Journal of Hepatology and Gastrointestinal disorders
Open Access
ISSN: 2475-3181
ISSN: 2475-3181
Review Article - (2022)Volume 8, Issue 6
Non-alcoholic Fatty Liver Disease (NAFLD) is believed to be amongst the maximum prevalent chronic liver disease globally secondary to pacey escalating obesity, Type 2 Diabetes Mellitus (T2DM), Metabolic Syndrome (MetS). In the form of a hepatic presentation of a metabolic disease NAFLD initiates with hepatic accrual along with propagationn towards hepatic inflammation; namely, Non-Alcoholic Steatohepatitis (NASH), hepatic fibrosis/ cirrhosis, with ultimate NAFLD associated Hepatocellular Carcinoma (NAFLD-HCC). Enhanced corroborate illustrated that gut microbiome possess an essential part in initiating besides propagatenag NAFLD via gut liver axis. The gut liver axis represents the reciprocal connection amongst gut as well as the liver constituted by the portal circulation, Bile Acids along with systemic circulation. This gut symbiosis aids in the generation of NAFLD by resulting in deregulation of gut - liver axis, causing escalated Intestinal permeability besides uncontrolled microbial metabolites transportation in the liver. Hence here we conducted a Thus here we conducted a systematic review utilizing search engine PubMed, Google scholar and others utilizing the MeSH terms like NAFLD; NASH; NAFLD–HCC; gut-liver axis; gut symbiosis Intestinal permeability; Bile Acids circulation ; portal circulation probiotics.; prebiotics; MT; individuals therapy treatment strategies, to update our earlier work from 2008 to 2022 till date. We found a total of 300 articles out of which we selected 103 articles for this update review. No meta-analysis was done. Thus we have described step by step the knowledge regarding gut micro biome symbiosis along with metabolomic alterations. Amongst steatosis NASH fibrosis along with NAFLD–HCC. Furthermore the different treatment strategies inclusive of probiotics. Prebiotics; FMT; individuals therapy treatment strategies, are detailed sides escalating Akkermansia muciniphilia amounts.
NAFLD; Gut-liver axis; Gut symbiosis; Portal circulation; Bile acids; Intestinal permeability; Probiotics; Prebiotics; FMT
Non-alcoholic Fatty Liver Disease (NAFLD) possesses the properties of lipid accrual in greater than 5% of the hepatocytes [1]. It represents an event that keeps propagateng in a continuous manner from Non-alcoholic fatty liver (alias simple steatosis) to Non-Alcoholic Steatohepatitis (NASH), along with ultimately towards NAFLD associated cirrhosis and Hepatocellular Carcinoma (HCC) [2]. NAFLD has assumed to be the most frequent etiological factor of chronic liver disease globally impacting 15%-40% of the general population [3]. This Prevalence of NAFLD might escalate to as high as 90% in cases of patients with obesity. NAFLD is believed to be the hepatic constituent of Metabolic Syndrome (MetS) besides being intricately correlated with metabolic disease like obesity, Type 2 Diabetes mellitus (T2DM) along with atherosclerosis [4,5]. Dependent on these characteristics, an International, expert consensus statement pointed that utilization of a new definition should be made regarding metabolic (impairment) correlated with fatty liver disease (namely MAFLD) [6]. The diagnostic criteria advocated were the existence of hepatic steatosis as well as obesity, T2DM or metabolic impairment [7]. Nevertheless, no consensus reached regarding these criteria thought to be not relevant regarding clinical scenario since certain no obese no diabetic patients with hepatic steatosis we’re not diagnosed in view of absence of laboratory investigations for metabolic impairment [8].
Environmental besides nutritional factors might contribute to the pathogenesis of NAFLD apart from its propagation to NASH, of which both might aid in the initiation of NAFLD besides its propagation to NASH, along with NAFLD HCC. Of the environmental factors Gut Micro biome symbiosis is the key factor believed to be implicated in the formation of NAFLD. The Gut Micro biota (GM) is believed to be an essential, organ that crosstalk’s with the host cells regarding metabolism [9]. The stability of the, community of GM is necessary for the sustenance of the homeostasis of body metabolism. That GM were responsible for controlling energy gleaning besides energy storage from dietary resources was illustrated in a study conducted in 2004 [10]. This represented an early study evaluating the implication of GM regarding control of host metabolism. Subsequently accumulating proof has demonstrated the key action of GM in the sustenance of host metabolism [11]. Hence symbiosis of this GM community possesses the capacity of directly/indirectly impacting host metabolism. Till date abnormalities of intestinal micro biota were documented to be correlated with numerous metabolic conditions inclusive of NAFLD [12].
The gut micro biota and their metabolites as well as some proinflammatory products can pass through the intestinal barrier and enter the liver through the portal system. In the liver, they can promote or inhibit the progression of NAFLD through different mechanisms. Meanwhile, liver can also regulate intestinal function and gut micro biota balance through the bile acid circulation, which is an important entero hepatic circulation in regulating NAFLD.
The gut-liver axis represents the shared connection amongst the intestine along with liver (Figure 1). This axis gets communicated by the i) Portal circulation, ii) Bile tract besides the iii) Systemic circulation [13,14]. The liver receives greater than 2/3rd of its blood from the Gastro Intestinal Tract (GIT) by the portal system. Via portal vein intestine obtained bacteria besides their constituents can arrive with ease. Stimulation of the hepatic immune cells by bacteria in liver cause activation of the inflammation pathways that ultimately progress to NAFLD/ NAFLD- HCC [15], thus corroborates the key part of the gutliver axis in the pathogenesis of NAFLD/ NAFLD- HCC. Therefore the provision of a potential therapeutic target is gutliver axis for NAFLD. Enhancement of gut-liver axis cann confer protection to liver from what are considered the pathogenic constituents of the intestine, while conversely probiotics along with certain advantageousmicrobial products axis cann confers protection to liver through the gut- liver axis. Thus overall this gut- liver axis possesses a key part in modulating the function of GM in the propagationn of NAFLD (Figure 2). Provision of probable approaches regarding the avoided well as treatment of NAFLD/NAFLD- HCC might become feasible by attempting to find the correlation of GM along with gut- liver axis in the generation of NAFLD.
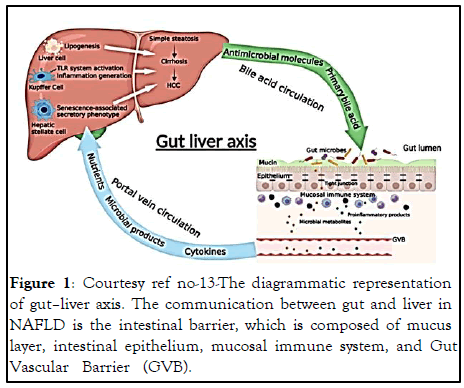
Figure 1: Courtesy ref no-13-The diagrammatic representation of gut–liver axis. The communication between gut and liver in NAFLD is the intestinal barrier, which is composed of mucus layer, intestinal epithelium, mucosal immune system, and Gut Vascular Barrier (GVB).
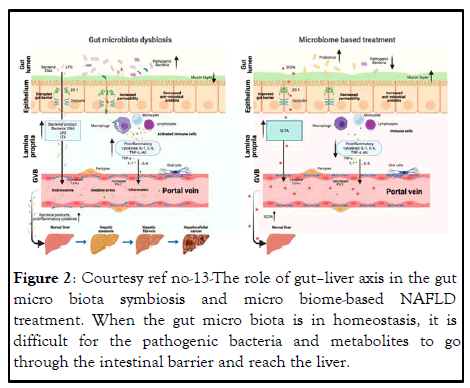
Figure 2: Courtesy ref no-13-The role of gut–liver axis in the gut micro biota symbiosis and micro biome-based NAFLD treatment. When the gut micro biota is in homeostasis, it is difficult for the pathogenic bacteria and metabolites to go through the intestinal barrier and reach the liver.
When the gut micro biota is symbiosis, the intestinal barrier is damaged (decreased mucus layer, increased epithelium, and endothelium layer permeability); thus, the pathogenic bacteria and metabolites can easily go through the intestinal barrier, come to the portal system, and finally reach the liver, where they can promote NAFLD progression. Micro biome-based treatment (probiotics and beneficial metabolites) can repair the intestinal barrier and the beneficial metabolites can reach the liver through the portal vein to prevent NAFLD progression.
This review subsequent to our earlier endeavors to elucidate the association of GM symbiosis along with microbial metabolites alterations in NAFLD besides NAFLD/NAFLD- HCC, the role of intestinal epithelial barrier besides the part of altered gut-liver axis associated with intestinal GM symbiosis in generating Neuro Degenerative Diseases (NDD), Neuropsychiatric Diseases (NPD) [16-20] here we attempt to further elaborate on the part of gut-liver axis along with their correlation with NAFLD as an update of what had been earlier described. Additionally, how to generate strategies for NAFLD that are dependent on microbes.
Gut microbiome sysbiosis correlation of NAFLD along with NAFLD HCC
Gut microbiome sysbiosis association with hepatic steatosis: Gut microbiome represents a key factor in the generation of hepatic steatosis. Like during Fecal Transplantation Experiment (FMT) implicating shift of GM from obese mice with hepatic steatosis towards Germ Free (GF) mice can cause induction of NAFLD changes like enhanced Triglycerides (TG), amounts along with upregulation of genes associated with lip genesis besides lipid uptake. Additionally, it was observed in a study where wild kind mice generated hepatic steatosis with ease simply by housing these mice together with mice with NASH. This phenotype was correlated with inflammasome modulated gut symbiosis. It was noticeable regarding FMT from obese patients with liver steatosis to mice can result in escalated liver TG accrual by just 2 weeks. Intriguingly researchers observed that obese infant mice kept on a western diet gained enhanced weight as well as exaggerated the propagationn of NAFLD. This pointed that gut obesity in maternal obesity correlated with infants generating childhood NAFLD is key for the same. Clarification exists dependent on these studies regarding GM being essential for aiding in forming hepatic steatosis.
Gut microbiome symbiosis association with NASH fibrosis: Possessing the information that Gut Microbiota (GM) is implicated, in the pathophysiology of NAFLD generation, microbiota dysbiosis might work as a dependable noninvasive gadget regarding early NAFLD diagnosis. In Europe contrasted to healthy individuals NAFLD patients possessed greater enrichment with Bradyrhizobium, Anaeroccus, Peptoniphylus Propriobacterium acnes along with Ruminococcus, however lesser amounts of Oscillospira as well as Rikenellacea. Furthermore intriguingly the microbiota dysbiosis kinds of NAFLD patients rely on variable different regions besidessex. Faecalibacterium Prausnitzii. In a Chinese cohort study the genera Lactobacillus, Oscillobacter, as well as Ruminoclostridium have been seen to be decreased in obese patients with NAFLD, whereas, Faecalibacterium Prausnitzii was the only spp existent in variable quantities amongst those with or without NAFLD. Regarding the women cohort, the quantities of variable separate genera like Subdoli granulum, Caprococcus, as well as Caprobacter possessed a negative association with hepatic steatosis. Provision of proof regarding gender variation in the GM within NAFLD patients was yielded by this study, thus GM might act as biomarker regarding gender backgrounds.
While NASH that is a greater formidable kind of NAFLD, NASH patients possess a unique Gut Microbiome contrasted to the ones just presenting with hepatic steatosis. A cross-sectional study illustrated the possession of NASH patients with greater quantities of fecal Clostridium coccoides along with lesser proportion of Bacteroides contrasted to the ones with steatosis however with out cancer. Accounting for hepatic fibrosis representing a robust NASH stage which required, clinical interference, numerous studies contrast the gut microbiome amongst non-fibrosis alongwith fibrosis NAFLD patients. In these studies researchers observed escalated quantities of Bacteroides besides reduction of variable separate genera like Prevotella . Generatied a universal gutmicrobiome- obtained signature for anticipating NAFLD cirrhosis. A combination of microbial spp, age alongwith serum estimates for developing a thorough detailed diagnostic signature regarding NAFLDcirrhosis patients. Nevertheless certain studies have illustrated certain contradictory gutmicrobiome- in NASH patients. Like, observed a reduction of Prevotella in F0/1 fibrosis stage NASH patients contrasted to F>2 fibrosis stage NASH patients, while conversely. Illustrated regarding patients with fibrosis advancements possessing greater quantities of Prevotella. This might be secondary to different genetic milieus of patients. EnrolledFrench, while. Enrolled Germans. A part from bacterial dysbiosis, fungal dysbiosis, is further existent in NASH patients. Observed that patient’s presentation with non-obese NASH or F2-4 fibrosis possessed unique fecal micro biome constitution contrasted to the ones with mild disease. Amelioration of NASH is feasible with utilization of antifungal therapies in mice. Hence intestinal fungi might be a promising target for mitigation of NASH.
The part of gut microbiome symbiosis on NASH propagation might be partly secondary to enhanced proneness towards escalation of intestinal permeability. Resultant to that translocation of certain proinflammatory agents obtained from GM might take place to portal vein besides liver. Like amounts of serum Lipo Poly Saccharides (LPS) binding protein were escalated in NASH patients contrasted to NAFLD patients. The enhanced amounts of serum endotoxin, in portal system besides plasma results in activation of Toll Like Receptor 4 (TLR4) in the liver of NASH patients. This was further corroborated by the greater amounts of TLR4+ macrophages in NASH contrasted to simple steatosis. TLR4 activation might facilityte generation of Reactive Oxygen Species (ROS) from macrophages along with enhance expression of pro Interleukin-1 (IL-1β,) aiding in proinflammatory milieu along with ultimately promotion of NASH. Thus pointing to the actions of LPS/TLR4 activation on the pathogenesis of NASH. Besides this mode a new study has demonstrated that gut obtained mmicrobial antigen might work in the form of a ligand which cause activation of intrahepatic B cells via myeloid differentiated in innate immuneTLR adaptor protein (My D88) pathway , that result in inflammation alongwith fibrosis at the time of NASH propagation.
Gut microbiome symbiosis association with NAFLD HCC: NAFLD-HCC is responsible for 10% of all HCC kinds. NAFLD-HCC occurs secondary to chronic inflammation modulated by lipotoxicity might be secondary to enhanced neutral lipids accrual in liver. Accounting for the action of gut microbiome on NAFLD propagationn, it is essential to evaluate the part of gut microbiome in NAFLD- HCC, An earlier clinical study demonstrated enhancement of Bacteroides as well as Ruminococcacea amounts, whereas reduction in Bifidobacterium amounts enrichment in NAFLD- HCC patients contrasted to cirrhosis patients not having propagated to NAFLDH CC. Additionally, correlationn amongst Gut Microbiota (GM) as well as robust proinflammatory cytokines like greater amounts of interleukin-8 (IL-8) along with chemokine (C-C motif) ligand (CCL) 3 in NAFLD HCC patients. Hence pointing to GM stimulated inflammation might exacerbate NAFLD HCC propagation. Additionally, GM might work in the form of co-factors in propagating NAFLD HCC, by crosstalk with immune chambers. In a recent study it was illustrated that the GM in 32 NAFLD- HCC patients possessed the capacity of reduction of expansion of CD8 + T cells, however enhance the expansion of 10+ Treg cells contrasted to 28 NAFLD cirrhosis patients along with 30 non NAFLD controls. By generation of a spontaneous NAFLD HCC mouse model by the group of Song, Odenwald etc, it was documented that GM symbiosis aids in NAFLD-HCC production by enhancement of amounts of, Mucispirillum, Desulfovibrio, Anaerotruncus along with Desulfovibrionaceae, with reduced amounts of Bacteroides and besides Bifidobacterium.
Gut Microbiota derived metabolites associated with NAFLD besides NAFLD HCC
Gut metabolomic alterationsin NAFLD, NASH: The gut microbiome metabolites are the necessary factors possessing the capacity of mediation of the pathogenesis of NAFLD along with NASH. Maximum microbial metabolites are basically obtained from carbohydrate along with protein fermentation. Short chain fatty acids (SCFA) represent oone of the maximum frequent microbial metabolites obtained from the indigestible carbohydrate. SCFA are advantageous regarding liver metabolism, being implicated in NAFLD propagationn. Like a recent study observed a type of acetate from commensal mmicrobes possessing the capacity of repression of NAFLD production by modulation of hepatic Free Fatty Acid Receptors (hepatic FFAR2) signaling in the liver of mice receiving High Fat Diet (HFD). Additionally, numerous studies illustrated the importance of another SCFA butyrate possessing the capacity of ameliorating NAFLD by controlling GM, intestinal tight junctions, and hepatic Glucagon Like Peptide 1 (GLP-1) receptor expression besides TLR4 pathways. Other SCFA substances like propionate further were attractive for mitigation of NASH propagationn. Nevertheless, there is a contradictory study illustrating Micro biota obtained acetate might facilitate hepatic lip genesis. This might be secondary to variation in diet. In a high fructose diet microbial acetate production can facilitate the lipogenic pools of acetyl-coA; nevertheless, in a HFdependent diet, FFAR2 signaling might be activated for hampering NAFLD propagationn.
Apart from SCFA other kinds of microbial metabolites illustrated a key part in NAFLD, in particular ethanol along with Bile Acids (BA’s). Ethanol obtained from microbiome is subsequent to fermentation following hydrolysis of sugars. It was iillustrated by preclinical studies that endogenous ethanol obtained from microbiota possesses the capacity of exacerbation of liver steatosis along with inflammation. Additionally, clinical corroboration regarding enhanced blood ethanol amounts was observed in NAFLD patients of greater significance the amounts of ethanol generating bacteria besides enhanced blood ethanol amounts observation in NASH patients. Hence pointing that ethanol obtained from microbiomeis necessary in facilitation of simple hepatic steatosis to NASH. Gut Microbiome is further implicated in BA’s metabolism. The GM has the ability of transformation of primary BA’s into secondary BA’s. In case of NAFLD this capacity of GM is hineared in view of reduction of the amounts of correlated bacteria. A reduction of the deconjugated BA’s might further lead to reduction of turbine, thus causing hepatic steatosis along with inflammation, targeting the Oxidative Stress (OS) associated genes. Furthermore, Farsenoid X Receptor (FXR), the receptor for BA’s is down regulated in NAFLD. The reduction of intestinal FXR further can result in reduction of the liberation of Fibroblast growth factor 15/19 (FGF15/19) both of which possess the capacity of decreasing liver steatosis. More microbial metabolites like amino acids awa choline have been further documented to modulate the same.
Gut metabolomic changes in NAFLD-HCC: What gut mmicrobial metabolites attribute towards colorectal cancer has been well elucidated. Only occasional studies regarding studying, the impact of bacterial metabolites on the propagationn of disease in NAFLD-HCC has been performed. In a recently conducted study it was observed that GM obtained, acetate butyrate propionate possessed a positive association with the propagationn of NAFLD-HCC that is contradictory to the observations regarding them having the capacity of mitigation of NASH propagation. The variable actions of SCFA in NASH besides NAFLD-HCC might probably be responsible for the distinctive microenvironments of NASH besides NAFLD-HCC. Thus greater evaluation is required for unearthing this query. Other than clinical proof, a study conducted recently by demonstrated the existence of changes in gut microbial metabolites like enhanced serum Taurocholic Acid (TCA) along with reduction of serum 3 indole Propionic Acid (IPA) in mice with NAFLD-HCC. From point of mode of action it was seen that IPA possessed the capacity of hampering lipid accrual along with cell proliferation, whereas TCA facilitated Triglycerides (TG) accrual in the liver. With little work in this issue the association amongst NAFLD-HCC along with Gut metabolomic alterations is not feasible with greater work required for resolving in this issue.
The crosstalk amongst gut along with liver in NAFLD besides NAFLD HCC
Role of intestinal permeability alterations: Intestinal permeability decides transportation of which substances might be feasible from gut to liver besides impacting NAFLD propagation. The intestinal permeability is based on the intestinal barrier constituted by mucus layer, intestinal epithelium, and mucosal immune system along with the GutVascular Barrier (GVB). The central part of intestinal barrier is represented by enterocytes along with GVB that are responsible for portal vein entry besides reaching the liver.
Enterocytes are intricately attached to each other via functional proteins like E-cadherin, Zonula Occludens 1 (ZO1) and claudin along with functional adhesion molecules. Reinforcement of intestinal coherence by generation of metabolites like SCFA is feasible by GM, that have the capacity of direct reinforcement of tight junctions, whereas GM symbiosis, results in interfered gut barrier with GM solidarity. The existent GM symbiosis in NAFLD patients have the capacity of interference of the tight junctions with ease resulting in enhanced intestinal permeability. Conversely avoidance of NAFLD is feasible by certain bacteria by impacting intestinal epithelial communication. Like escalated quantities of Akkermansia muciniphilia is associated with recovery of gut permeability along with NAFLD propagation via controlling tight junctions.
Interfered intestinal tight junctions might result in bacterial translocation as well as their metabolites from the gut lumen to lamina propria. In the lamina propria sustenance of intactness of GVB, which is comprised of endothelial cells, can avoid bacteria besides its toxic metabolites from arrival at the portal circulation. While at the time of High Fat Diet (HFD) induced symbiosis, GVB is interfered very early. The dysfunctional GVB might facilitate bacterial translocation to the liver as well as facilitate hepatic steatosis, inflammation as well as fibrosis. Of greater significance enhancement of GVB Farsenoid X nuclear receptor ligand agonist Obeticholic Acid (OCA) could mitigate NASH in mouse model as well as patients. OCA can cause recovery of NASH by escalating GVB function along with decreasing bacterial translocation in a mouse model. A multicenter, randomized, placebo controlled phase 3 clinical trial inclusive of 1968 NASH patients demonstrated that OCA could reduce NASH action along with fibrosis in NASH patients. This pointed that GVB targeting by OCA might prove to be an attractive strategy regarding NASH patients therapy. Thus disruption of these gut barrier constituents by diet & inflammation might cause enhanced permeability. Thus micro organisms along with micro organisms obtained molecules can reach with ease the portal vein, ultimately the liver. This process can be believed as the initial connection amongst Gut along with liver in NAFLD.
Role of portal vein circulation system: The portal vein is an essential Circulating system that directly communicates liver with the intestine. About 75% of the blood supply in the liver gets obtained from the portal vein draining blood from the intestinal mesenteric vein. In normal situations numerous nutrients along with advantageous mmicrobial metabolites arrive at the liver through the portal system. . Like absorption of microbiota obtained SCFA followed by shifting in liver occurs via the portal system. In certain pathological situations like gut inflammation, symbiosis, injured bacterial constituents (alias Damage–Associated Molecular Patterns (DAMP) (LPS), along with pro inflammatory bacterial metabolites (like amonnia, besides ethanol). Via the portal system such toxic factors might reach liver directly, stimulate immune cells, proinflammatory cytokines pathways with ultimate NAFLD generation. A preclinical study concentrated regarding mice fed HFD with intestinal inflammationn escalated microbial obtained LPS quantities in the portal system along with facilitated NASH propagation. It was illustrated that besides NASH metabolic changes in HCC patients had greater quantities of DL-3n phenyl acetic acid, L-tryptophan along with glycol alcoholic acid detectable in portal vein contrasted to healthy controls. These studies highlighted the central part of portal vein circulation on the connection amongst gut besides liver in NAFLD.
Role of bile acids (ba’s circulation: Another vital component of enterohepatic circulation is Bile Acids (BA’s) circulation in NAFLD. BA’s represent steroid molecules. They get generated from cholesterol in the hepatocytes implicating around 15 enzymes. Following that BA’s are liberated from liver cells via the biliary tree into Gall Bladder (GB) where they can get liberated into the small intestine at the time of inter digestive time. In the intestine GM can convert along with esterify BA’s by utilization of Bile Salt Hydrolysates (BSH). BSH possess activity in various bacterial genera like Lactobacillus, Bifidobacterium, Clostridium, besides Bacteroides that can generate free BA’s. Free BA’s possess the capacity of solubilization of intestinal lipids besides lesser, effectiveness of reabsorption. Hence greater quantities of BSH are intricately associated with decreased body weight along with decreased serum cholesterol and liver TG amounts. In NAFLD crosstalk amongst GM along with BA’s metabolism have a key influence on NAFLD propagation. Like NASH patients possessing gut symbiosis can synthesize BA’s. This was directly corroborated subsequent to antibiotic therapy. A preclinical study illustrated that antibiotic therapy could control bile acids/intestinal FXR axis, thus cause escalated hepatic lipids. Additionally provision of greater proof directly by GF mice regarding association of GM along with BA’s circulation in NAFLD was given. Knowledge regarding resistance of GF mice to HFD stimulated obesity is existent that can be reversed by GM which enhance weight along with lipids accrual in liver by FXR based mode. Thus targeting intestinal FXR might be an efficacious therapeutic target regarding NAFLD treatment. Nevertheless, as maximum studies were animal dependent it remains uncertain if variations exist amongst mouse along with human in impacting microbial BA’s metabolism. Hence greater human studies are required for resolution of the complex connection amongst GM, BA’s besides NAFLD pathogenesis.
Role of bile acids (ba’s )circulation: Another vital component of enterohepatic circulation is Bile Acids (BA’s) circulation in NAFLD. BA’s represent steroid molecules. They get generated from cholesterol in the hepatocytes implicating around 15 enzymes. Following that BA’s are liberated from liver cells via the biliary tree into Gall Bladder (GB) where they can get liberated into the small intestine at the time of inter digestive time. In the intestine GM can convert along with esterify BA’s by utilization of Bile Salt Hydrolysates (BSH). BSH possess activity in various bacterial genera like Lactobacillus, Bifidobacterium, Clostridium, besides Bacteroides that can generate free BA’s. Free BA’s possess the capacity of solubilization of intestinal lipids besides lesser, effectiveness of reabsorption. Hence greater quantities of BSH are intricately associated with decreased body weight along with decreased serum cholesterol and liver TG amounts. In NAFLD crosstalk amongst GM along with BA’s metabolism have a key influence on NAFLD propagation. Like NASH patients possessing gut symbiosis can synthesize BA’s. This was directly corroborated subsequent to antibiotic therapy. A preclinical study illustrated that antibiotic therapy could control bile acids/intestinal FXR axis, thus cause escalated hepatic lipids. Additionally provision of greater proof directly by GF mice regarding association of GM along with BA’s circulation in NAFLD was given. Knowledge regarding resistance of GF mice to HFD stimulated obesity is existent that can be reversed by GM which enhance weight along with lipids accrual in liver by FXR based mode. Thus targeting intestinal FXR might be an efficacious therapeutic target regarding NAFLD treatment. Nevertheless, as maximum studies were animal dependent it remains uncertain if variations exist amongst mouse along with human in impacting microbial BA’s metabolism. Hence greater human studies are required for resolution of the complex connection amongst GM, BA’s besides NAFLD pathogenesis.
Role of intestinal hormones: Besides direct connection amongst gut as well as liver via portal system/BA’s circulation, GM possess the capacity of impacting liver metabolism via impacting the liberation of intestinal hormones with the capacity of escalating glucose stimulated insulin liberation while hampering glucagon liberation. Like the microbiome obtained SCFA can stimulate Glucagon Like Peptide 1(GLP-1) liberation, an intestinal hormone liberated from intestinal L cells. Multiple GLP1 receptor agonists illustrated the capability of reversal of hepatic steatosis, thus acting as the newer alternate NAFLD therapy. Furthermore, GM evaluation revealed that liraglutide, a GLP1 receptor agonist had the capacity of modification of GM heterogeneity by reduction of Proteobacteria along with escalated Akkermansia muciniphilia amounts that was correlated with recovery of NAFLD. Besides GLP1, other intestinal hormones like FGF15/19 could mitigate HFD stimulated hepatic steatosis following engagement of GM. Additionally GM can impact other L cells obtained intestinal hormone, Insulin Like Peptide 5 (INSL5) that has been observed to be implicated in the pathophysiology of NAFLD. Dependent on these studies NAFLD generation might be influenced by the crosstalk amongst GM along with intestinal hormones in the gut-liver axis. Nevertheless, still absence of clinical proof to corroborate the connection amongst, GM along with G hormones in NAFLD is there.
Avoidance besides therapeutic approaches of NAFLD by modulation of Gut Microbiome
Probiotics: GM manipulation by probiotics is a generating besides attractive therapeutic approach regarding malfunctioned gut-liver axis in NAFLD. The definition recognized by Food and Agricultural Organization of the United Nations (FAO) and World Health Organization (WHO) working group experts is that probiotics are live strains of strictly selected microorganisms, which once administered in adequate amounts; give a health benefit to the host. This definition was accepted by the International Scientific Association of Prebiotics and Probiotics (ISAPP) in 2013. Regarding the gut-liver axis, the protection conferred by probiotics on NAFLD work basically by enhancement of the gut barrier. Like Lactobacillus rhamnosus GG, Lactobacillus planetariums, Lactobacillus Acidophilus La 5, Streptococcus Thermophilus have demonstrated their capability of activating tight junction proteins for escalating intestinal permeability. Recently a study illustrated that Probiotics stabilized mucosal immune function, thus protecting NAFLD patients from enhanced intestinal permeability.
In clinical scenario evaluation of different probiotics have been done for avoidance along with therapy of NAFLD in particular usually utilized like Lactobacillus Bifidobacterium, besides Streptococci. A study conducted by Wong, et al. For 6 mth with utilization of a mixture of variation of probiotics for NASH patients, observing a significant reduction in fat amounts of liver contrasted to placebo group in individualized treatment with probiotics. Moreover, clinical proof pointed that probiotics besides enhancing liver histology it further escalated serum Alanine Amino Transferase (ALT) as well as Aspartate Amino Transferase (AST) in NAFLD patients. Nevertheless, another clinical trial Ahn, etal. In 2019 it observed that probiotics only alleviated liver steatosis on treatment of NAFLD with numerous strains probiotic, without impacting liver enzymes. Hence these studies pointed that greater individualized probiotics had to be given with regards to effectiveness along with safety of the probiotics treatments in clinical scenario.
Prebiotics: A prebiotics is a nonviable food component which imparts health benefits on the host associated with micro biota modulation which might be a fiber, but all fibers are not necessarily a probiotic. Like usual prebiotics are inclusive of Fructoligosacchharides (FOS), inulin, Transgalactoligosacchharides (TOS), lactulose. Prebiotics possess the capacity of escalating growth besides activity of Probiotics, thus having efficacy as well as safety for controlling GM. Like Prebiotics might hamper the growth of pathogenic Escherichia Coli (E.Coli) Salmonella enteritis, Klebsiella pneumoniae, while activating advantageous bacteria simultaneously.
This characteristic further facilitates GM homeostasis, enhance gut barrier, and along with ultimately mitigate NAFLD propagation. Furthermore, prebiotics might confer protection in NAFLD via fermentation to generate SCFAs inclusive of acetate, butyrate, besides propionate that has been well evaluated for protection of gut-liver axis besides NAFLD. A newer soluble dietary fiber namely Larchwood Arabinogalactan (LA-AG) in the form of a candidate prebiotic. LA-AG possess the capacity of escalating accrual of organic acids via fermentation of hampering pathogenic bacteria action along with enhance gut health. Hence LA-AG might be robust for avoidance of NAFLD via control of gut-liver axis.
During clinical trials, numerous prebiotics were observed to be advantageous in NAFLD patients. With oligofructose like an example. Illustrated that oligofructose supplementation might aid in recovery of liver steatosis along with Non-Alcoholic Fatty Liver Activity Score (NAS) in NASH patients. Additionally, in recently conducted systematic review and meta-analysis of Randomized Placebo Controlled Trials (RCTs)it was llustrated that Prebiotics supplementation in NAFLD patients had the capacity of escalating their a anthropometric along with biochemical parameters inclusive of Body Mass Index (BMI), ALT, AST, along with fasting insulin besides Insulin Resistance (IR). However as planning further studies time period needs to be enough for evaluation of Prebiotics for treatment of NAFLD as well as NASH patients.
Antibiotics: Utilization of antibiotics in NAFLD therapy is dependent on the idea regarding antibiotics possessing the capacity of reduction of the impact of microbiota along with their metabolites on host metabolism gut-liver axis. A study in 2008 illustrated that neomycin along with polymixin B could robustly decrease hepatic lipid accrual by decreasing endotoxin transportation in a NAFLD mouse model. Furthermore, another preclinical study observed on antibiotics delivery antibiotics possessed the capacity of controlling the quantities of secondary BA’s by repression of gut bacteria, thus ameliorating inflammationn and fibrosis in the liver hence avoidance of NAFLD propagationn. Antibiotics further illustrate clinical effectiveness in avoidance of NAFLD. Like Solithromycin, a robust next generation macrolide antibiotic was observed to decrease NAS along with ALT regarding NASH patients in a phase II clinical trial. Nevertheless, antibiotics utilization required precautions as they have the capacity of depleting certain significant bacteria species correlated with healthy status besides resulted in certain antibiotics resistant bacteria.
Faecal Microbiota Transplantation (FMT): Faecal Microbiota Transplantation (FMT) represents a therapeutic strategy of shifting faucal microbiota besides metabolites from healthy donors to patients with requirement for re generation of a GM that is stable, has been generated for GIT disease like Clostridium difficult infection. Variable studies have iillustrated how FMT possessed effectiveness regarding bacterio therapy for NAFLD. Observed that FMT might improve HFD- stimulated NASH by controlling GM, enhancing SCFAs amounts, along with rescuing the gut barrier. More recently, demonstrated lesser hepatic lipid accrual, along with inflammation in GF mice that were recipient of FMT from normal chow fed mice contrasted to FMT from High Fat/High Cholesterol (HFHC) fed mice. In agreement with animal experimental studies, clinical trials conducted recently further observed that FMT possess the capacity of reduction of hepatic steatosis along with Intestinal permeability in NAFLD patients. Nevertheless, still certain side effects have been documented in FMT like bacteremia along with perforations. Hence greater clinical trials are warranted for enhancing effectiveness along with reduction of adverse actions regarding FMT therapeutic treatment strategies in NAFLD/ NASH.
Gut microbiome dependent individualized treatment: The Gut microbiome constitutes a key part regarding individualized treatment. Provision of individualized therapeutic treatment strategies that are Gut micro biome dependent in NAFLD by modulation of individualized micro biome alterations is feasible. This is inclusive of, targeting the gut barrier iintactness, intestinal symbiosis, and gut microbial metabolism, besides targeting individualized nutrition. Selective variable Gut microbiome dependent therapeutic treatment strategies regarding NAFLD patients depends on microbiome grouping based on microbial characteristic of patients Meta genomic along with metabolites outcomes. Additionally, factors at patient level need to be accounted in individualized treatment like patient age, sex, and clinical pathological factors. However adequate results are still not existent for evaluation of individualized therapeutic treatment strategies for NAFLD management along with avoidance (Figure 3).
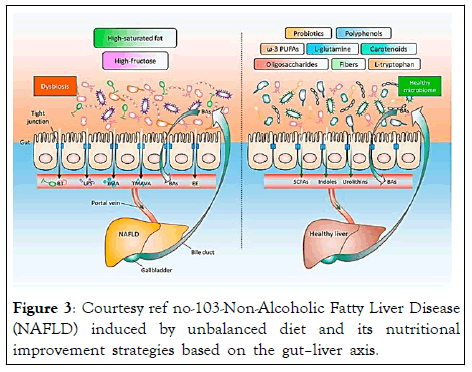
Figure 3: Courtesy ref no-103-Non-Alcoholic Fatty Liver Disease (NAFLD) induced by unbalanced diet and its nutritional improvement strategies based on the gut–liver axis.
Long-term high-saturated fat or high-fructose diet leads to an imbalanced intestinal flora, which in turn elicits an impaired gut barrier function and increased permeability, followed by Bacterial Translocation (BT), and additional bacterial components and metabolites (e.g., Lipopolysaccharides (LPS), Trimethylamine (TMA), N,N,N-Trimethyl-5-Aminovaleric Acid (TMAVA), and Endogenous Ethanol (EE)) entering into the liver through the portal vein. NAFLD patients exhibit abnormal Bile Acids (BAs) metabolism and its related signaling pathways. These factors together accelerate the occurrence and progression of NAFLD. By contrast, an appropriate consumption of probiotics, functional oligosaccharides, dietary fibers, ω-3 Polyunsaturated Fatty Acids (ω-3 PUFAs), functional amino acids (L-tryptophan and L-glutamine), carotenoids, and polyphenols, contributes (1) To the maintenance of the homeostasis of the intestinal flora and BAs, (2) To the enhancement of the intestinal barrier integrity, and (3) To the production of salutary metabolites (e.g., Short Chain Fatty Acids (SCFAs), indoles, and urolithins), there by supporting a healthy liver.
By generation of a spontaneous NAFLD-HCC mouse model by the group of Song, Odenwald etc, it was documented that GM symbiosis aids in NAFLD-HCC production by enhancing amounts of, Mucispirillum, Desulfovibrio, Anaerotruncus along with Desulfovibrionaceae, with reduced Bacteroides besides Bifidobacterium amounts.
SCFA generation like acetate, butyrate propionate were protective against NAFLD whereas of greater significance the amounts of ethanol generating bacteria enhanced blood ethanol amounts observed in NASH patients, causing facilitation of simple hepatic steatosis to NASH (Figure 4).
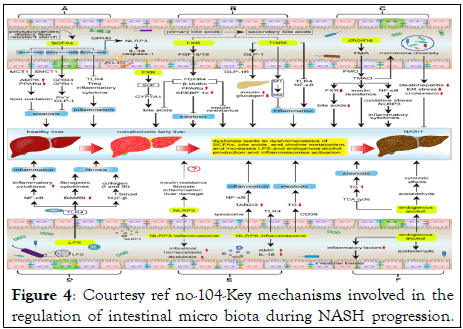
Figure 4: Courtesy ref no-104-Key mechanisms involved in the regulation of intestinal micro biota during NASH progression.
Intestinal symbiosis results in disruption of intestinal SCFAs, bile acids, and choline metabolic homeostasis, as well as increases LPS and endogenous alcohol production and NLPR3/6 activation, subsequently affecting the progression of ANSH; (A) SCFAs inhibit hepatic steatosis, inflammation, and protect the integrity of the intestinal barrier. Symbiosis decreases SCFA production, thereby promoting the NASH process; (B) The metabolism of bile acids is regulated by FXR and TGR5. FXR signaling suppresses hepatic steatosis and insulin resistance, as well as negative feedback inhibits bile acid synthesis; TGR5 can protect the liver from inflammation and insulin resistance. However, symbiosis will reduce the activity of FXR and TGR5 signaling; (C) Intestinal micro biota metabolizes choline to TMAO, but the effect of TMAO on NASH is controversial; (D) LPS mainly affects the progress of NASH through LPS-TLR4 and NF-κB signaling pathways, including hepatic inflammation, fibrosis and liver injury; (E) Activation of NLRP3 in the liver promotes liver damage, but NLRP3 in the intestine maintains intestinal homeostasis and improves intestinal symbiosis. NLRP6 inhibits NASH progression by inhibiting TLR4/NF-κB signaling and TG accumulation and promoting AMP and IL-18 secretion; (F) Intestinal micro biota increases the production of endogenous alcohol and promotes the progress of NASH. SCFAs, short chain fatty acids; MCT1, monocarboxylate transporter 1; SMCT1, sodium‐coupled monocarboxylate transporter 1; AMPK, AMP activated protein kinase; PPARα, Peroxisome proliferator-activated receptor α; GPR41/43, G protein-coupled receptor 41/43; IL-18, Interleukin 18; PYY, peptide YY; GLP1, Glucagon like peptide 1; TLR4, Toll-like Receptor 4; Treg, regulatory T; FXR, Farnesol X receptor; LRH-1, liver receptor homolog 1; CYP7A1, cholesterol 7a hydroxylated enzyme; FGF15/19, fibroblast growth factors 15/19; FGFR4, fibroblast growth factor receptor 4; SREBP-1c, sterol regulatory element-binding protein 1c; TGR5, Takeda G protein-coupled receptor 5; GLP-1R, GLP-1 receptor; NF-κB, nuclear factor-kappaB, TMA, trimethylamine; TMAO, trimethylamine-N-oxide; FMO, flavin monooxygenases; LPS, lipopolysaccharide; LBP, LPS binding protein; TGF-β, transforming growth factor-β; BAMBI, bone morphogenetic protein and active membrane-bound inhibitor; AMPs, antimicrobial peptides; NLRP3/6, nucleotide-binding domain, leucine-rich-repeat containing family, pyrin domain-containing 3/6; TAB2/3, TGF-β activated kinase 1 binding protein 2/3; TG, triglyceride; TCA, tricarboxylic acid.
A reduction of the deconjugated BA’s might further lead to reduction of turbine, thus causing hepatic steatosis along with inflammation, targeting the Oxidative Stress (OS) associated genes. The reduction of intestinal FXR can result in reduction of the liberation of FGF15/19 both of which possess the capacity of decreasing liver steatosis. More mmicrobial metabolites like amino acids and choline have been further documented to modulate the same.
From point of mode of action it was seen that 3 Indole Propionic Acid (IPA) possessed the capacity of hampering lipid accrual along with cell proliferation, whereas TCA Facilitated Triglycerides (TG) accrual in the liver. With little work in this issue the association amongst NAFLD-HCC along with Gut metabolomic alterations is not feasible with greater work required for resolving in this issue.
It was illustrated that besides NASH metabolic changes in HCC patients had greater quantities of DL-3n phenyl acetic acid, Ltryptophan along with glycol alcoholic acid detectable in portal vein contrasted to healthy controls. These studies highlighted the central part of portal vein circulation on the connection amongst gut besides liver in NAFLD.
Thus disruption of these gut barrier constituents like GVB by diet and inflammation might cause enhanced intestinal permeability. Thus microorganisms along with microorganisms obtained molecules reach with ease the portal vein and ultimately the liver. This process can be believed as the initial connection amongst gut along with liver in NAFLD.
Thus targeting intestinal FXR might be an efficacious therapeutic target regarding NAFLD treatment. Nevertheless, as maximum studies were animal dependent it remains uncertain if variations exist amongst mouse besides human in impacting microbial BA’s metabolism. Hence greater human studies are required for resolution of the complex connection amongst GM, BA’s besides NAFLD pathogenesis
Dependent on these studies NAFLD generation might be influenced by the crosstalk amongst GM besides intestinal hormones in the gut-liver axis. Nevertheless, still absence of clinical proof to corroborate the connection amongst, GM along with Gut hormones in NAFLD is there.
Therapy effect
Despite effect of probiotics illustrated earlier with liver ALT, AST recovery, nevertheless trial Ahn, et al. In 2019 it observed that probiotics only alleviated liver steatosis on treatment of NAFLD with numerous strains probiotic, without impacting liver enzymes. Hence these studies pointed that greater individualized probiotics had to be given with regards to effectiveness along with safety of the probiotics treatments in clinical scenario.
Additionally, in recently conducted systematic review and metaanalysis of RCTs it was illustrated that Prebiotics supplementation in NAFLD patients had the capacity of escalating their anthropometric along with biochemical parameters inclusive of Body Mass Index (BMI), ALT, AST, along with fasting insulin besides Insulin Resistance (IR). However as planning further studies time period needs tube enough for evaluation of Prebiotics for treatment of NAFLD as well as NASH patients.
Nevertheless, antibiotics utilization required precautions as they have the capacity of depleting certain significant bacteria species correlated with healthy status besides resulted in certain antibiotics resistant bacteria.
In agreement with animal experimental studies, clinical trials conducted recently further observed that FMT possess the capacity of reduction of hepatic steatosis along with Intestinal permeability in NAFLD patients. Nevertheless, still certain side effects have been documented in FMT like bacteremia along with perforations. Hence greater clinical trials are warranted for enhancing effectiveness along with reduction of adverse actions regarding FMT therapeutic treatment strategies in NAFLD/ NASH.
Adequate results are still not existent for evaluation of individualized therapeutic treatment strategies for NAFLD management along with avoidance.
There is a significant provision of a bidirectional connection amongst gut along with liver by the gut-liver axis. The GM symbiosis can result in changed intestinal permeability enhanced accrual of portal toxic metabolites, facilitate the hepatic inflammation, and hence result in generation of NAFLD along with NAFLD-HCC. Variable studies pointed that microbiota dependent pharmacological manipulation targeting the gut- liver axis appears an attractive therapeutic approach for the treatment of NAFLD patients. The gut-liver axis has a significant part in the generation of GM symbiosis along with microbiome dependent therapy of NAFLD. Nevertheless, the microbiota dependent treatment is still in preclinical budding stage regarding treatment of NAFLD- HCC, thus warranting further clinical evaluation. Additionally, dependent on the ideation of individualized macrobiotic treatment greater work needs to be put in for the generation of particular probiotics, advantageous metabolites, tryptophan, or hampering particular pathogenic microbes along with metabolites for NAFLD besides NAFLD-HCC. Furthermore, this concept of gut-liver axis has been applied regarding generation of neurological diseases as an extension of this idea.
Citation: Kaur KK, Allahbadia G, Singh M (2022) An update on the Association of Gut-Liver Axis with Gut Micro biome Dysbiosis Correlated NAFLD along with NAFLD- HCC with Potential Therapeutic Approaches: a Systematic Review. J Hepatol Gastroint Dis. 8:216.
Received: 19-May-2022, Manuscript No. JHGD-22-17578; Editor assigned: 23-May-2022, Pre QC No. JHGD-22-17578(PQ); Reviewed: 06-Jun-2022, QC No. JHGD-22-17578; Revised: 19-Jul-2022, Manuscript No. JHGD-22-17578(R); Published: 25-Jul-2022 , DOI: DOI: 10.35248/2475-3181.22.8.216
Copyright: © 2022 Kaur KK, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.