Research Article - (2016) Volume 2, Issue 1
An in vitro Investigation of the Antagonistic Effects of Multiple Strains of Lactobacillales on Salmonella Enterica Serovar Choleraesuis
*Corresponding Author: Cheng-Chih Tsai, Department of Food Science and Technology, Hungkuang University, Taichung City 43302, Taiwan, Tel: 886-4-26318652-5082, Fax: 886-4-26319176 Email:
Abstract
Objectives: The aim of this study was to investigate the conditions for viable lactic acid bacteria (LAB) cells and their heat-killed LAB, and the formulation as well as an alternative treatment to antibiotics to reduce infection, invasion and the inhibition of IL-8 secretion induced by S. choleraesuis for HT-29 cells.
Methods: To examined the efficacy of live LAB and heat-killed products in (1) inhibiting Salmonella choleraesuis growth by the plate agar-well diffusion assay, (2) adhering to swine intestinal cells and human intestinal Caco-2 cell line, (3) inhibiting the invasion of HT-29 cell line by Salmonella choleraesuis, and (4) inhibiting Salmonella choleraesuis induced interleukin (IL)-8 secretion by HT-29 cells
Results: The results indicated that viable LAB and heat-killed products significantly reduced S. choleraesuis infection, invasion in the HT-29 cell line, and had the adhesion ability of the LAB to the swine intestinal cells and human intestinal Caco-2 cell line. Both viable and heat-killed LAB powder possess an ability to reduce the S. choleraesuis induced interleukin (IL)-8 secretion by HT-29 cells, and viable bacteria powder products are the most effective.
Conclusion: Both viable and heat-killed LAB powder products were potentially useful for the protection of the epithelial cells against Salmonella infection, invasion and the induced inflammation for swine and human.
Keywords: Salmonella choleraesuis ; Lactic acid bacteria; Heat-killed; Swine; Interleukin-8
Introduction
Salmonella choleraesuis is one of the most common causes of foodborne diseases. S. choleraesuis is most often found in various food items, including meat, egg, and seafood, and can be contracted from having direct contact with infected animals [1]. In Taiwan, S. choleraesuis is the serotype of Salmonella most often isolated from sick pigs and exhibits the highest degree of invasiveness [2]. The use of antibiotics to improve the health of farm animals was widespread; however, the long-term use of antibiotics can lead to an imbalance in beneficial intestinal flora as well as bring about antibiotic-resistant pathogens [2,3]. Due to this, in 2006, the European Union implemented a ban on the use of antibiotics as feed additives and is actively developing the use of probiotics as an alternative to antibiotic treatments [4].
In 1989, Fuller proposed the use of probiotics as a food additive as it is beneficial in balancing the intestinal flora of animals [5,6]. Many researchers have also indicated that the use of probiotics was able to reduce diarrhea in piglets after weaning as well as increase their immune responses and feed conversion rates and reduce the rate of diseases caused by pathogens [7-10]. Probiotic products contain various bacterial species or strains that originate from different genera and are called multiple strains probiotics. Because different bacterial strains have different functions and characteristics, if they are mixed to form a microbial compound, attention must be paid to the compatibility of different strains to achieve a synergistic effect [11,12]. As the literature points out, multiple strains can increase the adhesion ability of Lactobacillales or give rise to numerous antibacterial substances. They can also help to regulate the immune response and reduce pathogenic invasions [13,14].
In recent years, researchers have found that after thermal death, the inhibitory and immunomodulatory effects of Lactobacillales remain unchanged or become even stronger [15,16]. Heat-killed LAB has advantages over viable LAB in that it has a long shelf-life and is therefore easy to store and transport [17]. Fourniat et al. found that after being heated to 100°C-105°C, L. acidophilus could still inhibit the adhesion of Escherichia coli to HeLa cells [18]. Furthermore, thermally killed Bifidobacteria cultured with macrophages showed that these dead bacteria were still able to stimulate an immune response as well as increase the levels of TNF-α and IL-6 [19].
In previous studies, strains of Lactobacillales were isolated from infant feces, pig intestines, and poultry appendices, and the strains that could stimulate macrophages’ ability to generate TNF-α had a high rate of adherence to epithelial colorectal cells (Caco-2) and could suppress S. typhimurium invasion in Caco-2 cells screened by cell-based assays to gain a better understanding of antibiotic resistance commonly found in livestock and clinical treatment of diseases [20]. The observations of the inhibitory effects of multiple strains of Lactobacillales toward S. Typhimurium showed that a combination of four strains (L. acidophlius LAP5, L. plantarum LPL05, L. fermentum LF33, and Enterococcus faecium TM39) could reduce S . typhimurium invasion of mice and broiler chicks [20,21].
In the present study, we explored the inhibitory effects of these four strains on diseases caused by the pathogen S. choleraesuis in pigs. The following tests were conducted to evaluate the ability of a combination of Lactobacillus bacteria to reduce invasion and infection by S. choleraesuis. The antimicrobial activity inhibition zone was investigated using the plate agar-well diffusion method. The absorption assay was conducted using Caco-2 and swine colorectal epithelial cells. The inhibition of S. choleraesuis invasion was tested using the colorectal epithelial cell line HT-29. In vitro tests were performed to assess the inhibition of S. choleraesuis invasion in HT-29 cells and the inhibition of IL-8 secretion induced by S. choleraesuis for HT-29 cells.
Materials and Methods
Bacteria strains, culture medium and growth conditions
This study used four LAB strains including L. acidophlius LAP5, L. plantarum LPL05, L. fermentum LF33, and Enterococcus faecium TM39 selected using their immunomodulatory activity [20]. Each strain was stock cultured and maintained at -80°C in 20 % glycerol. Bacterial cells were propagated twice in Lactobacilli MRS broth (Difco) containing 0.05% L-cysteine, each time for 24 h at 37°C before use. Four live and heat-killed LAB strain powders were manufactured on assignment from Grape King Bio LTD. (Taoyuan City, Taiwan). The LAB cells of each strain were heat-killed at 100°C for 30 min.
S. choleraesuis BCRC10743 (ATCC 13312) was obtained from the Bioresources Collection and Research Center (BCRC), Hsin-Chu, Taiwan. S. choleraesuis SCV26a, SCV28, SCV36 and SCV70 strains, identified by API 20E (bioMerieux, Inc., Hazelwood, MO), were obtained from the Department of Veterinary Medicine, National Ping- Tung University, Ping-Tung, Taiwan. Strains were grown on Tryptic Soy broth (TSB) and stored frozen at -80°C for future transfer.
The LAB inhibition zone on S. choleraesuis growth
The method was performed according to that described by Rammelsberg and Radler [22]. Pathogenic bacteria cells were grown in tryptic soy broth (TSB) overnight, with 100 μl bacterial culture transferred to 5 mL sterility TSB incubated after 2 hrs 15 mins (N×108 CFU per well, N=1~9), and then diluted to N×107 CFU ml-1 and spread onto the tryptic soy agar (TSA). One hundred μl of the mixture from add 1g LAB powder into the 9 ml 1×PBS (N×109 CFU per well) dropped into the well on the TSA and the plates were incubated at 37°C for 12 h. The inhibition zone diameters were then measured.
Similar studies were performed for heat treated (100°C, 15 min) and protease treated supernatants. The LAB was incubated at 37°C for 1 h with α-amylase (200 μg ml-1), catalase (0.5 μg ml-1), chymotrypsin (200 μg ml-1), lactate dehydrogenase (250 μg ml-1), pepsin (200 μg ml-1), proteinase K (1 mg ml-1), or trypsin (200 μg ml-1) to test its sensitivity to protease.
The Caco-2 epithelial cell line culture and adhesion assay
The epithelial-like Caco-2 cell line was obtained from BCRC, Hsin- Chu, Taiwan. Caco-2 cells were grown in Dulbecco’s modified Eagle’s medium (DMEM)(GIBCO) supplemented with 10% (v v-1) fetal bovine serum, 1% non-essential amino acid (NEAA), 0.01 mg ml-1 transferrin and 50 unit ml-1 Penicillin-Streptomycin (GIBCO). The Caco-2 cell line was cultured in 75 cm2 plastic tissue culture flasks (GIBCO). The cells were washed twice with PBS and then transferred (4×104 cells ml-1) with 0.05% trypsin into a 24-well multi-dish containing fresh tissue culture medium without Penicillin- Streptomycin. The mixtures were kept at 37°C in 5 % CO2/ 95% air atmosphere until cell lines formed a monolayer in each well. Prior to the adhesion test all bacterial strains were washed twice with PBS and centrifuged for 5min at 5,000 g. One hundred μl of the mixture from the added 1g LAB powder into the 9 ml 1×PBS were transferred to the 24-well multidish (N×108 CFU per well, N=1~9) and incubated for 2 h at 37°C in 5% CO2. After incubation cells were washed twice with PBS, fixed with 10% formalin for 30 min, washed four times with PBS and then stained with crystal violet for 5 min. The numbers of LAB cells adhered to the cultured cell lines were counted according to the method of Banerjee et al. [23].
LAB adhesion to the intestinal epithelial cells from swine
The procedures described by Annika et al. [24] were used for the preparation of the intestinal epithelial cells from swine. Segments of the ileum were opened, washed with PBS (pH 7.2) and held in PBS at 4°C for 30 min to loosen the surface mucus. The segments were then washed three times with PBS and the epithelial cells scraped off with the edge of a microscope slide. The cells were suspended in PBS and examined microscopically to ensure that the adherent bacteria had been removed. The cell number in the suspension was about 104 cells ml-1.
The adhesion test was performed according to the method of Yu and Tsen [25]. LAB cells in the overnight broth culture were centrifuged and resuspended in PBS to give a cell density of 1×108 CFU ml-1. One hundred μl of the LAB preparation was added to 100 μl of the epithelial cell suspension at 37°C for 30 min. Adhesion was measured by observing crystal violet stained preparations of the bacteria-epithelial cell mixtures with a phase contrast microscope. Positive adhesion was recorded if more than 15 bacterial cells were seen to adhere to one epithelial cell [26].
LAB powder antagonistic effect against S. choleraesuis invasion to HT-29 cells
The antagonistic effect of LAB against cell invasion by S. choleraesuis methods were those described by Kim and Wei [27]. The HT-29 cell line was obtained from BCRC, Hsin-Chu, Taiwan. HT-29 cells were grown in Eagle’s minimal essential medium (EMEM) (GIBCO) supplemented with 10% (v v-1) fetal bovine serum, 1% nonessential amino acid (NEAA), and 50 unit ml-1 Penicillin-Streptomycin (GIBCO). For competitive exclusion assay, HT-29 cell monolayer were cultured and washed as previously described and incubated with or without LAB strains (108 cfu per well) for 1 hr. Afterward one hundred μl of S. choleraesuis (107 cfu ml-1) was added and incubation was continued for a further 2 h at 37°C in a 5% CO2 incubator. The wells were then washed three times with PBS and incubated for another 2 h in fresh EMEM containing 200 μg of gentamicin per ml. After incubation each well was washed with PBS three times and then lysed with 1% Triton X-100. Appropriate dilutions were spread onto Brilliant Green agar and the plates were incubated at 37°C overnight. The colonies that showed the typical morphological characteristics of the target species were counted.
HT-29 cell exposure to LAB powder or to LAB powder and S. choleraesuis simultaneously, determination of IL-8 secretion by ELISA
HT-29 cells were cultured in triplicate at a density of 3 × 105 cells ml-1 of EMEM and 10% FBS without penicillin-streptomycin, in each well of the 24-well tissue culture plates. The mixtures were kept at 37°C in 5% CO2/ 95% air atmosphere until cell lines formed a monolayer in each well. Aliquots of 100 μl of each of these viable or heat-killed single LAB strains or multi-strains LAB were pipetted to stimulate the HT-29 (N×108 CFU per well) with or without 100 μl of S. choleraesuis (N×107 cells ml-1). The HT-29 cells were co-incubated with each of the abovedescribed LAB preparations with or without 100 μl of S. choleraesuis, at 37°C for 6 h. The cell culture medium containing IL-8 was collected and then stored at -80°C until analyzed.
The IL-8 concentrations were assayed using the Biosciences OptEIA™ Set Human IL-8 kit (BD, CA, USA). The assay was performed according to the manufacturer’s specifications. Equivalent levels of IL-8 were calculated by comparison with a reference curve generated using IL-8 standards. The results were expressed as the concentration of the cytokine in serum (pg mL-1).
Statistical analysis
All data are expressed as mean ± SD and subjected to one-way analysis of variance using SPSS 12.0 for Windows (SPSS). Mean treatment group values were compared using one way ANOVA (analysis of variance) and Duncan’s multiple range test and differences were considered statistically significant at P<0.05. The figure was drawn using SigmaPlot 4.0.
Results
Antimicrobial activity inhibition zone test using the plate agar-well diffusion assay
This study used premanufactured Lactobacillus powder dissolved in PBS to investigate its antibacterial activity. Table 1 shows the antimicrobial ability of the viable LAB powder. The LF 33 strain had the best growth inhibition effect against six strains of S. choleraesuis, having the largest inhibition zone of 34 mm. TM39 and LPL5 had the second largest inhibition zones of 29 mm. The inhibitory effect of LAP5 was the weakest, but it had an inhibition zone of 24 mm toward the Scv 70 pathogen strain. All of heat-killed LAB powder lost the antimicrobial ability (zone diameter of inhibition less than 11 mm, data not shown).
| Salmonella Choleraesuisstrains | Inhibition zone (mm)§ | ||||
|---|---|---|---|---|---|
| - | LF 33 | TM 39 | LAP 5 | LPL 5 | Multistrain probiotics |
| SC | 20 (++) | 14 (+) | - | 16 (+) | 16 (+) |
| Scv 26a | 24 (+++) | 19 (++) | 12 (+) | 24 (+++) | 25 (+++) |
| Scv 28 | 18 (++) | 12 (+) | 12 (+) | 14 (+) | 15 (+) |
| Scv 36 | 19 (++) | 16 (+) | - | 15 (+) | 15 (+) |
| Scv 41 | 24 (+++) | 12 (+) | - | 16 (+) | 14 (+) |
| Scv 70 | 34 (+++) | 29 (+++) | 24 (+++) | 29 (+++) | 28 (+++) |
§Interpretation of zone diameter of inhibition. -, less than 11 mm; +, 11-16 mm; ++, 17-22 mm and +++, more than 23 mm
Table 1: Antimicrobial activity of living LAB powdered product against Salmonella choleraesuis.
The influence of enzyme digestion on Lactobacillales products’ antibacterial effects
In this study, we selected LF 33 strain that exhibited the best antibacterial properties in the aforementioned experiment and added various types of proteolytic enzymes to perform hydrolysis as a means of assessing its influence on the probiotics’ inhibitory effects against pathogenic bacteria. As is shown in table 2, many results maintained stability in antibacterial effect between the experimental group and the control group after adding the proteolytic enzyme to LF 33 viable bacteria powder. After adding lactate dehydrogenase (all experimental groups) and catalase (except SC and Scv36), inhibitory effect maintained stability, thereby suggesting that lactic acid and hydrogen peroxide may not play a role in this inhibitory function.
| Enzyme | Zones of inhibition (mm)§ | |||||
|---|---|---|---|---|---|---|
| SC | Scv 26a | Scv 28 | Scv 36 | Sc 41 | Scv 70 | |
| Control | 19 (++) | 25 (+++) | 20 (++) | 17 (++) | 17 (++) | 26 (+++) |
| Pepsin | 19 (++) | 25 (+++) | 17 (++) | 16 (+) | 16 (+) | 24 (+++) |
| Catalase | 16 (+) | 25 (+++) | 20 (++) | 16 (+) | 17 (++) | 23 (+++) |
| Lactate dehydrogenase | 18 (++) | 25 (+++) | 20 (++) | 17 (++) | 17 (++) | 24 (+++) |
| a-amylase | 18 (++) | 25 (+++) | 18 (++) | 17 (++) | 17 (++) | 24 (+++) |
| Trypsin | 18 (++) | 25 (+++) | 19 (++) | 16 (+) | 22 (++) | 23 (+++) |
| Chymotrypsin | 18 (++) | 25 (+++) | 17 (++) | 18 (++) | 20 (++) | 23 (+++) |
| Proteinase K | 18 (++) | 25 (+++) | 19 (++) | 16 (+) | 18 (++) | 23 (+++) |
§The inhibition zones <11 mm, 11–16 mm, 17–22 mm and >23 mm, were classified as strains of no -; mild +; strong ++; and very strong +++ inhibition, respectively.
Table 2: Effect of different enzyme treatments on the antimicrobial activity of probiotics LF33 powdered product against Salmonella choleraesuis.
Adherence assay of probiotic products to intestinal cell lines
In this study, we used Caco-2 cells to test the adhesion of Lactobacillales products. As the literature indicates, each cell can be adhered by 15 or more bacteria. These bacteria are identified as having adhesion ability. As shown in table 3, regardless of whether viable or heat-killed LAB powder was used, all four strains of Lactobacillales had adhesion ability. Among them, viable L. plantarum LPL05 powder had the highest adhesion ability, with each cell being adhered by 35 bacteria on average. The second highest was L. acidophlius LAP5, with each cell being adhered by 24 bacteria on average. The heat-killed LAB powders with the best adhesion ability were L. acidophlius LAP5 and E. faecium TM39, with each cell being adhered by 35 and 26 bacteria on average, respectively. This indicates that both viable and heat-killed LAB powders possess good adhesion ability; thus, protecting the intestinal cells and reducing the potential for pathogenic infection.
| LAB strains | Adherence to the per Caco-2 cell (CFU per cell)§ | |
|---|---|---|
| Viable | Heat-killed | |
| LF 33 | 19.5 ± 9.22ab | 11.4 ± 6.17a |
| TM 39 | 14.5 ± 5.67a | 26.5 ± 9.53b |
| LPL 5 | 35.5 ± 3.03c | 14.0 ± 5.93a |
| LAP 5 | 24.8 ± 7.50b | 35.9 ± 3.98c |
| §Adhesion assays were monitored after 2 h of incubation. Ten of the Caco-2 cells were used to calculate the average number of the adhering LAB cells per epithelial cell. a, b, c Values in the same column with different superscripts mean significant difference (P<0.05). | ||
Table 3: Adhesion of various lactic acid bacteria powdered product onto the Caco-2 cell line.
Adhesion assay of probiotic products to pig intestinal primary epithelial cells
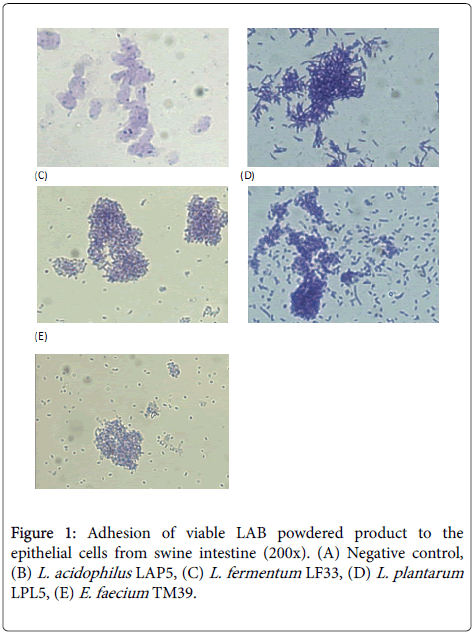
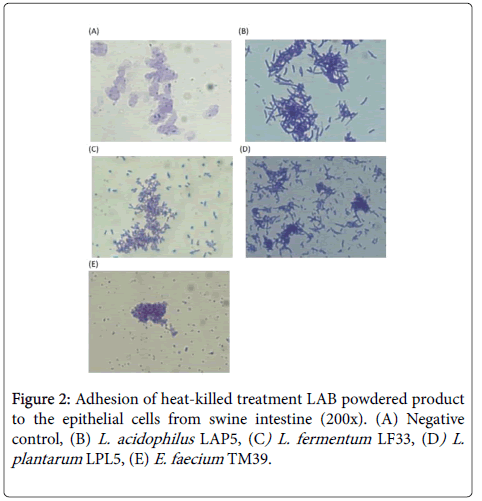
In vitro adhesion assays of probiotics have been widely used to screen for those that potentially have good intestinal adhesion in hosts and resistance to inflammation induced by pathogens. The most commonly used human intestinal epithelial cell lines are Caco-2, HT-29, and T84. Many researchers look for cells from hosts of their main research, such as piglet intestinal epithelial cells, IPEC-J2, to better evaluate the studies on inhibiting pathogens [28]. To understand which probiotic products have effective adhesion in the pig intestines, in the present study, we used pig intestinal primary cells for in vitro testing. The results in Figures 1 and 2 shows that four strains of manufactured viable and heat-killed LAB powder have good adhesion to swine intestinal cells. These findings should be further assessed for use in animal feed additives and for their efficacy in animal growth and inhibition of pathogens.
Test of the inhibition of S. choleraesuis invasion by probiotics
When Salmonella enters the body, it survives on the epithelial cells of the intestinal wall and is adhered to the host cell; thus, having an invasive ability. When the cytoskeleton is damaged and the protective barrier of the intestine is breached, Salmonella can proliferate in the gut and then spread infection throughout the body [29]. In this experiment, we used intestinal epithelial cells (HT-29) to test whether probiotic products can inhibit pathogens isolated from infected pigs. As seen in table 4, the number of SC 41, Scv 26a, Scv 28, and Scv 36 bacteria strains that invaded HT-29 cells was approximately 4.9-5.9 log CFU mL-1. When the invasion experiment was performed with the addition of viable or heat-killed LAB powder, there was a significant reduction in S. choleraesuis invasion. As can be seen from this, both viable and heat-killed LAB powder can inhibit pathogenic invasion in cells.
| Salmonella Choleraesuisstrains | Bacterial counts (log CFU mL-1) | ||
|---|---|---|---|
| Only Salmonella Choleraesuis | Live multistrain LAB powdered +Salmonella Choleraesuis | Heat-killed multistrain LAB powdered +Salmonella Choleraesuis | |
| ATCC 13312 (SC 41) | 4.97 ± 0.47 | <1 | <1 |
| SC | <1 | <1 | <1 |
| Scv 26a | 4.98 ± 0.47 | <1 | <1 |
| Scv 28 | 5.94 ± 0.46 | <1 | <1 |
| Scv 36 | 5.89 ± 0.38 | <1 | <1 |
| Scv 70 | <1 | <1 | <1 |
Table 4: Effect of multi-LAB powdered product on the Salmonella choleraesuis invasion to the HT-29 cell line.
The inhibitory effects of probiotics on IL-8 secretion in HT-29 cells induced by S. Choleraesuis
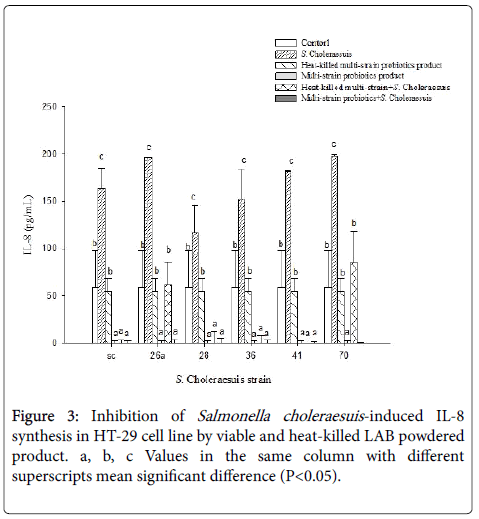
As indicated in the literature, S. choleraesuis can induce IL-8 secretion in pig intestinal cells [30]. Our study used HT-29 cell line to test whether Lactobacillales can reduce IL-8 secretion caused by S. choleraesuis by adding a mixture of four strains of viable or heat-killed LAB. As seen in Figure 3, IL-8 secretion was significantly higher in the group with six strains of Salmonella than that in the control group and the Lactobacillales group (P < 0.05).
Discussion
Probiotics protect the host from pathogenic infection. Beside its immunoregulatory effect, competitive absorption, improvement of barrier function, and secretion of antibacterial substances are also the conditions for screening probiotics. The antimicrobial metabolites of Lactobacillales can be divided into two categories: (a) low-molecular weight compounds (<1000 Da), such as organic acids, which normally exist in an undissociated form, exercize antimicrobial activity by penetrating microbial cells and reducing the internal pH and (b) antimicrobial proteins called bacteriocins (>1000 Da) synthesize peptides for ribosomes or protein complexes and can bind to the inner membrane of microorganisms to cause their death. They also have an application in the food industry as a natural preservative [31,32].
Conconnier et al. demonstrated that the supernatant of L. acidophilus cultures could inhibit the pathogens S. typhimurium , E. coli and Shigella flexneri. After adding trypsin or proteinase K to the supernatant, there was a slight decrease in the inhibitory effect [33]. The literature suggests that the bacteriocins (nisin Z and nisin A) secreted by Lactococcus lactis have a tolerance toward pepsin but sensitivity toward the proteolytic enzymes trypsin, papain, α- chymotrypsin, and pancreatin [34]. In another study, Bernet-Camard et al. investigated the inhibitory effects of the supernatant of L. acidophilus against S. typhimurium through in vitro experiments. Their results showed that L. acidophilus can inhibit the invasion of Caco-2 cells. Additionally, after adding pronase, trypsin, proteinase K, or pepsin to the supernatant for hydrolysis, the inhibitory effect remained unchanged, suggesting that the antimicrobial substance is not affected by proteases [35]. In this study, we added proteolytic enzymes, such as pepsin, trysin, chymotrypsin, or proteinase K, to the LF33 and our results were similar to the study mentioned above. Therefore, we speculate that the antibacterial components that participate in this antibacterial effect may be resistant to proteolytic enzymes, but the exact underlying antimicrobial mechanism requires further investigation.
The epithelial cells of the digestive tract provide a natural barrier against invasion by foreign objects. When a bacterial strain enters the body and reaches the intestine, to avoid being discharged from the body through peristalsis, it is adhered to the epithelial cells of the intestinal walls, thereby maintaining the balance of intestinal flora and reducing the invasion and adhesion of pathogens. It can also promote the host’s immune functions. Thus, this adhesion ability could be used to screen for probiotic strains that are beneficial to hosts [36].
Current studies have indicated that the mechanisms and related factors driving the adhesion of Lactobacillales to the epithelial cells include hydrophobic interaction, lipoteichoic acids (LTA), and exopolysaccharides [37,38]. Granato et al. found that the surface of L. johnsonii cell walls contains LTA, which is also present in the supernatant of the Lactobacillales culture, and that if LTA is isolated or purified, it can inhibit cell adhesion in a dose-dependent manner [37]. Exopolysaccharides usually are attached to the cell surface or secreted into the extracellular culture medium. Sun et al. reported that after purifying exopolysaccharides from the cell surface of L. plantarum, there was a remarkable decrease in adhesion. Furthermore, they also found that trypsinization had an effect on the adhesion of Lactobacillales. Thus, it was observed that proteins play a role in the adhesion of L. plantarum [38].
Ishikawa et al. used HeLa cells and 5–6-week-old mice to test the ability of thermally killed L. plantarum bacteria to inhibit S. enterica serovar Typhimurium infection both in vitro and in vivo. The results showed that thermally killed Lactobacillale s can inhibit S. typhimurium invasion and adhesion in HeLa cells; thus, reducing Salmonella infection in mice [39]. The present study verified that both viable and heat-killed LAB has good adhesion ability through the adhesion assay. This may indicate that Lactobacillales can be used to competitively inhibit the adhesion of pathogens, thereby reducing pathogenic invasion and infection of host cells.
The literature confirms that HT-29 cells produce IL-8 and are the most commonly used cells to detect cytokines and inflammatory bowel disease [40]. We found similar results in our research. In the Lactobacillales group with a mixture of four strains, although there was no notable reduction in IL-8 production from the cells themselves, there was a remarkable reduction in IL-8 produced by HT-29 cells that were stimulated by the six strains of Salmonella (P < 0.05). Furthermore, in the group with four strains of viable Lactobacillales powder, a significant reduction was seen in IL-8 production regardless of whether it was reduced in cells themselves or inhibited in intestinal epithelial cells stimulated by the six strains of S . choleraesuis compared with the heat-killed LAB group. This shows that both dead and viable bacteria powder products possess an ability to slow the pathogeninduced inflammatory response, and of these, viable LAB powder products are the most effective.
It is known that IL-8 is a trigger for the innate immune response. Its purpose is to attack and eliminate pathogens, and it can attract neutrophils to the site of inflammation to promote the inflammatory response. With continued production of proinflammatory factors, neutrophils continue to infiltrate into tissues, leading to epithelial cell damage [41,42]. Candela et al. reported that B. longum Bar33 and L. acidophilus Bar13 can reduce IL-8 production in HT-29 cells induced by LPS in a concentration-dependent manner. Both strains of Lactobacillales could competitively eliminate pathogen adhesion to Caco-2 cells. It was also found that HT-29 and LPS cultured with a proportionate combination of the two strains of probiotics reduced the concentration of IL-8 more than one Lactobacillales strain alone [43].
Conclusion
The results presented in this study show that both viable and heatkilled LAB powders possess good adhesion ability to human and swine epithelial cells, protecting S. choleraesuis invasion into the intestinal cells and reducing the potential for pathogenic infection. Both heatkilled and viable multi-strain LAB powder products possess the ability to reduce S. choleraesuis induced IL-8 cytotoxicity. These heat-killed or viable multi-strain LABs may have potential for use as effective feed supplements to reduce S. choleraesuis infection and the induced inflammation of hosts.
Acknowledgements
This study was supported by the MOST 101-2324-B-241-002 project from Ministry of Science and Technology, R.O.C.
References
- Foley SL, Lynne AM, Nayak R (2008) Salmonellachallenges: prevalence in swine and poultry and potential pathogenicity of such isolates.J AnimSci 86: E149-162.
- Chiu CH, Su LH, Chu C (2004) Salmonella enterica serotype Choleraesuis: epidemiology, pathogenesis, clinical disease, and treatment.ClinMicrobiol Rev 17: 311-322.
- Ross GR, Gusils C, Oliszewski R, de Holgado SC, González SN (2010) Effects of probiotic administration in swine.J BiosciBioeng 109: 545-549.
- Castanon JI (2007) History of the use of antibiotic as growth promoters in European poultry feeds.PoultSci 86: 2466-2471.
- Fuller R (1989) Probiotics in man and animals.J ApplBacteriol 66: 365-378.
- Manson JE, Tosteson H, Ridker PM, Satterfield S, Hebert P, et al. (1992) The primary prevention of myocardial infarction.N Engl J Med 326: 1406-1416.
- Casey PG, Gardiner GE, Casey G, Bradshaw B, Lawlor PG, Lynch PB, et al. (2007) A five-strain probiotic combination reduces pathogen shedding and alleviates disease signs in pigs challenged with Salmonella entericaserovarTyphimurium. Appl Environ Microbiol 73: 1858-1863.
- Huang CH, Qiao SY, Li D, Piao XS, Ren J (2004) Effects of Lactobacilli on the performance, diarrhea incidence, VFA concentration and gastrointestinal microbial flora of weaning pigs. Asian-Australas J AnimSci 17: 401-409.
- Scharek L, Altherr BJ, Tölke C, Schmidt MF (2007) Influence of the probioticBacillus cereus var. toyoi on the intestinal immunity of piglets.Vet ImmunolImmunopathol 120: 136-147.
- Yu HF, Wang AN, Li XJ, Qiao SY (2008) Effect of viable Lactobacillus fermentum on the growth performance, nutrient digestibility and immunity of weaned pigs. J Anim Feed Sci 17: 61-69.
- Timmerman HM, Koning CJ, Mulder L, Rombouts FM, Beynen AC (2004) Monostrain, multistrain and multispecies probiotics--A comparison of functionality and efficacy.Int J Food Microbiol 96: 219-233.
- Timmerman HM, Niers LE, Ridwan BU, Koning CJ, Mulder L, et al. (2007) Design of a multispecies probiotic mixture to prevent infectious complications in critically ill patients.ClinNutr 26: 450-459.
- Ouwehand AC, Isolauri E, Kirjavainen PV, Tölkko S, Salminen SJ (2000) The mucus binding of Bifidobacteriumlactis Bb12 is enhanced in the presence ofLactobacillus GG and Lact. delbrueckii subsp. bulgaricus.LettApplMicrobiol 30: 10-13.
- Viljanen M, Pohjavuori E, Haahtela T, Korpela R, Kuitunen M, et al. (2005) Induction of inflammation as a possible mechanism of probiotic effect in atopic eczema-dermatitis syndrome.J Allergy ClinImmunol 115: 1254-1259.
- Kobayashi N, Saito T, Uematsu T, Kishi K, Toba M, Kohda N, Suzuki T (2011) Oral administration of heat-killed Lactobacillus pentosus strain b240 augments protectionagainst influenza virus infection in mice. IntImmunopharmacol 11: 199-203.
- Kim MS, Yoon YS, Seo JG, Lee HG, Chung MJ, et al. (2013) A study on the prevention of salmonella infection by using the aggregation characteristics of lactic Acid bacteria.Toxicol Res 29: 129-135.
- Ou CC, Lin SL, Tsai JJ, Lin MY (2011) Heat-killed lactic acid bacteria enhance immunomodulatory potential by skewing the immune response toward Th1 polarization.J Food Sci 76: M260-267.
- Fourniat J, Colomban C, Linxe C, Karam D (1992) Heat-killed Lactobacillus acidophilus inhibits adhesion of Escherichia coli B41 to HeLa cells.Ann Rech Vet 23: 361-370.
- Marin ML, Lee JH, Murtha J, Ustunol Z, Pestka JJ (1997) Differential cytokine production in clonal macrophage and T-cell lines cultured with bifidobacteria.J Dairy Sci 80: 2713-2720.
- Tsai CC, Liang HW, Yu B, Hsieh CC, Hwang CF, Chen MH, Tsen HY (2011) The relative effcacy of different strain combinations of lactic acidbacteria in the reduction of populations of Salmonella entericaTyphimuriumin the livers and spleens of mice. FEMS Immunol Med Microbiol 63: 44-53.
- Chen CY, Tsen HY, Lin CL, Yu B, Chen CS (2012) Oral administration of a combination of select lactic acid bacteria strains to reduce the Salmonella invasion and inflammation of broiler chicks.PoultSci 91: 2139-2147.
- Rammelsberg M, Radler F (1990) Antibacterial polypeptides of Lactobacillusspecies. J ApplBacteriol 69: 177-184.
- Banerjee P, Merkel GJ, Bhunia AK (2009) Lactobacillus delbrueckii ssp. bulgaricus B-30892 can inhibit cytotoxic effects and adhesion of pathogenic Clostridium difficile to Caco-2 cells.Gut Pathog 1: 8.
- Mäyrä-Mäkinen A, Manninen M, Gyllenberg H (1983) The adherence of lactic acid bacteria to the columnar epithelial cells of pigs and calves.J ApplBacteriol 55: 241-245.
- Yu B, Tsen HY (1993)Lactobacillus cells in the rabbit digestive tract and the factors affecting their distribution.J ApplBacteriol 75: 269-275.
- Pedersen K, Tannock GW (1989) Colonization of the porcine gastrointestinal tract by lactobacilli.Appl Environ Microbiol 55: 279-283.
- Kim SH, Wei CI (2007) Invasiveness and intracellular growth of multidrug-resistant salmonella and other pathogens in Caco-2 cells.J Food Sci 72: M72-78.
- Brosnahan AJ, Brown DR (2012) Porcine IPEC-J2 intestinal epithelial cells in microbiological investigations.Vet Microbiol 156: 229-237.
- Gagnon M, ZihlerBerner A, Chervet N, Chassard C, Lacroix C (2013) Comparison of the Caco-2, HT-29 and the mucus-secreting HT29-MTX intestinal cell models to investigateSalmonella adhesion and invasion.J Microbiol Methods 94: 274-279.
- Cho WS, Chae C (2003) Expression of inflammatory cytokines (TNF-alpha, IL-1, IL-6 and IL-8) in colon of pigs naturally infected with Salmonella typhimuriumand S. choleraesuis.J Vet Med A PhysiolPatholClin Med 50: 484-487.
- Collado MC, Gueimonde M, Salminen S (2010) Chapter 23 - Probiotics in adhesion of pathogens: mechanisms of action. In: Bioactive Foods in Promoting Health, R.R. Watson, and VR Preedy(edn.), Boston Academic Press, pp. 353-370.
- García P, Rodríguez L, Rodríguez A, Martínez B (2010) Food biopreservation: promising strategies using bacteriocins, bacteriophages and endolysins. Trends Food Sc Tech 21:373-382.
- Coconnier MH, Lievin V, Bernet-Camard MF, Hudault S, Servin AL (1997) Antibacterial effect of the adhering human Lactobacillus acidophilus strain LB. Antimicrob Agents Chemother 41: 1046-1052.
- Matsusaki H, Sonomoto K, Ishizaki A (1998) Some characteristics of Nisin Z, a peptide antibiotic produced by Lactoctococcuslactis IO-1. Food SciTechnolInt 4: 290-294.
- Bernet-Camard MF, Liévin V, Brassart D, Neeser JR, Servin AL, et al. (1997) The human Lactobacillusacidophilus strain LA1 secretes a nonbacteriocin antibacterial substance(s) active in vitro andin vivo.Appl Environ Microbiol 63: 2747-2753.
- Li XJ, Yue LY, Guan XF, Qiao SY (2008) The adhesion of putative probiotic lactobacillito cultured epithelial cells and porcine intestinal mucus.J ApplMicrobiol 104: 1082-1091.
- Granato D, Perotti F, Masserey I, Rouvet M, Golliard M, et al. (1999) Cell surface-associated lipoteichoic acid acts as an adhesion factor for attachment of Lactobacillus johnsonii La1 to human enterocyte-like Caco-2 cells.Appl Environ Microbiol 65: 1071-1077.
- Sun J, Le GW, Shi YH, Su GW (2007) Factors involved in binding of Lactobacillus plantarum Lp6 to rat small intestinal mucus.LettApplMicrobiol 44: 79-85.
- Ishikawa H, Kutsukake E, Fukui T, Sato I, Shirai T, Kurihara T, et al. (2010) Oral Administration of heat-killedLactobacillus plantarum strain b240 protected mice against Salmonella entericaserovarTyphimurium. BiosciBiotechnolBiochem 74: 1338-1342.
- Verhagen AM, Pakusch M, Silke J, Vaux DL (2001) TNF and CD95 promote IL-8 gene transactivation via independent elements in colon carcinoma cells.Cytokine 15: 108-112.
- Burkey TE, Skjolaas KA, Dritz SS, Minton JE (2007) Expression of Toll-like receptors, interleukin 8, macrophage migration inhibitory factor, and osteopontin in tissues from pigs challenged withSalmonella entericaserovarTyphimurium or serovarCholeraesuis.Vet ImmunolImmunopathol 115: 309-319.
- Malago JJ, Tooten PC, Koninkx JF (2010) Anti-inflammatory properties of probiotic bacteria on Salmonella-induced IL-8 synthesis in enterocyte-like Caco-2 cells.Benef Microbes 1: 121-130.
- Candela M, Perna F, Carnevali P, Vitali B, Ciati R, et al. (2008) Interaction of probiotic Lactobacillus and Bifidobacterium strains with human intestinal epithelial cells: adhesion properties, competition against enteropathogens and modulation of IL-8 production. Int J Food Microbiol 125: 286-292.
Copyright: © 2016 Tsai CC, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.