
Journal of Clinical Chemistry and Laboratory Medicine
Open Access
ISSN: 2736-6588

ISSN: 2736-6588
Research Article - (2024)Volume 7, Issue 2
Aim: The study aims to examine the correlation between alcohol consumption and the risk of gastric cancer in the Wuwei population with different levels of H. pylori infection.
Methods: The study included 3123 participants aged 40-70 years who had resided in Wuwei city for at least 5 years. Participants completed questionnaires on personal information, diet, alcohol consumption, family and medical history and H. pylori detection. All participants underwent 13C-Urea Breath Test (13C-UBT) for H. pylori infection and upper gastrointestinal endoscopy and pathology were performed. Testing was conducted before diagnosis to avoid recall bias.
Results: Alcohol increases the risk of gastric cancer, but it decreases the risk in those who are infected. Those aged 50-70 without H. pylori who consume alcohol and smokers who quit with H. pylori infection have a higher risk of gastric cancer.
Conclusion: Alcohol consumption represents a significant risk factor for the development of gastric cancer. This risk is particularly pronounced with advancing age and following the eradication of H. pylori.
Gastric cancer; Alcohol consumption; H. pylori; 13C-Urea Breath Test (13C-UBT)
Gastric Cancer (GC) is the fifth most common cancer and the fourth leading cause of cancer-related deaths worldwide [1]. According to the latest report from the International Agency for Research on Cancer (IARC), China accounted for 44.0% of new cases and 48.6% of deaths related to GC in 2020, with 479,000 new cases and 374,000 deaths. Although the incidence and mortality rates for GC have decreased in China over the last decade, it remains the most common malignant tumor and the third leading cause of death. The high incidence of gastric cancer in China poses a significant threat to public health [2-3]. Effective prevention, early detection and improved treatment strategies are urgently needed to combat this disease.
Several factors contribute to the high prevalence of gastric cancer in China [4]. First, dietary habits play an important role, with a preference for high-salt and high-fat foods, as well as preserved and pickled foods. These dietary choices are known to increase the risk of developing gastric cancer. Second, infection with H. pylori, a bacterium commonly found in the stomach lining, is a major risk factor for gastric cancer and is prevalent in the Chinese population. In addition, lifestyle factors such as smoking and alcohol consumption also contribute to the development of gastric cancer. Rapid industrialization and urbanization in China have led to an increase in these unhealthy habits, further exacerbating the problem [5-9].
Alcohol consumption is a known risk factor for gastric cancer. However, the presence of H. pylori infection may modify this relationship. H. pylori is a bacterium that causes gastric cancer and is prevalent in Wuwei, China [10-12]. Previous studies have examined the effects of alcohol consumption and H. pylori infection separately. However, few have examined how they interact and affect gastric cancer risk. Understanding this interaction could provide insights into the prevention and treatment of gastric cancer. Further research is needed to determine whether the combined effects of H. pylori infection and alcohol consumption are additive or multiplicative and to identify potential treatments for gastric cancer [13,14].
This study examines the combined impact of H. pylori infection and alcohol consumption on the development of gastric cancer. To gain a comprehensive understanding of this association, large-scale epidemiologic studies are essential. A case-control study was conducted in Wuwei, China, to investigate the association between risk factors for gastric cancer and to inform screening strategies. Given the high prevalence of H. pylori infection in Wuwei, gastric cancer is a significant concern. The objective of this study is to analyse the effect of alcohol consumption on gastric cancer among individuals with different H. pylori infection statuses, with a focus on the Wuwei population. The findings of this study will offer valuable insights into preventive strategies and make a significant contribution to future research and clinical interventions.
Study population and sample size
The National Public Welfare Industry Research Project (prospective evaluation study of upper gastrointestinal cancer screening) involved 10,035 participants. They were selected according to China Cancer Screening's Early Diagnosis and Treatment Technical Scheme. The study was conducted in various villages in Liangzhou District, Wuwei City, Gansu Province from November 2015 to November 2017. Wuwei Cancer Hospital performed upper gastrointestinal endoscopy and pathology for screening purposes. All participants provided informed consent before the study and the study was approved by the ethics committee of Wuwei Cancer Hospital. Expert physicians standardized the gastroscopy and pathology examinations. To ensure accuracy, all personnel involved in the survey received standardized training on survey methods and techniques. During the research phase, the questionnaire was entered twice using EpiData3.1 software to identify and correct inconsistencies and errors in the original data. The study included a total of 3123 participants, 777 in the case group and 2346 in the control group as seen in (Figure 1). The study focused on participants aged 40-70 years who had lived in Wuwei city for at least 5 years prior to diagnosis or interview. Participants completed a questionnaire on personal information, including diet, alcohol consumption, garlic consumption, family and medical history and H. pylori detection. All participants underwent 13C-UBT testing for H. pylori infection after fasting for at least 6 hours using a specific method. Positive results would lead to further testing and possible treatment for H. pylori. Testing was done before diagnosis to avoid recall bias.
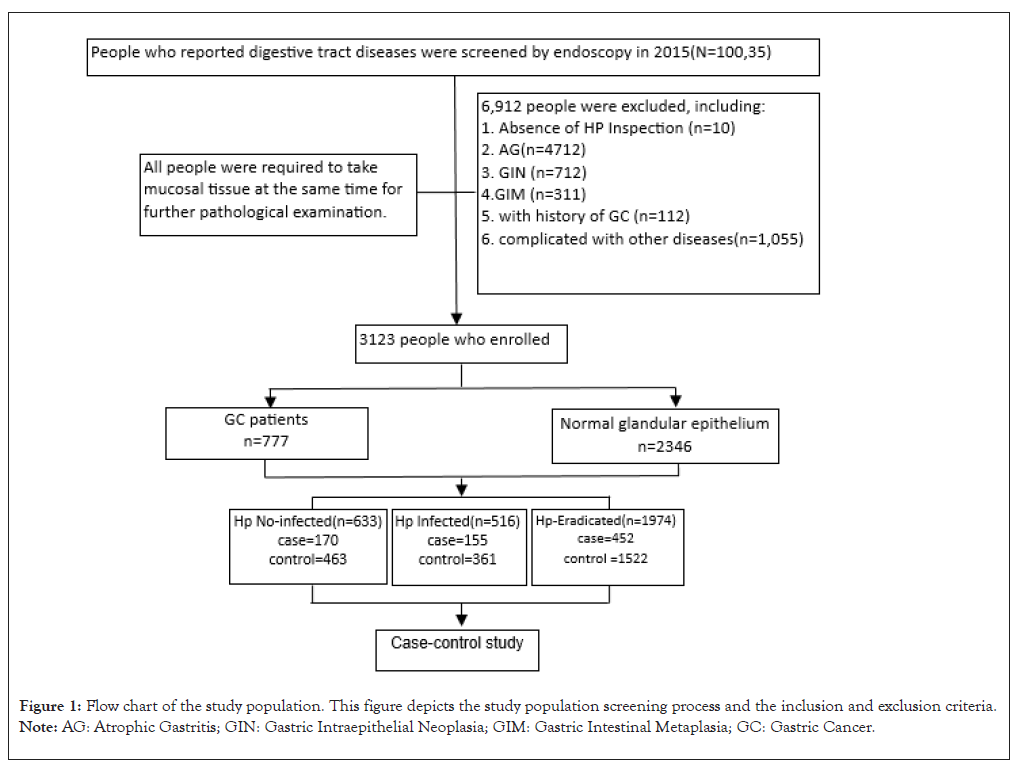
Figure 1: Flow chart of the study population. This figure depicts the study population screening process and the inclusion and exclusion criteria. Note: AG: Atrophic Gastritis; GIN: Gastric Intraepithelial Neoplasia; GIM: Gastric Intestinal Metaplasia; GC: Gastric Cancer.
Data collection
Trained interviewers conducted face-to-face interviews with patients suspected of having gastric cancer before a final diagnosis was made. The objective was to minimize the influence of biased information. The interviews employed a structured questionnaire comprising five categories of questions. These included demographic information, dietary habits, lifestyle factors, a history of gastric disease and a family history of gastric cancer. The questionnaire defined technical terms and collected data on various factors, including age, weight, dietary preferences, smoking and alcohol consumption, physical activity and history of gastric disorders. It also inquired about a family history of gastric cancer. The questionnaire was designed to collect unbiased data on lifestyle and dietary habits. It defined smoking as at least one cigarette per day for six months or longer and drinking as consuming more than one or two alcoholic beverages per day for one year or longer. The frequency of food consumption was categorized as daily, often, occasionally and never. Certain foods, such as coarse grains, fruits and vegetables, were identified as beneficial, while pickled foods and hard and crunchy foods were mentioned as preferences for some individuals. A family history of gastric cancer was defined as having a first-degree relative diagnosed with the disease. Body Mass Index (BMI) ranges were also provided to classify weight status. According to the guidelines for the prevention and management of overweight and obesity in adults in China, a Body Mass Index (BMI) below 18.5 kg/m² is indicative of low weight, a BMI between 18.5 kg/m² and 23.9 kg/m² denotes normal weight and a BMI of 24.0 kg/m² or above is indicative of overweight and obesity [15].
Inclusion and exclusion criteria
This study mainly investigated the relationship between alcohol consumption, H. pylori and gastric cancer. Based on gastroscopy, pathology and H. pylori examination, exclusion conditions were set for subjects who did not meet the requirements of this study:
No H. pylori test results, since the subjects of this study were gastric cancer patients, the patients with gastrointestinal metaplasia, atrophic gastritis and non-atrophic gastritis were excluded. Other diseases other than gastrointestinal diseases.
The inclusion criteria for the case and control groups were as follows: Inclusion conditions of the case group of gastric cancer was diagnosed by gastroscopy and pathological biopsy, patients who completed the health questionnaire, permanent residents of the Wuwei area.
The inclusion conditions of the control group: Gastroscopy showed normal gastric glandular epithelium, People who completed the health questionnaire, permanent population of the Wuwei area.
Statistics analysis
Baseline description: Continuous variables were analysed with normal distribution and expressed as mean ± standard deviation (x ± s). One-way Analysis of Variance (ANOVA) test and Student's T-test (T-test) were used for comparison. Categorical variables are expressed as counts and percentages, compared by chi-squared test (χ²) analysis or Fisher's exact test.
Multivariate regression analysis: The effect of alcohol consumption on gastric cancer risk was analysed using unconditional logistic regression to calculate Odds Ratios (OR) and 95% Confidence Intervals (95% CI). Multiple logistic regression models were adjusted for significant variables identified by univariate analysis. These variables included education level (no formal education, primary school, junior high school or above), per capita income (<5000, 5000-10000, >10000), smoking status (never, current smoker, former smoker), frequency of garlic consumption (never, occasionally, often, daily) and H. pylori infection (uninfected, infected, eradicated). The study used an unconditional logistic regression model to analyse the interactions between alcohol consumption and several variables such as age, sex and H. pylori infection. All statistical analyses were performed with SPSS 25.0 software using a two-tailed test with a test level of α=0.05.
Ethics approval and consent to participate
This study was conducted in accordance with the tenets of the declaration of Helsinki and all procedures involving study participants were approved by the ethics committee of the Wuwei Cancer Hospital of Gansu Province, confirming that all experiments were conducted in accordance with relevant guidelines and regulations.
Univariate analysis and multivariate analysis of risk factors for gastric cancer
The univariate analysis and multivariate analysis of risk factors for gastric cancer are presented in Table 1. In this study, the proportion of patients aged 50 years-59 years and 60 years -69 years, in the gastric cancer group was 35.7% and 38.2%, respectively, which were higher than those in the control group (33.9% and 17.7%). Most subjects were male. There were 488 males (62.8%) and 289 females (37.2%) in the gastric cancer group. In the control group, there were 947 males (40.4%) and 1399 females (59.6%). The proportion of highly educated subjects was relatively small. In the gastric cancer group, 244 people, accounting for 31.4%, had not formally attended school. Primary school culture 233 people, accounting for 30.0%; middle school education 290 students, accounting for 37.3%; junior and above 10 students, accounting for 1.3%; In the control group, 560 people did not go to school formally, accounting for 23.9%; primary school education 746 students, accounting for 31.8%; middle school education 1,006 students, accounting for 42.9%; college and above 34 people, accounting for 1.4%. According to the analysis results, age, gender, education attainment, total annual family income, smoking, drinking, garlic consumption and H. pylori infection were associated with the occurrence of gastric cancer, with significant statistical differences (P<0.01).
| Variables | Cases N (%) | Controls N (%) | P-value |
|---|---|---|---|
| (t/ χ²) | |||
| Age | 55.81 ± 7.77 | 51.21 ± 7.48 | - |
| 40~ | 203 (26.1) | 1136 (48.4) | <0.01 |
| 50~ | 277 (35.7) | 795 (33.9) | |
| 60~70 | 297 (38.2) | 415 (17.7) | |
| Gender | |||
| Female | 488 (62.8) | 947 (40.4) | <0.01 |
| Male | 289 (37.2) | 1399 (59.6) | |
| BMI | 24.04 ± 3.394 | 23.94 ± 3.230 | |
| Underweight | 20 (2.6) | 64 (2.7) | 0.98 |
| Normal | 389 (50.1) | 1180 (50.3) | |
| Overweight | 287 (36.9) | 867 (37.0) | |
| Obesity | 81 (10.4) | 235 (10.0) | |
| Education attainment | |||
| Illiterate | 244 (31.4) | 560 (23.9) | <0.01 |
| Primary school | 233 (37.3) | 746 (31.8) | |
| Middle school or above | 290 (30) | 1006 (42.9) | |
| Junior college or above | 10 (1.3) | 34 (1.4) | |
| Family size | 4.22 ± 1.74 | 4.30 ± 1.51 | 0.25 |
| Per-capita income | |||
| <5000 | 80 (10.3%) | 248 (10.6%) | <0.01 |
| 5000-10000 | 479 (61.6%) | 1512 (64.5%) | |
| >10000 | 218 (28.1%) | 586 (25.0%) | |
| Alcohol consumption | |||
| Non-drinker | 625 (80.4) | 2073 (88.4) | <0.01 |
| Drinker | 152 (19.6) | 273 (11.6) | |
| Smoking | |||
| Never | 398 (51.2) | 1655 (70.5) | <0.01 |
| Still | 306 (39.4) | 567 (24.2) | |
| Stopped smoking | 73 (9.4) | 124 (5.3) | |
| Bean food | |||
| Every day | 22 (2.8) | 70 (3) | 0.987 |
| Often | 287 (36.9) | 866 (36.9) | |
| Sometimes | 449 (57.8) | 1357 (57.8) | |
| Never | 19 (2.4) | 53 (2.3) | |
| Garlic | |||
| Every day | 115 (14.8) | 327 (13.9) | 0.019 |
| Often | 314 (40.4) | 837 (35.7) | |
| Sometimes | 277 (35.6) | 892 (38) | |
| Never | 71 (9.1) | 290 (12.4) | |
| Hard food | |||
| Every day | 19 (2.4) | 39 (1.7) | 0.06 |
| Often | 56 (7.2) | 161 (6.9) | |
| Sometimes | 468 (60.2) | 1532 (65.3) | |
| Never | 233 (30) | 613 (26.1) | |
| H. pylori infection | |||
| No | 168 (21.7) | 452 (19.4) | 0.001 |
| Infected | 155 (20) | 361 (15.5) | |
| Eradicated | 452 (58.3) | 1522 (65.2) | |
Note: P<0.05, statistical analysis was performed by t-test/χ²-test; BMI: Body Mass Index;
χ²: Chi-square; P-value: Probability value.
Table 1: Univariate analysis and multivariate analysis of risk factors for gastric cancer.
General risk factors associated with gastric cancer
After adjusting for age and gender as shown in Table 2. BMI, education level, family size, coarse food and H. pylori infection were not significantly associated with gastric cancer risk. However, the drinking group (OR=1.33; 95% CI=1.04-1.70) had a significantly higher risk of developing gastric cancer than those who never drank alcohol. The smoking group (OR=1.37; 95% CI=1.04-1.79) had a significantly higher risk of gastric cancer than those who had never smoked. In addition, in the per capita income group >50000 (OR=1.39; 95% CI=1.01-1.89), the risk of gastric cancer was significantly higher than that of the group with per capita income <5000; the group that never ate garlic (OR=0.64; 95% CI=0.45-0.91) had a significantly lower risk of gastric cancer than those who ate garlic regularly.
| General characteristics | Cases N (%) | Controls N (%) | Age and sex-adjusted OR (95% CI) |
|---|---|---|---|
| BMI | |||
| Underweight (<18.5) | 20 (2.6) | 64 (2.7) | 1.0 (reference) |
| Normal (18.5 ≤ BMI ≤ 23.9) | 389 (50.1) | 1180 (50.3) | 1.42 (0.81-2.48) |
| Overweight (24.0 ≤ BMI ≤ 27.9) | 287 (36.9) | 867 (37.0) | 1.35 (0.77-2.37) |
| Obesity (≥ 28) | 81 (10.4) | 235 (10.0) | 1.31 (0.64-2.67) |
| Family size | 4.22 ± 1.74 | 4.30 ± 1.51 | 0.99 (0.94-1.04) |
| Education attainment | |||
| Illiterate | 244 (31.4) | 560 (23.9) | 1.0 (reference) |
| Primary school | 233 (37.3) | 746 (31.8) | 0.91 (0.71-1.17) |
| Middle school or above | 290 (30) | 1006 (42.9) | 0.81 (0.62-1.07) |
| Junior college or above | 10 (1.3) | 34 (1.4) | 0.58 (0.27-1.23) |
| Per-capita income | |||
| <10000 | 37 (4.8) | 55 (2.3) | 1.0 (reference) |
| 1000-50000 | 475 (61.1) | 1526 (65.1) | 1.12 (0.84-1.49) |
| >50000 | 265 (34.1) | 765 (32.6) | 1.39 (1.01-1.89) |
| Smoking | |||
| Never | 398 (51.2) | 1655 (70.5) | 1.0 (reference) |
| Still | 306 (39.4) | 567 (24.2) | 1.37 (1.04-1.79) |
| Stopped smoking | 73 (9.4) | 124 (5.3) | 1.11 (0.76-1.62) |
| Alcohol consumption | |||
| Non-drinker | 625 (80.4) | 2073 (88.4) | 1.0 (reference) |
| Drinker | 152 (19.6) | 273 (11.6) | 1.33 (1.04-1.70) |
| Hard food | |||
| Every day | 19 (2.4) | 39 (1.7) | 1.0 (reference) |
| Often | 56 (7.2) | 161 (6.9) | 0.70 (0.36-1.37) |
| Sometimes | 468 (60.2) | 1532 (65.3) | 0.61 (0.34-1.12) |
| Never | 233 (30) | 613 (26.1) | 0.76 (0.41-1.39) |
| Garlic | |||
| Every day | 115 (14.8) | 327 (13.9) | 1.0 (reference) |
| Often | 314 (40.4) | 837 (35.7) | 1.08 (0.83-1.41) |
| Sometimes | 277 (35.6) | 892 (38) | 0.86 (0.66-1.12) |
| Never | 71 (9.1) | 290 (12.4) | 0.64 (0.45-0.91) |
| H. pyloriinfection | |||
| No | 168 (21.7) | 452 (19.4) | 1.0 (reference) |
| Infected | 155 (20) | 361 (15.5) | 1.19 (0.91-1.57) |
| Eradicated | 452 (58.3) | 1522 (65.2) | 0.82 (0.66-1.03) |
Note: P<0.05, statistical analysis was performed by t-test /χ²-test. OR: Odds Ratio; Cl: Confidence Interval; BMI: Body Mass Index.
Table 2: General risk factors associated with gastric cancer.
Stratified analysis and interaction test of alcohol consumption status and risk of gastric cancer
In the multivariate analysis of the association between drinking status and gastric cancer risk, drinkers had a higher risk of gastric cancer than nondrinkers and this association was statistically significant (OR=1.33; 95% CI=1.04-1.70). Stratification by age and gender showed similar trends in the association between gastric cancer risk and alcohol consumption status for all participants ass shown in (Table 3). However, stratification by H. pylori infection status showed that in the uninfected and eradicated H. pylori groups, drinkers had a higher risk of developing gastric cancer, whereas, in the infected H. pylori group, drinkers had a lower risk of gastric cancer than non-drinkers (OR=0.51, 95% CI=0.25-1.03), although this was not statistically significant. In addition, interaction analysis showed that there was an interaction between H. pylori infection and alcohol consumption (P<0.05).
| Alcohol consumption status | Cases N (%) | Controls N (%) | Age and gender adjusted OR (95% CI) | Multivariate adjusted OR (95% Cl) | Pinteraction |
|---|---|---|---|---|---|
| H. pylori infection | |||||
| No | 0.04 | ||||
| Non-drinker | 126 (23.9) | 402 (76.1) | 1.0 (reference) | 1.0 (reference) | |
| Drinker | 42 (45.7) | 50 (54.3) | 2.50 (1.49-4.20) | 2.57 (1.48-4.45) | |
| Infected | |||||
| Non-drinker | 134 (29.8) | 316 (70.2) | 1.0 (reference) | 1.0 (reference) | |
| Drinker | 21 (31.8) | 45 (68.2) | 0.76 (0.41-1.41) | 0.51 (0.25-1.03) | |
| Eradicated | |||||
| Non-drinker | 365 (21.3) | 1345 (78.7) | 1.0 (reference) | 1.0 (reference) | |
| Drinker | 87 (33.0) | 177 (67.0) | 1.195 (0.87-1.64) | 1.13 (0.82-1.56) | |
| Gender | |||||
| Male | 0.14 | ||||
| Non-drinker | 338 (67.2) | 693 (32.8) | 1.0 (reference) | 1.0 (reference) | |
| Drinker | 150 (37.1) | 254 (62.9) | 1.38 (1.08-1.78) | 1.35 (1.05-1.75) | |
| Female | |||||
| Non-drinker | 287 (17.2) | 1380 (82.8) | 1.0 (reference) | 1.0 (reference) | |
| Drinker | 2 (9.5) | 19 (90.5) | 0.51 (0.12-2.23) | 0.44 (0.10-1.95) | |
| Age | |||||
| 40~ | 0.23 | ||||
| Non-drinker | 153 (13.2) | 1005 (86.8) | 1.0 (reference) | 1.0 (reference) | |
| Drinker | 50 (27.6) | 131 (72.4) | 1.64 (1.10-2.44) | 1.47 (0.97-2.23) | |
| 50~ | |||||
| Non-drinker | 216 (23.9) | 688 (76.1) | 1.0 (reference) | 1.0 (reference) | |
| Drinker | 61 (36.3) | 107 (63.7) | 1.182 (0.80-1.74) | 1.08 (0.73-1.61) | |
| 60~70 | |||||
| Non-drinker | 256 (40.3) | 380 (59.7) | 1.0 (reference) | 1.0 (reference) | |
| Drinker | 41 (53.9) | 35 (46.1) | 1.13 (0.68-1.87) | 1.06 (0.63-1.79) | |
Note: P<0.05, statistical analysis was performed by t-test /χ²-test; OR: Odds Ratio; CI: Confidence Interval.
Table 3: Stratified analysis and interaction between alcohol consumption status and the risk of developing gastric cancer.
Subgroup analysis
Subgroup analysis showed that the risk of gastric cancer was 2.45 times that of non-drinkers (OR=2.45, 95% CI=1.07-5.59, P<0.05) in the 50 age group without H. pylori infection as shown in Table 4. After adjustment, the risk of gastric cancer was 2.42 (OR=2.42, 95% CI=1.02-5.73, P<0.05), which was still statistically significant. In the 60-70 age group, the risk of gastric cancer was 3.26 (OR=3.26, 95% CI=1.12-9.50, P<0.05) higher than that of non-drinkers. The adjusted risk of gastric cancer among drinkers was 3.80 (OR=3.80, 95% CI=1.50-10.95, P<0.05), which was still statistically significant. In the state of H. pylori infection seen in Table 5, the risk of gastric cancer in smokers who had quit smoking was 1.65 times that of non-smokers (OR=1.65, 95% CI=1.11-2.28, P<0.05), which was statistically significant. The adjusted risk of gastric cancer was 1.34 times higher in drinkers than in non-drinkers (OR=1.34, 95% CI=0.66-2.39, P>0.05), but this result was not statistically significant. With H. pylori eradication seen in Table 6, the risk of gastric cancer was 1.74 times that of non-drinkers (OR=1.74, 95% CI=1.04-2.89, P<0.05). The adjusted risk of gastric cancer was 1.64 times that of non-drinkers (OR=1.64, 95% CI=1.01-2.78, P<0.05), which was still statistically significant.
| Alcohol consumption status | Cases N (%) | Controls N (%) | Age and sex adjusted OR (95% CI) | Multivariate adjusted OR (95% Cl) |
|---|---|---|---|---|
| Age | ||||
| 40~ | ||||
| Non-drinker | 625 (23.2) | 2073 (76.8) | 1.0 (reference) | 1.0 (reference) |
| Drinker | 152 (35.8) | 273 (64.2) | 1.90 (0.78-4.61) | 1.87 (0.72-4.86) |
| 50~ | ||||
| Non-drinker | 43 (23.1) | 143 (76.9) | 1.0 (reference) | 1.0 (reference) |
| Drinker | 17 (45.9) | 20 (54.1) | 2.45 (1.07-5.59) | 2.42 (1.02-5.73) |
| 60~70 | ||||
| Non-drinker | 51 (38.6) | 81 (61.4) | 1.0 (reference) | 1.0 (reference) |
| Drinker | 14 (70.0) | 6 (30.0) | 3.26 (1.12-9.50) | 3.80 (1.50-10.95) |
| Smoking | ||||
| Never | ||||
| Non-drinker | 92 (23.2) | 304 (76.8) | 1.0 (reference) | 1.0 (reference) |
| Drinker | 4 (20.0) | 16 (80.0) | 0.75 (0.23-2.45) | 0.76 (0.23-2.52) |
| Still | ||||
| Non-drinker | 24 (24.0) | 76 (76.0) | 1.0 (reference) | 1.0 (reference) |
| Drinker | 35 (52.2) | 32 (47.8) | 4.09 (2.02-8.29) | 4.99 (2.29-10.91) |
| Stopped smoking | ||||
| Non-drinker | 10 | 22 | 1.0 (reference) | 1.0 (reference) |
| Drinker | 3 | 2 | 1.17 (0.44-2.64) | 1.07 (0.32-2.51) |
Note: P<0.05, statistical analysis was performed by t-test /χ²-test; CI: Confidence Interval; OR: Odds Ratio.
Table 4: Subgroup analysis of alcohol consumption status and gastric cancer risk without H. pylori infection.
| Alcohol consumption status | Cases N (%) | Controls N (%) | Age and sex adjusted OR (95% CI) | Multivariate adjusted OR (95% Cl) |
|---|---|---|---|---|
| Age | ||||
| 40~ | ||||
| Non-drinker | 32 (80.0) | 146 (88.0) | 1.0 (reference) | 1.0 (reference) |
| Drinker | 8 (20.0) | 20 (12.0) | 1.09 (0.41-2.87) | 0.817 (0.29-2.29) |
| 50~ | ||||
| Non-drinker | 44 (28.6) | 110 (71.4) | 1.0 (reference) | 1.0 (reference) |
| Drinker | 6 (23.1) | 20 (76.9) | 0.63 (0.22-1.81) | 0.51 (0.15-1.68) |
| 60~70 | ||||
| Non-drinker | 58 (49.2) | 60 (50.8) | 1.0 (reference) | 1.0 (reference) |
| Drinker | 7 (58.3) | 5 (41.7) | 0.78 (0.22-2.77) | 0.69 (0.18-2.61) |
| Smoking | ||||
| Never | ||||
| Non-drinker | 78 (23.9) | 249 (76.1) | 1.0 (reference) | 1.0 (reference) |
| Drinker | 2 (16.7) | 10 (83.3) | 0.48 (0.92-2.51) | 0.32 (0.56-1.87) |
| Still | ||||
| Non-drinker | 45 (46.9) | 51 (53.1) | 1.0 (reference) | 1.0 (reference) |
| Drinker | 16 (32.0) | 34 (68.0) | 0.57 (0.27-1.17) | 0.52 (0.24-1.10) |
| Stopped smoking | ||||
| Non-drinker | 11 (40.7) | 16 (59.3) | 1.0 (reference) | 1.0 (reference) |
| Drinker | 3 (75.0) | 1 (25.0) | 1.65 (1.11-2.28) | 1.34 (0.56-2.39) |
Note: P<0.05, statistical analysis was performed by t-test /χ²-test; CI: Confidence Interval; OR: Odds Ratio.
Table 5: Subgroup analysis of alcohol consumption and risk of gastric cancer under H. pylori infection.
| Alcohol consumption status | Cases N (%) | Controls N (%) | Age and sex adjusted OR (95% CI) | Multivariate adjusted OR (95% Cl) |
|---|---|---|---|---|
| Age | ||||
| 40~ | ||||
| Non-drinker | 89 (11.7) | 674 (88.3) | 1.0 (reference) | 1.0 (reference) |
| Drinker | 31 (26.5) | 86 (73.5) | 1.74 (1.04-2.89) | 1.64 (1.01-2.78) |
| 50~ | ||||
| Non-drinker | 129 (23.0) | 432 (77.0) | 1.0 (reference) | 1.0 (reference) |
| Drinker | 37 (35.6) | 67 (64.4) | 1.00 (0.61-1.63) | 0.93 (0.56-1.53) |
| 60~70 | ||||
| Non-drinker | 58 (49.2) | 60 (50.8) | 1.0 (reference) | 1.0 (reference) |
| Drinker | 7 (58.3) | 5 (41.7) | 0.78 (0.22-2.77) | 0.69 (0.18-2.61) |
| Smoking | ||||
| Never | ||||
| Non-drinker | 208 (16.8) | 1029 (83.2) | 1.0 (reference) | 1.0 (reference) |
| Drinker | 14 (25.5) | 41 (74.5) | 0.93 (0.46-1.87) | 0.86 (0.43-1.73) |
| Still | ||||
| Non-drinker | 118 (31.8) | 255 (68.4) | 1.0 (reference) | 1.0 (reference) |
| Drinker | 66 (36.3) | 116 (63.7) | 1.36 (0.92-2.00) | 1.33 (0.89-1.97) |
| Stopped smoking | ||||
| Non-drinker | 39 (39.0) | 61 (61.0) | 1.0 (reference) | 1.0 (reference) |
| Drinker | 7 (25.9) | 20 (74.1) | 0.61 (0.22-1.63) | 0.57 (0.21-1.58) |
Note: P<0.05, statistical analysis was performed by t-test /χ²-test;CI: Confidence Interval; OR: Odds Ratio.
Table 6: Subgroup analysis of alcohol consumption and risk of gastric cancer under H. pylori eradication.
The univariate analysis and multivariate analysis of risk factors for gastric cancer. The interaction between alcohol consumption and the H. pylori infection is an area that merits further investigation. The data indicates that both alcohol consumption and H. pylori infection are associated with an increased risk of gastric cancer [16]. However, the nature of the combined effect of these two factors is not fully understood. One hypothesis is that alcohol may act synergistically with H. pylori to promote gastric carcinogenesis. Chronic alcohol exposure can result in gastric mucosal inflammation and atrophy, creating an environment conducive to H. pylori colonization and persistence. Conversely, H. pylori infection itself can induce oxidative stress and Deoxy Ribo Nucleic Acid (DNA) damage, potentially amplifying the carcinogenic effects of alcohol metabolites. An alternative hypothesis is that alcohol and H. pylori operate through distinct pathways, independently elevating cancer risk through different mechanisms. In this scenario, the effects of alcohol and H. pylori could be additive rather than synergistic. To gain a more comprehensive understanding of this relationship, it would be beneficial to stratify the data based on H. pylori infection status and alcohol consumption patterns. This could elucidate whether individuals who consume alcohol in excess face a disproportionately elevated risk if they are also infected with H. pylori, compared to individuals who consume alcohol in moderation or who are not infected with the bacteria [17-21].
Conversely, examining the impact of alcohol among H. pylori-negative individuals could provide insight into its independent carcinogenic potential. Furthermore, investigating potential effect modifiers, such as smoking, diet and socioeconomic factors, could provide insights into how these variables interact to influence gastric cancer development. For example, it would be of interest to ascertain whether certain dietary components (e.g., garlic, as indicated in the data) act to mitigate or exacerbate the combined effects of alcohol and H. pylori. Ultimately, a more profound comprehension of these intricate interactions could facilitate the development of targeted prevention strategies and personalized risk assessments for gastric cancer. While the current data provides insight into the key risk factors, further research is necessary to elucidate the specific mechanisms underlying the interplay between alcohol, H. pylori and other variables in gastric carcinogenesis.
In the analysis of general risk factors for gastric cancer, after adjusting for age and gender. One of the most significant findings is the correlation between alcohol consumption and an increased risk of gastric cancer. The odds ratio of 1.33 for the drinking group in comparison to non-drinkers is a noteworthy finding. This finding is consistent with previous research indicating a correlation between alcohol consumption and an increased risk of developing various cancers, including gastric cancer. The potential mechanisms underlying this association may involve alcohol's capacity to act as an irritant to the gastric mucosa, as well as its metabolites, which may induce oxidative stress and DNA damage [22].
Similarly, the higher odds ratio of 1.37 for smokers compared to non-smokers is not unexpected, given the well-established carcinogenic effects of tobacco smoke. The chemicals in cigarette smoke can lead to DNA mutations and impaired cellular repair mechanisms, thereby increasing cancer susceptibility in various organs, including the stomach [23].
The finding that higher per capita income is associated with increased gastric cancer risk is intriguing [24]. It would be of interest to investigate the potential contributing factors to this observation, such as dietary patterns, stress levels or access to healthcare screening and early detection.
Conversely, the protective effect of garlic consumption against gastric cancer is noteworthy, with an odds ratio of 0.64 for those who never ate garlic compared to regular consumers. This finding is consistent with previous studies that have demonstrated the anti-cancer properties of garlic, which may be attributed to its organosulfur compounds and antioxidant effects [25,26].
While our study did not find a significant association between H. pylori infection and gastric cancer risk, it's important to note that H. pylori is a well-established risk factor for gastric cancer, particularly in cases of chronic infection and atrophic gastritis. The interaction between H. pylori and other risk factors, such as diet and lifestyle, could be an interesting avenue for further research [27,28].
Overall, our findings highlight the complex interplay of various factors in gastric cancer development and underscore the importance of adopting a healthy lifestyle, including limiting alcohol consumption, avoiding smoking and incorporating protective dietary components like garlic. Additionally, regular screening and early detection efforts could play a crucial role in reducing the burden of gastric cancer in high-risk populations.
The multivariate analysis of the association between drinking status and gastric cancer risk, indicated that, in general, individuals who consume alcohol have a higher risk of developing gastric cancer compared to those who do not drink alcohol. This association was found to be statistically significant. When stratified by age and gender, the observed trend remained consistent across all participant groups. However, when the data were stratified by H. pylori infection status, the results were quite different. In the uninfected and eradicated H. pylori groups, drinkers still exhibited a higher risk of gastric cancer, as anticipated. It is noteworthy that in the infected H. pylori group, drinkers exhibited a lower risk of gastric cancer than non-drinkers, although this finding was not statistically significant.
The interaction analysis demonstrated that there was indeed an interaction between H. pylori infection and alcohol consumption. This suggests that the effect of alcohol on gastric cancer risk may be moderated by the presence or absence of H. pylori infection [29,30].
This finding is intriguing and raises several questions. First, it is necessary to determine why alcohol consumption may have a protective effect against gastric cancer in the presence of H. pylori infection. One potential explanation is that alcohol may possess antimicrobial properties that inhibit the growth or virulence of H. pylori, thereby reducing the risk of gastric cancer development. However, this is purely speculative and further research would be necessary to explore this hypothesis [31,32].
A further question that arises is whether the type of alcoholic beverage consumed (e.g., beer, wine, spirits) or the pattern of consumption (e.g., binge drinking vs. moderate consumption) might influence the association between alcohol and gastric cancer risk in the context of H. pylori infection [33-36].
It is also important to consider the potential confounding factors that may have influenced the observed results. For instance, dietary habits, smoking status, socioeconomic factors and other lifestyle or environmental factors may interact with both alcohol consumption and H. pylori infection, thereby influencing the observed associations.
Overall, these findings underscore the intricate interrelationship between diverse risk factors and their potential interactions in the pathogenesis of gastric cancer. While the study provides valuable insights, further research is necessary to replicate these findings in different populations and to elucidate the underlying mechanisms behind the observed interactions [37].
Furthermore, it would be of interest to ascertain whether analogous interactions exist between alcohol consumption and other infectious agents or chronic inflammatory conditions that may contribute to gastric cancer risk. Such research could potentially lead to a more comprehensive understanding of the multifactorial etiology of gastric cancer and inform more targeted prevention and intervention strategies.
In the subgroup analysis, it is noteworthy that in the 50-70 age group without H. pylori infection. Alcohol consumption was associated with a significantly higher risk of gastric cancer, even after adjusting for potential confounders. This indicates that alcohol may be an independent risk factor for gastric cancer development, particularly in older individuals without H. pylori infection [38]. However, in the presence of H. pylori infection, the risk of gastric cancer was significantly elevated among former smokers, but not among drinkers. This suggests the potential for an interaction between smoking, alcohol consumption and H. pylori infection in the development of gastric cancer [39-41]. The analysis also investigated the influence of H. pylori eradication on the risk of gastric cancer among drinkers. It is notable that even after the successful eradication of the bacteria, alcohol consumption remained a significant risk factor for gastric cancer development. These findings prompt several thought-provoking questions that warrant further investigation. Firstly, what are the potential mechanisms through which alcohol consumption increases the risk of gastric cancer, particularly in the absence of H. pylori infection? It is conceivable that chronic alcohol exposure may result in gastric mucosal damage, inflammation or alterations in the gastric microenvironment, thereby promoting carcinogenesis. Secondly, the reasons for the apparent discrepancy between the interaction between alcohol consumption and H. pylori infection and the interaction between smoking and H. pylori infection in terms of gastric cancer risk remain unclear. It is conceivable that there are discrete pathways or synergistic effects involved [42-46].
Moreover, the persistence of elevated gastric cancer risk among drinkers even after H. pylori eradication raises questions about the reversibility of alcohol-induced gastric mucosal changes and the potential for residual risk factors beyond bacterial infection. It would be of interest to explore potential confounders or effect modifiers that could influence the observed associations, such as dietary patterns, socioeconomic status or genetic predispositions. Furthermore, investigating the dose-response relationship between alcohol consumption and gastric cancer risk across different subgroups could provide valuable insights.
In summary, this analysis illuminates the intricate interrelationship between alcohol consumption, H. pylori infection and gastric cancer risk, underscoring the necessity of considering a multitude of factors and their potential interactions in cancer prevention strategies. While this study offers valuable insights, it is essential to consider potential limitations and areas for future research. For instance, the study relied on self-reported data for alcohol consumption and other lifestyle factors, which may be subject to recall bias or underreporting. Moreover, the case-control design of the study precludes the ability to establish causality. Prospective cohort studies or interventional studies could provide more robust evidence.
Overall, this study contributes to the growing body of knowledge on the complex etiology of gastric cancer and the interplay between various risk factors. The findings underscore the importance of considering individual risk profiles, including H. pylori infection status, when assessing the impact of alcohol consumption on gastric cancer risk. Further research is needed to elucidate the underlying mechanisms and develop tailored prevention and management strategies for different populations.
Alcohol consumption represents a significant risk factor for the development of gastric cancer. This risk is particularly pronounced with advancing age and following the eradication of H. pylori. The study involved 3,123 long-term residents of Wuwei city, aged between forty and seventy years, who participated by providing detailed information on personal demographics, lifestyle factors including diet and alcohol consumption, medical history and detection of H. pylori infection. Rigorous diagnostic procedures such as upper gastrointestinal endoscopy, histology and the 13C-Urea Breath Test were employed to screen all participants for H. pylori infection, ensuring accurate results and minimizing the influence of recall bias in the study findings.
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
Citation: Olivier BM, Feng B, Zhou Y, Zhang M, Wang Y, Lv Z, et al. (2024) Analysis of Alcohol Consumption on Gastric Cancer in Different Infectious States of Helicobacter pylori in the Wuwei Population, China. J Clin Chem Lab Med. 7:290
Received: 12-Jul-2024, Manuscript No. JCCLM-24-32870 ; Editor assigned: 15-Jul-2024, Pre QC No. JCCLM-24-32870 (PQ); Reviewed: 29-Jul-2024, QC No. JCCLM-24-32870 ; Revised: 05-Aug-2024, Manuscript No. JCCLM-24-32870 (R); Published: 13-Aug-2024 , DOI: 10.35248/2736-6588.24.7.290
Copyright: © 2024 Olivier BM, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution and reproduction in any medium, provided the original author and source are credited