
Journal of Clinical and Cellular Immunology
Open Access
ISSN: 2155-9899

ISSN: 2155-9899
Commentary - (2021)Volume 12, Issue 1
Clear cell renal cell carcinoma (RCC) is the most common form of kidney cancer and is known to be an immunogenic neoplasm with a consistent T-cell infiltrate. Immune checkpoint blockade (ICB) is a first line therapy for advanced RCC, however complete responses to ICB remain rare [1]. In order to advance immunotherapy for RCC, including ICB and adoptive cell therapies, an in vitro model for studying autologous T-cell and tumor cell interactions using fresh tumor tissue is needed. Recently, we have reported a novel method for consistently expanding tumor infiltrating lymphocytes (TIL) from RCC [2]. Prior to this, the prospect of TIL therapy for RCC had largely been abandoned since the termination of an international Phase III clinical trial in the 1990s due to the high failure rate of TIL production from RCC [3]. The high success rate and optimal phenotype of the TIL product created after adherent cell depletion has renewed the prospect of adoptive cell transfer of tumor infiltrating lymphocytes for RCC.
An overlooked aspect of this method which has significant basic and translational science implications is that not only do we expand TILs with this method, but as a byproduct of adherent cell depletion the consistent creation of autologous, primary adherent tumor cell lines was also accomplished. In fact, this method originated from attempts to create both TIL and tumor cell lines from the small tumor specimens which are often all that is obtained for research purposes; it was then anecdotally noted that there was a high success rate and phenotypes appeared different when using this method. As a result, we designed a prospective study to compare methods of TIL expansion from RCC [2]. In this published study, the success rate for generating primary adherent tumor cell cultures paralleled that of the TIL culture success rate (>90%). Primary tumor cells were routinely able to be cultured for a minimum of 3 to 4 passages and multiple cell lines were grown for 10 to 20 passages and presumed to be spontaneously immortalized for in vitro culture. Critically, these primary tumor cell cultures were shown to bear driver VHL mutations identical to the primary tumor as well as other common RCC mutations. However, at progressively higher passages it became clear that tumor heterogeneity was lost, and a dominant clonal population of tumor cells grew out as shown by all mutant allele frequencies approaching 100%.
The consistent generation of autologous, low passage tumor and TIL cell lines from surgical samples creates an in vitro model, as shown in Figure 1, with increased clinical relevance relative to using immortalized tumor cell lines and allogeneic T-cells. This autologous model allows for direct tumor cell/TIL co-culture assays which could be used to screen the effects of checkpoint inhibition, co-stimulation augmentation, small molecule signaling modulators and other methods of tumor and/or T-cell modification. The model may also help decipher the complex interplay between tumor cells and immune cells within the tumor microenvironment (TME). By creating separate, autologous cultures, the phenotype and function of each can be evaluated independently and in response to soluble factors or extracellular vesicles (EVs) which are secreted by the opposing primary culture through various conditioned media and/or purified EV supplementation experiments.
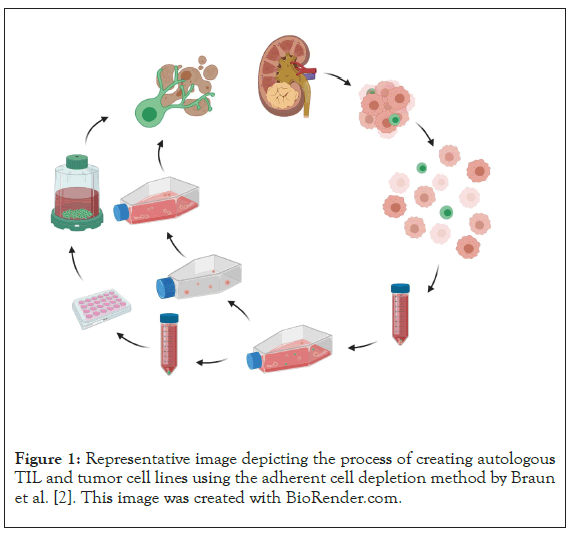
Figure 1: Representative image depicting the process of creating autologous TIL and tumor cell lines using the adherent cell depletion method by Braun et al. [2]. This image was created with BioRender.com.
By obtaining peripheral blood from RCC patients prior to surgery an additional tangent of in vitro experimentation becomes possible. T-cell receptors expressed by dysfunctional/differentiated TILs can be identified and transduced into less differentiated/multifunctional peripheral blood T-cells–creating a gene modified autologous TCR cell product. The screening of chimeric antigen receptor (CAR) T-cell constructs against the patient’s own tumor cells also becomes possible–once again creating a relevant preclinical model.
All of these are ongoing areas of investigation using this model and we hope that the detailed description of our method is utilized by others to help advance immunotherapy for patients with renal cell carcinoma.
Citation: Braun MW, Godwin AK (2021) An in vitro Model for Evaluating Autologous T-cell and Tumor Cell Interactions in Renal Cell Carcinoma. J Clin Cell Immunol. 12:609.
Received: 14-Jan-2021 Accepted: 28-Jan-2021 Published: 04-Feb-2021 , DOI: 10.35248/2155-9899.21.12.609
Copyright: © 2021 Braun MW, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.