terms of reaction conversion. Below room temperature we have not tried.
In order to accelerate the reaction rate, the mole ratio of catalyst has been studied. From 0.25 to 1 mole of catalyst and Entry 3a and 3b were chosen for the study. The results reveals that the reaction yield is almost similar for 0.5 and 0.75 mole, and lower conversion was observed with 0.25 mole of catalyst. Higher side catalyst (1.0 mole) loading reaction shows some uncharacterized impurities and ended with low isolated yields compare with 0.5 or 0.75 mole loading (Table 1).
| Product | Isolated Yield (%) | |||
|---|---|---|---|---|
| 0.25 Mole (%) | 0.5 Mole (%) | 0.75 Mole (%) | 1 Mole (%) | |
| 3a | 82 | 97 | 97 | 90 |
| 3b | 80 | 97 | 95 | 88 |
Table 1: Effect of catalyst loading.
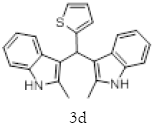
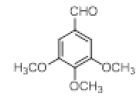
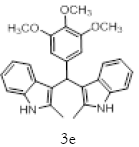
With this optimal reaction conditions in hand further substrate scope for the synthesis of 3 was investigated. A variety of substituted indoles and aldehydes has been selected and reacted with this optimized reaction conditions, yield and the products are tabulated (Table 2). The catalytic system is so powerful that the substrate electronic factors are not playing any notable role. Heterocyclic aldehyde such as thiophene-2-carboxaldehyde also furnishes the desired product in good yield (Table 2, Entry 4). Few of substrates (Table 2, Entry 11, 12) are not completely soluble in the reaction medium, progressed slow and yielded low with optimized time duration. Except low soluble substrates in MDC rest of all substrates reacted smoothly in the MDC medium and furnishes good yield. Except handling loss reaction yielded excellent. Recyclability of catalyst were tried but was not successful. All the isolated products were characterized and confirmed by using NMR, IR and mass spectroscopy techniques.
| Entry | Indoles (1) | Aldehyde (2) | Product (3) | Yield (%) |
|---|---|---|---|---|
| 1 |  |
 |
 |
97 |
| 2 | 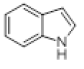 |
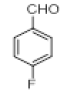 |
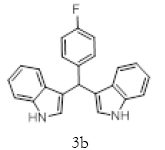 |
97 |
| 3 |  |
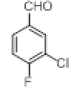 |
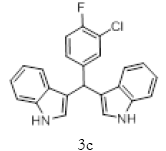 |
97 |
| 4 | 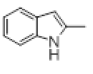 |
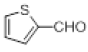 |
 |
96 |
| 5 | 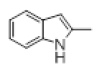 |
 |
 |
96 |
| 6 | 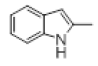 |
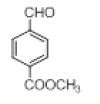 |
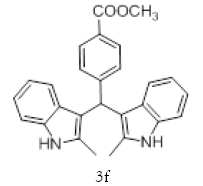 |
96 |
| 7 | 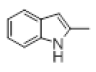 |
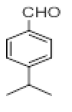 |
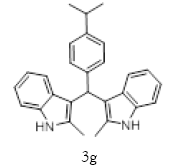 |
96 |
| 8 | 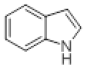 |
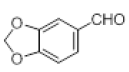 |
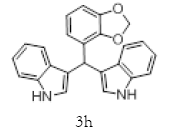 |
95 |
| 9 | 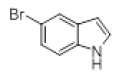 |
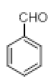 |
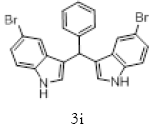 |
95 |
| 10 | 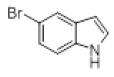 |
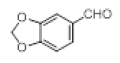 |
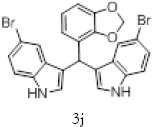 |
92 |
| 11 | 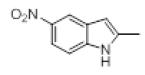 |
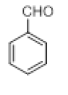 |
 |
60 |
| 12 | 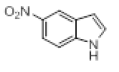 |
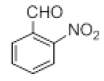 |
 |
58 |
Table 2: Synthesis of various Bis (indolyl) methanes catalyzed by Nickel iodide.
Spectral data’s for selected compounds
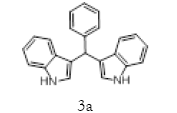
Table 2; Entry 1 creamy solid
1H NMR (400 MHz, DMSO-d6): δ10.80(s, 2H), 7.34 (t, J=7.6 Hz, 4H), 7.26 (t, J=7.6 Hz, 4H), 7.16 (m, 1H), 7.02 (t, J=7.6 Hz, 2H), 6.87-6.81 (m, 4H), 5.82(s, 1H). 13C NMR (100MHz, DMSO-d6): δ144.9, 136.5, 128.2, 127.9, 126.5, 125.7, 123.4, 120.8, 119.0, 118.1, 118.0 and 111.3. MS (EI): Calculated mass for C23H18N2 is 322.40 found 321.60 (M+1)-. IR (KBr): 3409.06, 3080, 1617.30, 1455.57, 1092.54, 743.70, 596.22 cm-1 (Figures 1-3; Tables 3 and 4).
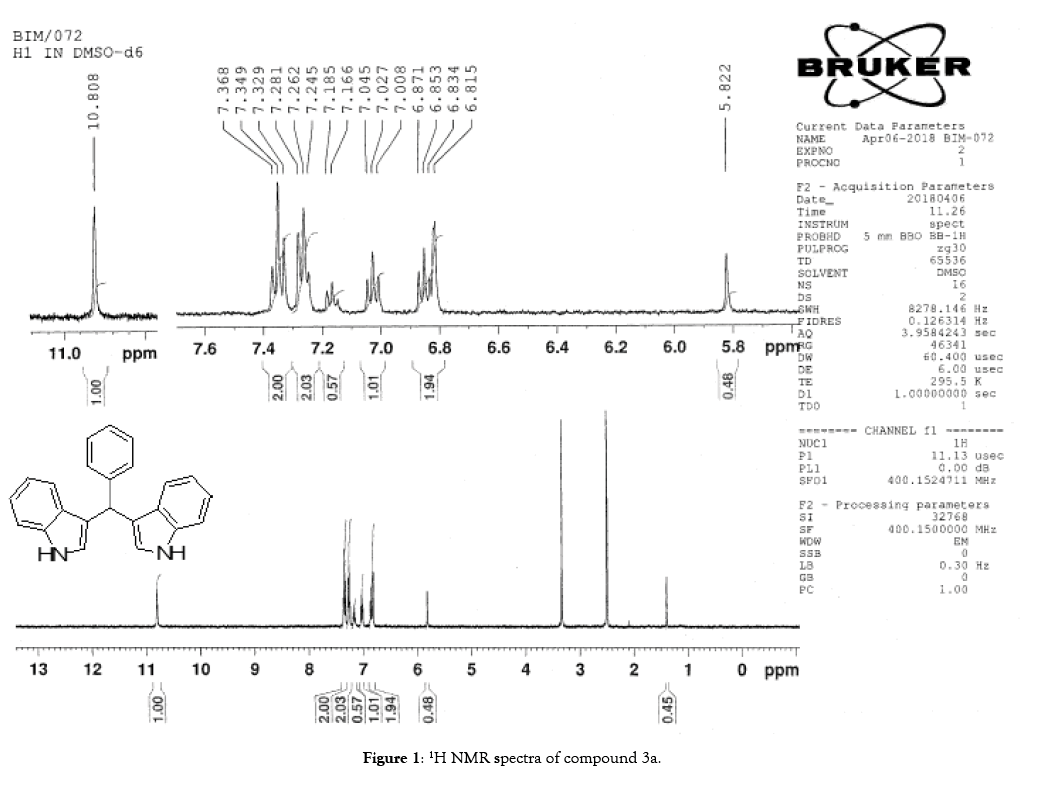
Figure 1: 1H NMR spectra of compound 3a.
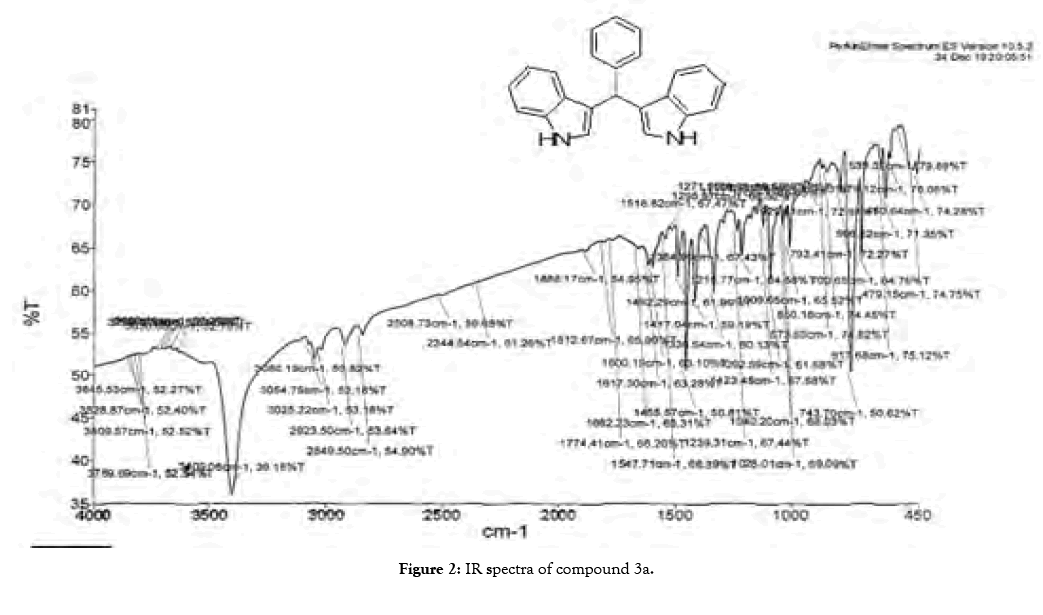
Figure 2: IR spectra of compound 3a.
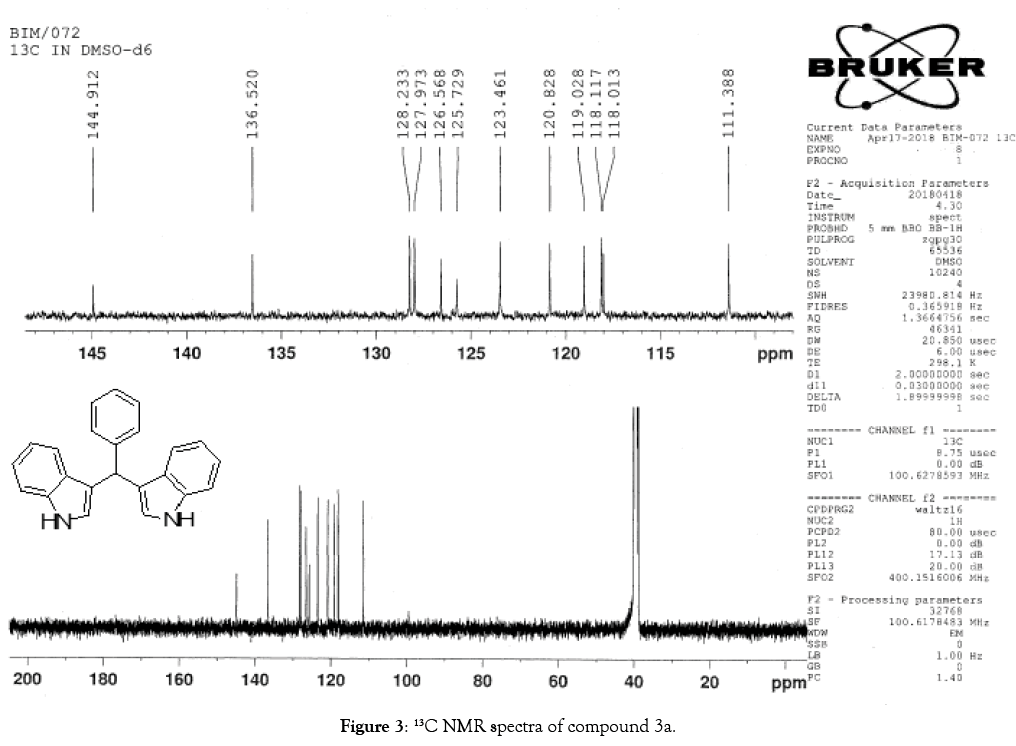
Figure 3: 13C NMR spectra of compound 3a.
| Source Spectra Results | |
|---|---|
| Spectrum Name | Number Of Peaks |
| 0181 | 54 |
Table 3: IR spectra results of compound 3a.
| List of Peak Area/Height | ||
|---|---|---|
| Peak Number | X (cm-1) | Y (%T) |
| 1 | 3845.53 | 52.27 |
| 2 | 3828.87 | 52.4 |
| 3 | 3809.57 | 52.52 |
| 4 | 3789.69 | 52.54 |
| 5 | 3719.31 | 53.14 |
| 6 | 3698.97 | 53.17 |
| 7 | 3682.18 | 53.27 |
| 8 | 3660.33 | 53.08 |
| 9 | 3636.68 | 52.78 |
| 10 | 3409.06 | 36.18 |
| 11 | 3080.19 | 53.82 |
| 12 | 3054.75 | 52.18 |
| 13 | 3025.22 | 53.16 |
| 14 | 2923.5 | 53.64 |
| 15 | 2849.5 | 54.9 |
| 16 | 2508.73 | 59.65 |
| 17 | 2344.54 | 61.26 |
| 18 | 1888.17 | 64.95 |
| 19 | 1812.67 | 65.99 |
| 20 | 1774.41 | 66.2 |
| 21 | 1662.23 | 65.31 |
| 22 | 1617.3 | 63.28 |
| 23 | 1600.19 | 63.1 |
| 24 | 11547.71 | 166.39 |
| 25 | 1518.82 | 67.47 |
| 26 | 1492.29 | 61.99 |
| 27 | 1455.57 | 50.81 |
| 28 | 1417.04 | 59.19 |
| 29 | 1384.99 | 67.43 |
| 30 | 1336.54 | 60.13 |
| 31 | 1295.97 | 68.32 |
| 32 | 1271.95 | 69.55 |
| 33 | 1239.31 | 67.44 |
| 34 | 1216.77 | 64.58 |
| 35 | 1177.17 | 68.85 |
| 36 | 1150.73 | 69.47 |
| 37 | 1123.48 | 67.88 |
| 38 | 1092.59 | 61.68 |
| 39 | 1060.16 | 69.23 |
| 40 | 1040.2 | 68.03 |
| 41 | 1028.01 | 69.09 |
| 42 | 1009.65 | 65.52 |
| 43 | 929.11 | 72.91 |
| 44 | 873.66 | 74.82 |
| 45 | 850.18 | 74.45 |
| 46 | 793.41 | 72.27 |
| 47 | 743.7 | 50.62 |
| 48 | 700.65 | 64.76 |
| 49 | 617.68 | 75.12 |
| 50 | 596.22 | 71.35 |
| 51 | 579.12 | 76.06 |
| 52 | 539.32 | 79.89 |
| 53 | 1479.15 | 74.75 |
| 54 | 460.64 | 74.28 |
Table 4: IR spectra values of compound 3a.
Table 2; Entry 2 Brown solid
1H NMR (400 MHz, DMSO-d6): δ10.88 (s, 1H), 7.47 (d, J=7.2 Hz, 1H), 7.34 (t, J=8 Hz, 3H), 7.3-7.27 (m, 3H), 7.04 (t, J=7.6, 2H), 6.90– 6.86 (m, 4H), 5.89 (s, 1H). 13C NMR (100 MHz, DMSO-d6): δ145.41, 138.52, 128.73, 127.97, 126.96, 126.72, 124.56, 121.62, 119.82, 118.71, 117.91, 111.68. 34.43 MS (EI): Calculated mass for C23H17N2F is 340.14 found 339.2 (M+1)-. IR (KBr): 3332.5, 3395.25, 1590.81, 1504.55, 1303.16, 1117.24, 1008.77, 753.02 cm-1 (Figures 4 and 5; Tables 5 and 6).
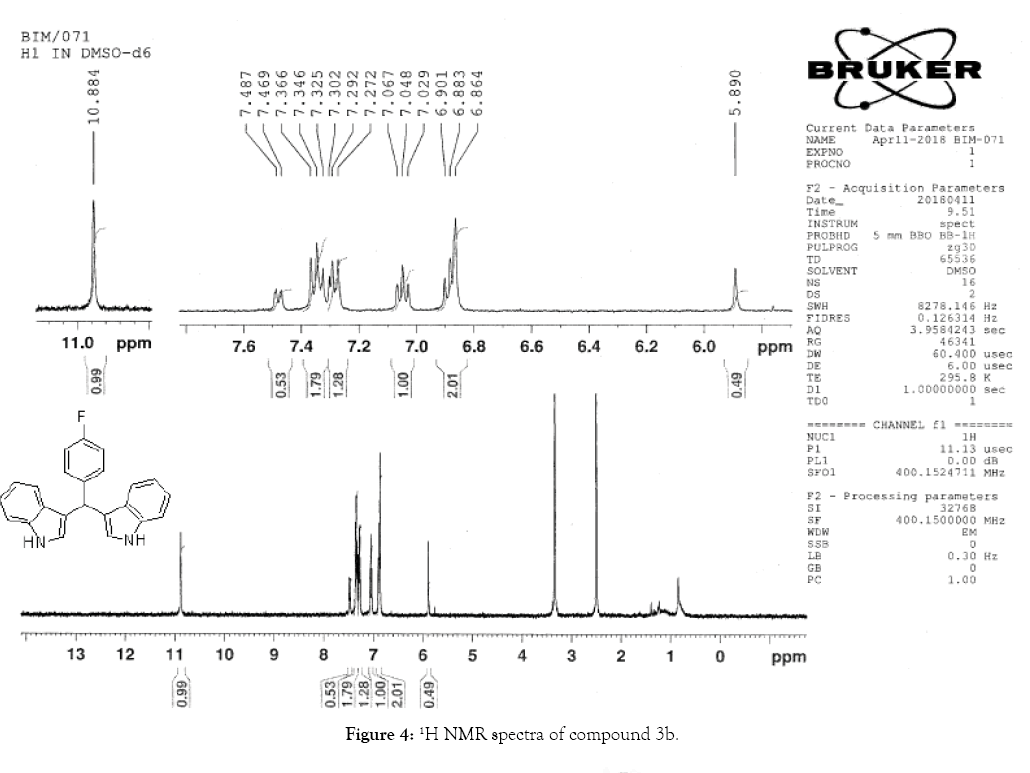
Figure 4: 1H NMR spectra of compound 3b.
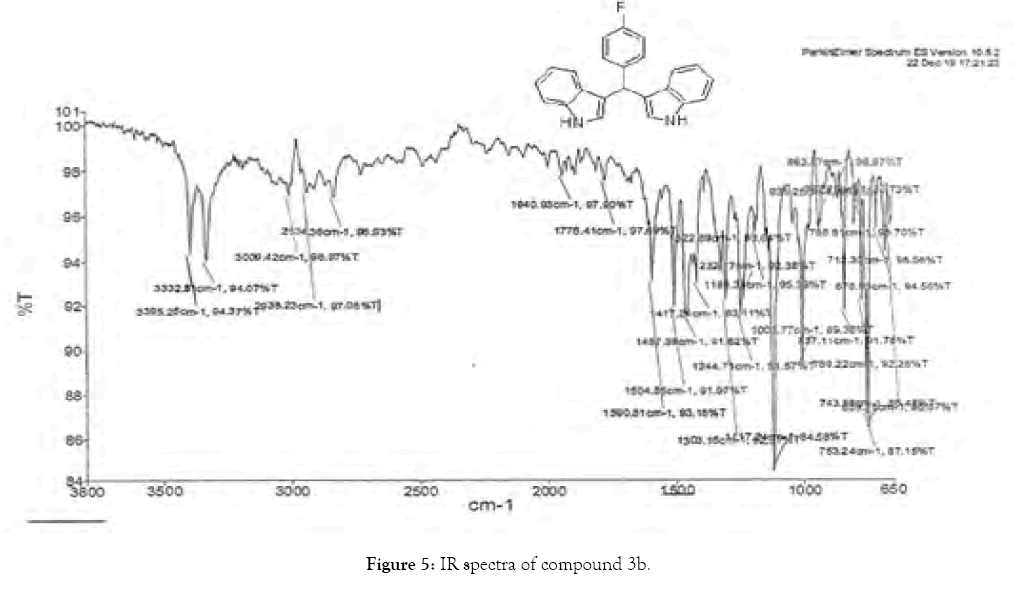
Figure 5: IR spectra of compound 3b.
| Source Spectra Results | |
|---|---|
| Spectrum Name | Number Of Peaks |
| 178 | 29 |
Table 5:IR spectra of compound 3b.
| List of Peak Area/Height | ||
|---|---|---|
| Peak Number | X (cm-1) | Y (%T) |
| 1 | 3395.25 | 94.37 |
| 2 | 3332.51 | 94.07 |
| 3 | 3009.42 | 96.97 |
| 4 | 2938.23 | 97.08 |
| 5 | 2834.36 | 96.93 |
| 6 | 1940.93 | 97.9 |
| 7 | 1776.41 | 97.69 |
| 8 | 1590.81 | 93.18 |
| 9 | 1504.55 | 91.97 |
| 10 | 1457.39 | 91.62 |
| 11 | 1417.21 | 93.11 |
| 12 | 1322.89 | 93.64 |
| 13 | 1303.16 | 92.31 |
| 14 | 1244.71 | 91.57 |
| 15 | 1232.17 | 92.38 |
| 16 | 1188.35 | 95.36 |
| 17 | 1117.24 | 84.58 |
| 18 | 1003.77 | 89.38 |
| 19 | 935.25 | 95.66 |
| 20 | 863.87 | 96.97 |
| 21 | 837.11 | 91.77 |
| 22 | 797.28 | 95.73 |
| 23 | 785.81 | 96.7 |
| 24 | 769.22 | 92.28 |
| 25 | 753.24 | 87.15 |
| 26 | 743.86 | 86.48 |
| 27 | 712.33 | 95.56 |
| 28 | 1676.98 | 194.56 |
| 29 | 659.15 | 95.67 |
Table 6: IR spectra values of compound 3b.
Table 2; Entry 4 brownish solid
1H NMR (400 MHz, DMSO-d6): δ10.78 (s, 2H), 7.33 (dd, J=4.8, 1.2 Hz, 1H), 7.21 (d, J=8 Hz, 2H), 6.95-6.88 (m, 5H), 6.74 (m, 3H), 6.11 (s, 1H), 2.15 (s, 6H). 13C NMR (400 MHz, DMSO-d6): δ149.3, 135.0, 132.0, 127.7, 126.5, 125.1, 124.0, 119.7, 118.4, 118.1, 112.6, 110.4, 34.2 and 11.92. MS (EI): Calculated mass for C23H20N2S is 356.44 found 355.2 (M+1)-. IR (KBr): 3395.95, 3044.94, 1461.21, 1219.80, 985.87, 813.59, 751.06, 710.54, 667.13 cm-1 (Figures 6-8; Tables 7 and 8).
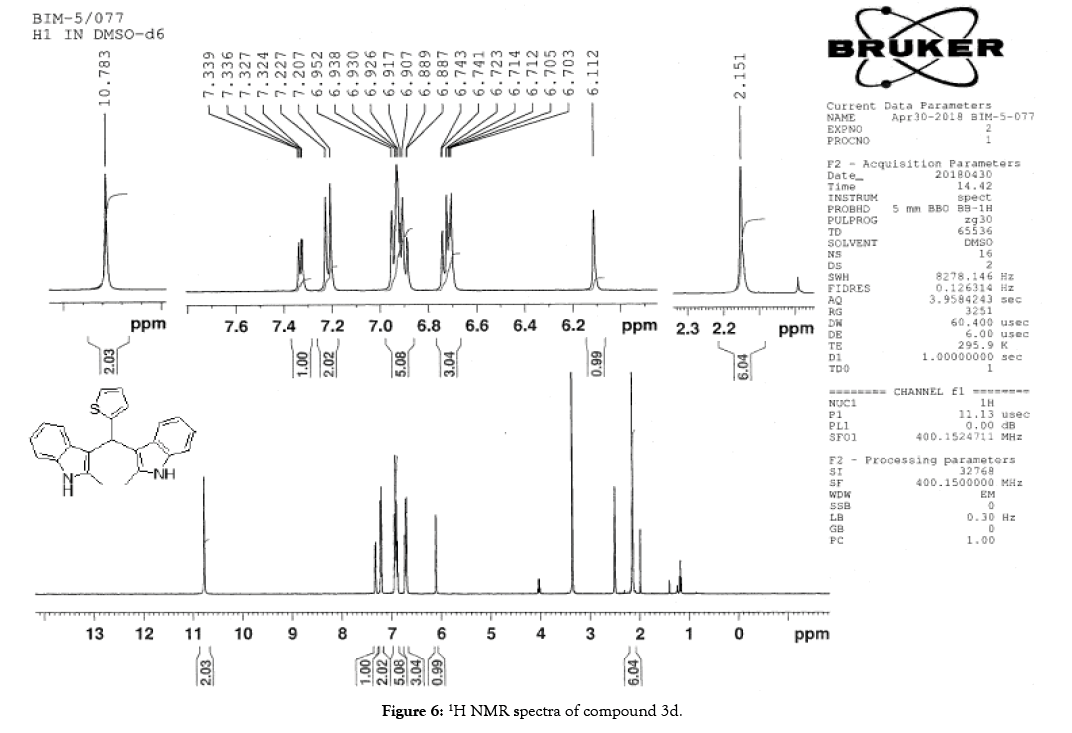
Figure 6: 1H NMR spectra of compound 3d.
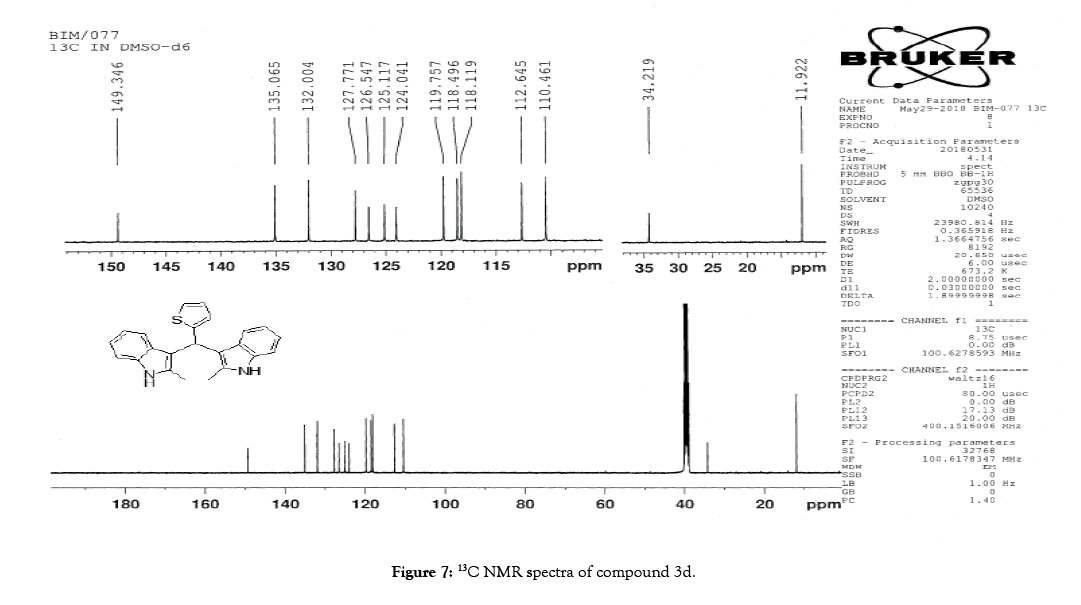
Figure 7: 13C NMR spectra of compound 3d.
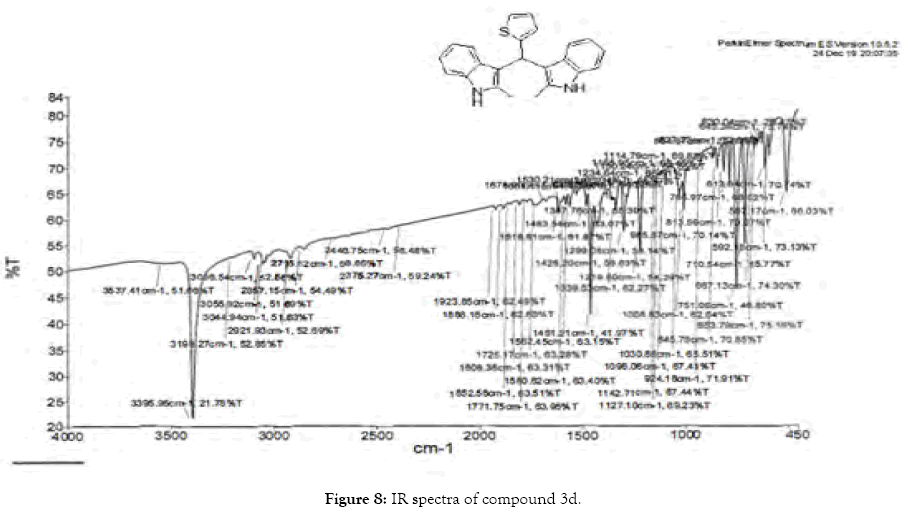
Figure 8: IR spectra of compound 3d.
| Source Spectra Results | |
|---|---|
| Spectrum Name | Number Of Peaks |
| 0183 | 58 |
Table 7: IR spectra results of compound 3d.
| List of Peak Area/Height | ||
|---|---|---|
| Peak Number | X (cm-1) | Y (%T) |
| 1 | 3537.41 | 51.66 |
| 2 | 3395.95 | 21.78 |
| 3 | 3198.27 | 52.85 |
| 4 | 3096.54 | 52.55 |
| 5 | 3055.92 | 51.69 |
| 6 | 3044.94 | 51.83 |
| 7 | 2921.93 | 52.69 |
| 8 | 2857.15 | 54.49 |
| 9 | 2705.82 | 56.65 |
| 10 | 2446.75 | 58.48 |
| 11 | 2375.27 | 59.24 |
| 12 | 1923.85 | 62.49 |
| 13 | 1886.16 | 62.6 |
| 14 | 1852.56 | 63.51 |
| 15 | 1808.38 | 63.31 |
| 16 | 1771.75 | 63.95 |
| 17 | 1725.17 | 63.28 |
| 18 | 1678.9 | 64.07 |
| 19 | 1618.61 | 61.87 |
| 20 | 1601.41 | 63.97 |
| 21 | 1580.62 | 63.4 |
| 22 | 1562.45 | 63.15 |
| 23 | 1530.21 | 65.34 |
| 24 | 1483.54 | 63.07 |
| 25 | 1461.21 | 41.97 |
| 26 | 1428.2 | 59.69 |
| 27 | 1387.76 | 65.39 |
| 28 | 1360.92 | 64.32 |
| 29 | 1350.69 | 64.33 |
| 30 | 1339.53 | 62.27 |
| 31 | 1299.05 | 58.14 |
| 32 | 1281.21 | 64.97 |
| 33 | 1234.64 | 66.61 |
| 34 | 1219.8 | 54.39 |
| 35 | 1165.95 | 68.46 |
| 36 | 1150.24 | 67.85 |
| 37 | 1142.71 | 67.44 |
| 38 | 1127.1 | 69.23 |
| 39 | 1114.79 | 69.83 |
| 40 | 1098.06 | 67.41 |
| 41 | 1030.86 | 65.51 |
| 42 | 11008.83 | 62.64 |
| 43 | 985.87 | 170.14 |
| 44 | 924.18 | 71.91 |
| 45 | 1864.67 | 72.67 |
| 46 | 845.76 | 70.85 |
| 47 | 837.73 | 112.96 |
| 48 | 813.59 | 70.07 |
| 49 | 785.97 | 68.02 |
| 50 | 751.06 | 46.8 |
| 51 | 1710.54 | 155.77 |
| 52 | 667.13 | 74.3 |
| 53 | 653.79 | 75.16 |
| 54 | 1645.34 | 175.74 |
| 55 | 630.04 | 76.47 |
| 56 | 1613.64 | 70.74 |
| 57 | 592.18 | 173.13 |
| 58 | 507.17 | 66.03 |
Table 8: IR spectra values of compound 3d.
Table 2; Entry 5 off white solid
1H NMR (400 MHz, DMSO-d6): δ10.71 (s, 1H), 7.20 (d, J=7.6 Hz, 2H), 6.88 (t, J=7.6 Hz, 4H), 6.70 (t, J=7.6 Hz, 2H), 6.55 (s, 2H), 5.89 (s, 1H), 3.65 (s, 3H), 3.53 (s, 6H), 2.07 (s, 3H). 13C NMR (100 MHz, DMSO-d6): δ152.5, 139.8, 135.9, 134.9, 131.8, 128.2, 119.5, 118.3, 117.8, 112.2, 110.2, 106.5, 60.1 and 55.7. MS (EI): Calculated mass for C28H28N2O3 is 440.53 found 439.2 (M+1)-. IR (KBr): 3406.18, 2853.70, 1493.10, 1455.33, 1215.80, 1038.80, 740.35 cm-1 (Figures 9-12; Tables 9 and 10).
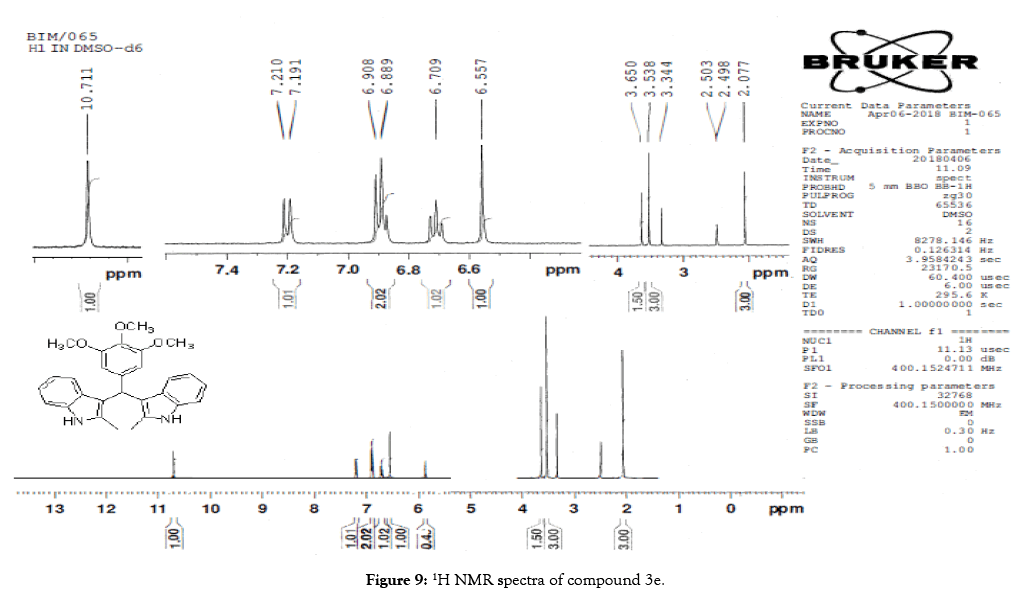
Figure 9: 1H NMR spectra of compound 3e
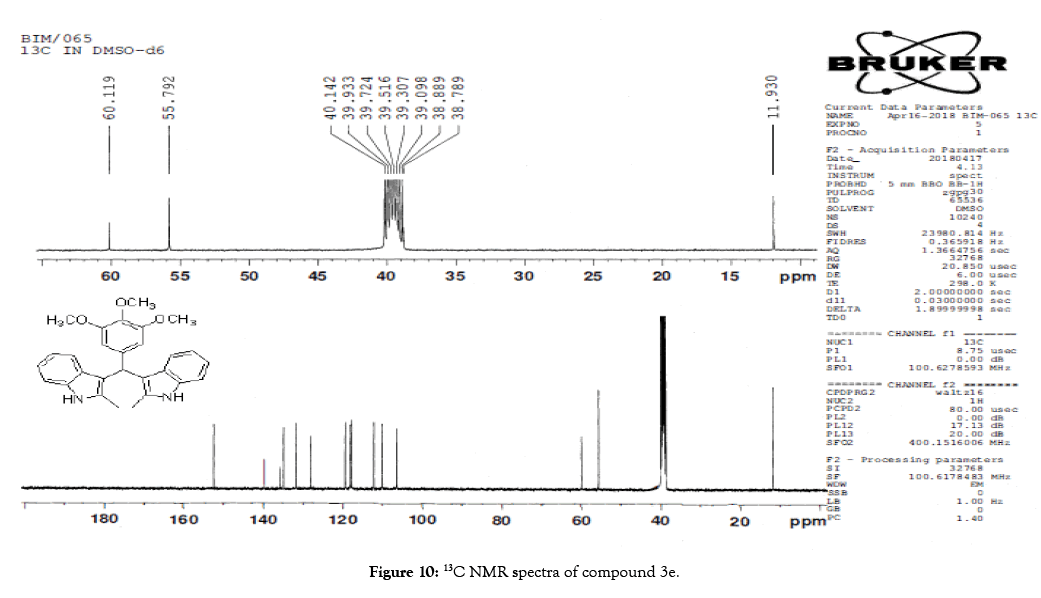
Figure 10: 13C NMR spectra of compound 3e.
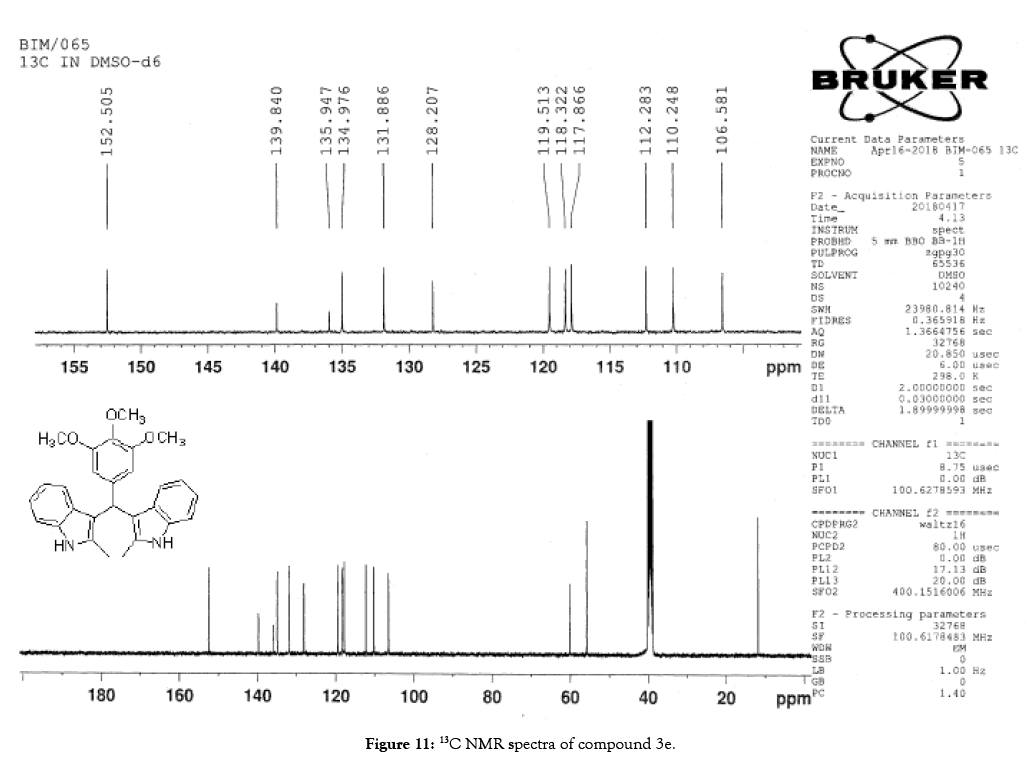
Figure 11: 13C NMR spectra of compound 3e.
Figure 12: IR spectra of compound 3e.
| Source Spectra Results | |
|---|---|
| Spectrum Name | Number Of Peaks |
| 0179 | 25 |
Table 9: IR spectra results of compound 3e.
| List of Peak Area/Height | ||
|---|---|---|
| Peak Number | X (cm-1) | Y (%T) |
| 1 | 3406.18 | 93.87 |
| 2 | 3052.61 | 96.81 |
| 3 | 2924.88 | 92.97 |
| 4 | 2853.7 | 94.69 |
| 5 | 2505.17 | 98.76 |
| 6 | 1892.19 | 98.21 |
| 7 | 1598.21 | 95.63 |
| 8 | 1549.07 | 96.72 |
| 9 | 1493.1 | 85.21 |
| 10 | 1455.33 | 87.62 |
| 11 | 1417.27 | 92.01 |
| 12 | 1337.24 | 91.98 |
| 13 | 1296.34 | 96.31 |
| 14 | 1244.33 | 91.42 |
| 15 | 1215.8 | 91.85 |
| 16 | 1123.14 | 95.85 |
| 17 | 1092.98 | 90.63 |
| 18 | 1057.63 | 91.03 |
| 19 | 1038.8 | 91.9 |
| 20 | 1009.89 | 89.99 |
| 21 | 907.23 | 92.96 |
| 22 | 823.25 | 93.65 |
| 23 | 786.92 | 88.58 |
| 24 | 740.35 | 75.59 |
| 25 | 691.61 | 90.58 |
Table 10: IR spectra values of compound 3e.
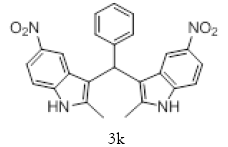
Table 2; Entry 11 yellow Solid;
1H NMR (400 MHz, DMSO-d6): δ10.84 (s, 2H), 7.83 (dd, J=7.6,1.2 Hz, 1H), 7.56-7.54 (m, 1H), 7.49 (t, J=7.6 Hz,1H), 7.26 (d, J=7.6 Hz, 1H), 7.22 (d, J= 7.6 Hz, 2H), 6.92-6.88 (m-2H), 6.74-6.67 (m- 4H), 6.58 (s,1H), 2.03 (s, 6H). 13C NMR (100 MHz, DMSO-d6): δ150.21, 137.88, 135.16, 132.78, 132.48, 130.61, 128.20, 124.63, 120.01, 118.49, 117.83, 110.70, 109.95, 34.05, and 11.70. MS (EI): Calculated mass for C25H20N4O4 is 440.15 found 441.4 (M+1)-. IR: 3454.70, 3392.43, 1527.07, 1458.52, 1300.35, 753.02, 580.39 cm-1 (Figures 13-15; Tables 11 and 12).
Figure 13: 1H NMR spectra of compound 3k.
Figure 14: 13C NMR spectra of compound 3k.
Figure 15: IR spectra of compound 3k.
| Source Spectra Results | |
|---|---|
| Spectrum Name | Number Of Peaks |
| 0184 | 56 |
Table 11: IR spectra results of compound 3k.
| List of Peak Area/Height | ||
|---|---|---|
| Peak Number | X (cm-1) | Y (%T) |
| 1 | 3536.07 | 50.53 |
| 2 | 3454.7 | 37.55 |
| 3 | 3392.43 | 27.54 |
| 4 | 3082.84 | 52.32 |
| 5 | 3056.19 | 51.04 |
| 6 | 2917.82 | 51.98 |
| 7 | 2707.36 | 55.13 |
| 8 | 1975.02 | 60.71 |
| 9 | 1934.58 | 60.74 |
| 10 | 1898.68 | 60.88 |
| 11 | 1785.67 | 62.22 |
| 12 | 1687.68 | 62.89 |
| 13 | 1615.71 | 60.28 |
| 14 | 1603.38 | 60.9 |
| 15 | 1577.19 | 60.56 |
| 16 | 1560.87 | 60.54 |
| 17 | 1551.93 | 59.9 |
| 18 | 1527.07 | 39.81 |
| 19 | 1484.71 | 58.79 |
| 20 | 1458.52 | 41.25 |
| 21 | 1425.86 | 56.55 |
| 22 | 1370.36 | 52.43 |
| 23 | 1322.28 | 62.94 |
| 24 | 1300.35 | 52.74 |
| 25 | 1257.56 | 64.68 |
| 26 | 1244.47 | 60.76 |
| 27 | 1223.09 | 63.14 |
| 28 | 1191.34 | 64.89 |
| 29 | 1160 | 63.76 |
| 30 | 1143.9 | 66.38 |
| 31 | 1134.49 | 66.02 |
| 32 | 1127.02 | 66.23 |
| 33 | 1102.95 | 66.58 |
| 34 | 1093.58 | 65.88 |
| 35 | 1038.27 | 66.29 |
| 36 | 1019.77 | 61.47 |
| 37 | 968.5 | 68.89 |
| 38 | 958.55 | 68.54 |
| 39 | 934.05 | 68.64 |
| 40 | 907.07 | 69.16 |
| 41 | 877.1 | 70.51 |
| 42 | 866.03 | 69.3 |
| 43 | 854.95 | 68.67 |
| 44 | 839.47 | 67.14 |
| 45 | 775.43 | 65.9 |
| 46 | 753.92 | 52.13 |
| 47 | 742.43 | 49.75 |
| 48 | 690.74 | 67.5 |
| 49 | 666.28 | 71.78 |
| 50 | 628.18 | 71.85 |
| 51 | 598.4 | 66.78 |
| 52 | 580.39 | 73.48 |
| 53 | 529.85 | 75.05 |
| 54 | 502.32 | 69.38 |
| 55 | 481.11 | 75.77 |
| 56 | 453.73 | 67.8 |
Table 12: IR spectra values of compound 3k.
Table 2; Entry 12 yellow Solid;
1H NMR (400 MHz, DMSO-d6): δ11.67 (s, 2H), 8.32 (d, J=2 Hz, 2H), 7.97 (dd, J =9.2,9 Hz, 2H), 7.54 (d, J=8.8 Hz, 2H), 7.40 (d, J=7.6 Hz, 2H), 7.32 (t, J= 7.2 Hz, 2H), 7.22 (t, J=7.2 Hz, 1H), 7.14 (d, J=1.6 Hz, 2H), 6.20 (s, 1H). 13C NMR (100 MHz, DMSO-d6): δ143.7, 140.2, 139.8, 129.3, 128.6, 128.5, 128.2, 127.6, 126.4, 125.8, 120.5, 116.7, 116.2 and 112.2. MS (EI): Calculated mass for C23H16N4O4 is 412.40 found 411.40 (M+1)-. IR (KBr): 3306.95, 1623.05, 1513.56, 1325.03, 1088.74 cm-1, 776.55 cm-1, 734.29 cm-1.
We have developed a new efficient, mild synthetic protocol for the preparation of Bis(indolyl)methane by using NiI2 which can be applied to wide range of aldehydes and indoles. Reactions were performed at room temperature. We have demonstrated the complete conversion with 0.5 moles catalyst loading, where us many of the reported literatures used equimolar ratio of Lewis acids for the higher yield. Another important advantage of this reaction is that the entire reaction is taking place in short period of time. Therefore we believe that the present reaction protocol will certainly fulfil the alternate needs to the existing procedure for the synthesis of Bis(indolyl)methanes.
We thank the management of National College, Tiruchirapalli for providing the facility to carry out our research work.
Citation: Ramesh S, Saravanan D (2020) An Efficient Method for the Synthesis of Bis(indolyl)methane Catalyzed by Nickel (II) Iodide. Organic Chem Curr Res. 9: 199. doi:10.35248/2161-0401.19.9.199
Received: 22-Oct-2019 Accepted: 10-Jan-2020 Published: 17-Jan-2020 , DOI: 10.35248/2161-0401.19.9.199
Copyright: © 2020 Ramesh S, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.