Medicinal & Aromatic Plants
Open Access
ISSN: 2167-0412
ISSN: 2167-0412
Research Article - (2019)Volume 8, Issue 4
The medicinal plant garden of Hoshi University located in southern Tokyo is home to many medicinal plants, and analysis of odor and measurement of antioxidant activity of cultivated plant have been carried out. In this study compounds originated from Curcuma rhizomes, i.e., Curcuma longa, Curcuma aromatica, Curcuma zedoaria, Curcuma
xanthorrhiza, were investigated and antioxidant activities of rhizome extracts were measured. Volatile compounds originating from the Curcuma rhizomes were analyzed using thermal desorption (TD)-GC-MS with solid-phase micro extraction fiber as an adsorption device. p-Cymene, 1,8-cineol, β-elemene, and β-caryophyllene were the predominant constituents in most cases. Curcuminoids, which were not identified by TD-GC-MS, were detected using direct analysis in real time time-of-flight MS. Curcumin and demethoxycurcumin were detected from both C. longa and C. xanthorrhiza. The antioxidant activity of each Curcuma species rhizome was confirmed using the electron spin-resonance spin-trapping method with potent scavenging activity against superoxide anion radicals. Extracts from Curcuma rhizomes cultivated in the medicinal plant garden exhibited antioxidant activities, and the order of the activity of methanol extracts was: C. longa>C. xanthorrhiza>C. aromatica>C. zedoaria. Phenolic compounds, particularly curcumin, are known to possess potent antioxidant activity and are extracted with methanol, thus it reflects the intensity of the antioxidant activity.
Curcuma longa; Curcuma aromatica; Curcuma zedoaria; Curcuma xanthorrhiza; Component; Thermal desorption-GC-MS; Direct analysis in real time-TOFMS; Antioxidant activity; Electron spin-resonance spectrometry
Curcuma plants belong to the family Zingiberaceae and are widely distributed throughout the tropics, particularly in South Asia. Rhizomes of Curcuma species located underground have been used for cooking, dyes, healthcare products, and traditional medicines in Asian countries and are endowed with antioxidative properties [1].
In the medicinal plant garden of Hoshi University Curcuma longa, Curcuma aromatica, Curcuma zedoaria, and Curcuma xanthorrhiza are cultivated. Turmeric is the rhizome of C. longa. The main rhizome is nearly ovoid with a yellow brown to yellow-red color. It contains the greatest volume of curcuminoids, i.e., curcumin (1), demethoxycurcumin (2), and bisdemethoxycurcumin, compared with other Curcuma species. The C. longa rhizome is commonly used for cooking such as a spice in curry dishes and for coloring and flavoring. It has also been used for medicinal purposes, e.g. as a cholagogue and as an anti-inflammatory, anti-angiogenic, antibacterial, anti-fungal, analgesic, immunomodulatory, vasodilatory, anti-diabetes, and anti-Alzheimer’s disease agent [2,3].
Compared with C. longa, the activities of other Curcuma species have not been thoroughly investigated even though they also show a wide range of medicinal properties. C. aromatica has been used in cosmetic formulation and exhibits various pharmacological properties, including anti-inflammatory, antioxidant, antiangiogenic, and anti-carcinogenic effects as well as anti-bacterial activity. It contains monoterpenoids, sesquiterpenoids, and curcuminoids [4,5]. Zedoary is the rhizome of C. zedoaria. It is nearly ovoid and externally grayish yellow brown to grayish-brown in color and is a perennial rhizomatous herb indigenous to Bangladesh, Sri Lanka, and India. Zedoary exhibits anti-inflammatory, antibabesial, cytotoxic, and anti-fungal activities. In Indonesia, it is used traditionally in the treatment of parasitic organisms. Lotion made from its extract has been used for skin care as an effective whitener [6-9]. C. xanthorrhiza, which is native to Indonesia and commonly known as “java ginger” or “temulawak,” has been shown to exert anti-bacterial, anti-fungal, anti-halitosis, anti-inflammatory, and anti-oxidant activities. In particular, xanthorrhizol (3) exhibits strong activity, and inhibitory effects on periodontitis were also reported Figure 1 [10].
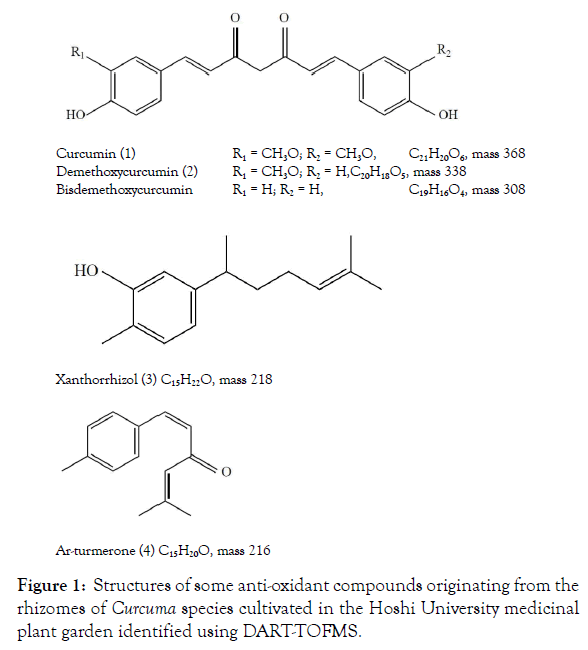
Figure 1: Structures of some anti-oxidant compounds originating from the rhizomes of Curcuma species cultivated in the Hoshi University medicinal plant garden identified using DART-TOFMS.
Although the medicinal plant garden of Hoshi University is home of medicinal plants, there is a lack of information about odor and functional activity of the plants. Thus, we have investigated the odor, which is composed of several volatile compounds, and analyzed anti-oxidant activities of several plants cultivated in the garden [11-13] because essential oil compositions, odor component, functional activity are affected by the cultivar, time of harvest, and environment.
Although we have already reported about volatile compounds and antioxidant activity of rhizome extract of C. longa [12], comparative information among the different Curcuma species, i.e., C. longa, C. aromatica, C. zedoaria, and C. xanthorrhiza, are required. Our investigations evaluate the differences between Curcuma plants species in the volatile compounds, and non-volatile curcuminoids, sesquiterpenoids, and antioxidant activity of extracts from rhizomes of different species. We compared the compounds obtained from Curcuma species rhizomes in the medicinal plant garden with those presented in the previous reports [14,15].
For analysis of volatile compounds originating from fresh rhizomes of Curcuma plants, thermal desorption (TD)-GC-MS was used. As the device for adsorption of volatile compounds Solid-Phase Micro Extraction (SPME) fiber coated with carboxene (CAR)/ polydimethylsiloxane (PDMS) was used. Subsequently analyses of Curcuma rhizomes using Direct Analysis in Real Time (DART) Timeof- Flight (TOF) MS was performed. DART is one of the ionization techniques of MS and is conducted in the open air, allowing for the rapid and direct analysis of samples in the gas, liquid, and solid states [16,17]. We expected DART-TOFMS to detect curcuminoids and sesquiterpenoids, which were not detected by TD-GC-MS, without complicated sample preparation prior to measurement.
Antioxidants are important because they prevent oxidative cell damage by acting as free radical scavengers. The superoxide anion radical (O2∙ˉ), hydroxyl radical (∙OH), and hydrogen peroxide are produced continuously in the human body. The harmful reactive oxygen species may be related to cancer, cardiovascular disease, diabetes, neurodegenerative diseases, and aging. Although endogenous enzymes such as Superoxide Dismutase (SOD) usually control the levels of free radicals, oxidative stress occurs under high levels of free radicals. To counter the effects of oxidative stress, many people take antioxidants. As there are limitations on the use of synthetic antioxidants such as butylated hydroxy anisole, butylated hydroxytoluene, and tert-butyl hydroquinone because of their production of toxins or action as carcinogens, identifying antioxidants in natural materials is required. Several plants are rich in antioxidant compounds with no toxic effects. For measuring the antioxidant activity of Curcuma rhizomes cultivated in the medicinal plant garden, the Electron Spin-Resonance (ESR) spin-trapping method was used. Superoxide anion radical (O2∙ˉ) is trapped by a spin-trapping agent such as 5,5-dimethyl-1-pyrroline- N-oxide (DMPO) to form a spin adduct such as DMPO–OOˉ. A decrease in the ESR signal intensity of DMPO-OOˉ with an addition of a sample extract to the reaction system reflects the antioxidant activity.
Plant material
C. longa, C. aromatica, C. zedoaria, and C. xanthorrhiza were purchased from a domestic market in Japan and are cultivated in the medicinal plant garden of Hoshi University located in southern Tokyo. Fresh rhizomes of Curcuma species harvested from the garden in November were washed with water, peeled, cut into small pieces or grated prior to analyses.
Chemicals
For TD-GC-MS measurements, the standard reagent β-caryophyllene was purchased from Fujifilm Wako Pure Chemical Corporation (Tokyo, Japan); p-cymene, (E)-β-farnesene, β-myrcene, and α-terpinolene were from Tokyo Chemical Industry Co., Ltd. (Tokyo, Japan); and 1,8-cineol was from Sigma-Aldrich (St. Louis, MO, USA). Naginata criteria sample Mix II in dichloromethane, necessary for the determination of retention indices (RIs), was purchased from Hayashi Pure Chemical Industries, Ltd. (Osaka, Japan). For DART-TOFMS measurement, the standard reagent Polyethylene Glycol (PEG) with average molecular weight of 400 was purchased from Tokyo Chemical Industry Co., Ltd.
For ESR measurement, Hypoxanthine (HPX) and Xanthine Oxidase (XOD) from bovine milk were obtained from Sigma- Aldrich, SOD and sodium dihydrogen phosphate were from Fujifilm Wako Pure Chemical Corporation, DMPO was from Labotec (Tokyo, Japan), methanol (MeOH) and sodium hydroxide were from Kanto Chemical Co., Inc. (Tokyo, Japan). For preparing aqueous solutions water filtered through Autopure WD501UV from Yamato (Tokyo, Japan) was used.
Instrumentation
TD-GC-MS analyses: TD unit equipped with a CIS4 programmed temperature vaporization inlet (Gerstel, Mülheim an der Ruhr, Germany); 7890A gas chromatograph (Agilent Technologies, Palo Alto, CA, USA); and JMS-700 mass spectrometer (JEOL, Tokyo, Japan) was used. DB-5 column (30-m × 0.25-mm internal diameter) coated with a 0.25-μm film consisting of 5% phenyl and 95% dimethyl polysiloxane (Agilent Technologies) was equipped.
DART-TOFMS analyses: JMS-T100LP mass spectrometer (JEOL) in positive-ion mode was used. The DART ion source conditions were needle voltage, 2500 V; electrode, lens 1: 100 V and lens 2: 250 V; helium gas flow, 3 L/min; and temperature, 300°C. The TOFMS was set as follows: 1st orifice lens, 15 V; 2nd orifice lens, 5 V; ling lens, 10 V; peak voltage, 500 V; bias voltage, 27 V; pusher bias voltage, –0.45 V; and detector voltage, 2200 V. PEG 400 was used as a calibration standard.
ESR analyses: JES-RE1X ESR spectrometer (JEOL) was used. The measurement conditions were: magnetic field, 335.7 ± 5 mT; sweep width, 5 × 1; microwave power, 9 mW; modulation width, 0.063 mT; sweep time, 30 s; and time constant, 0.03 s. The signal intensity was normalized as the relative height against the standard signal intensity of the manganese oxide marker.
TD-GC-MS analyses
For adsorption of volatile compounds, SPME fiber coated with a 100-μm-thick film of CAR/PDMS (Supelco, Bellefone, PA, USA) was used as the device. The plant material was harvested, cut into pieces (ca. 1 g per sample), and transferred to a 40-mL vial equipped with a clean pinhole septum (Thermo Fischer Scientific, Waltham, MA, USA) immediately. SPME fiber was fixed in the headspace of the vial in which each rhizome was placed, and headspace sportive extraction was carried out at room temperature for 60 min. The adsorption device was removed and injected into the TD-GC-MS system. The conditions of TD-GC-MS were same as mentioned before [12].
DART-TOFMS analyses
Each rhizome was broken into fine pieces using a grater and then dipped with a glass rod. Then it was placed between the inlet of the MS analyzer and the DART ion source for direct analysis. The ions were observed down to the fourth decimal place using PEG 400 as a calibration standard.
ESR spin-trapping analyses
Each sample solution was prepared by extracting grated rhizome of 0.1 g with either 1 mL of sodium phosphate buffer–water solution (PB; pH 7.8) or with 1 mL of MeOH and was stored at –78°C until further analysis. Superoxide anion radical-scavenging activity (SOSA) was measured by ESR using the same method as mentioned before [11,12,18–20]. The SOSA of the extracts was compared as the SOD equivalent (U/mg) using SOD as a standard.
Volatile compounds originating from freshly harvested rhizomes using TD-GC-MS
Odor compositions of Curcuma rhizome species cultivated in the medicinal plant garden are shown in Table 1. Volatile compounds were identified by comparison of the mass spectra with those in the National Institute of Standards and Technology (NIST) library (Gaithersburg, MD, USA) and by comparing RIs with those reported in the literature in the Aroma Office database [21]. When authentic standards were available, the compounds were further confirmed by comparing retention times and mass spectra with those of the authentic standards.
p-Cymene, 1,8-cineol, β-elemene, and β-caryophyllene were the predominant constituents in most cases. ar-Curcumene and β-bisabolene were found from both C. longa and C. xanthorrhiza.
The compounds obtained from Curcuma species rhizomes harvested in the medicinal plant garden were compared with those in essential oil obtained by hydrodistillation presented in the previous reports [14,15,22]. In C. longa rhizome in the garden β-elemene was newly identified using this procedure. In C. zedoaria rhizome limonene, 2-nonanone, camphor, and (E)-β-farnesene were newly obtained. In C. aromatica rhizome β-myrcene, limonene, 2-nonane, linalool, terpinen-4-ol, β-caryophyllene, α -humulene were newly detected. In C. xanthorrhiza rhizome p-cymmene, limonene, α-terpinolene, β-caryophyllene, and ar-turmerone were newly identified (Table 1).
| aCompounds | bRI | cRIlit | C. longa | C. zedoaria | C. aromatica | C. xanthorrhiza | dID |
|---|---|---|---|---|---|---|---|
| ß-Myrcene | 990 | 990 | — | 〇 | 〇 | 〇 | MS, RI, Std |
| p -Cymene | 1024 | 1023 | 〇 | 〇 | 〇 | 〇 | MS, RI, Std |
| Limonene | 1027 | 1027 | — | 〇 | 〇 | 〇 | MS, RI, Std |
| 1, 8-Cineol | 1029 | 1030 | 〇 | 〇 | 〇 | 〇 | MS, RI, Std |
| ß-cis -Ocimene | 1037 | 1037 | — | 〇 | — | — | MS, RI |
| 2-Nonanone | 1090 | 1090 | — | 〇 | 〇 | — | MS, RI, Std |
| a-Terpinolene | 1089 | 1089 | 〇 | — | — | 〇 | MS, RI, Std |
| Linalool | 1099 | 1099 | — | — | 〇 | — | MS, RI, Std |
| Camphor | 1143 | 1143 | — | 〇 | — | — | MS, RI, Std |
| Terpinen-4-ol | 1175 | 1175 | — | — | 〇 | — | MS, RI, Std |
| a-Terpineol | 1189 | 1189 | — | 〇 | 〇 | — | MS, RI, Std |
| 2-Undecanone | 1292 | 1292 | — | 〇 | — | — | MS, RI |
| d-Elemene | 1338 | 1338 | — | 〇 | 〇 | — | MS, RI |
| ß-Elemene | 1349 | 1349 | 〇 | 〇 | 〇 | 〇 | MS, RI |
| ß-Caryophyllene | 1423 | 1423 | 〇 | 〇 | 〇 | 〇 | MS, RI, Std |
| a-Humulene | 1456 | 1456 | — | — | 〇 | — | MS, RI, Std |
| (E )- ß-Farnesene | 1457 | 1457 | 〇 | 〇 | — | 〇 | MS, RI, Std |
| ar-Curcumene | 1485 | 1485 | 〇 | — | — | 〇 | MS, RI |
| ß-Bisabolene | 1512 | 1512 | 〇 | — | — | 〇 | MS, RI |
| g-Cadinene | 1517 | 1517 | — | 〇 | — | — | MS, RI |
| d-Cadinene | 1526 | 1526 | — | 〇 | — | — | MS, RI |
| ß-Sesquiphellandrene | 1531 | 1531 | — | — | — | 〇 | MS, RI |
| ar-Turmerone | 1663 | 1664 | — | 〇 | — | 〇 | MS, RI |
Table 1: Volatile compounds originating from Curcuma species rhizomes cultivated in the garden, identified by TD-GC-MS using CAR/PDMS SPME fiber as an adsorption device.
Distinctive compounds such as curcuminoids in Curcuma rhizomes using DART-TOFMS
Because curcumin (1), the main compound exhibiting antioxidant activity in C. longa, was not detected using the TD-GC-MS procedure, DART-TOFMS was carried out subsequently. For investigating the relation between components and antioxidant activities of rhizomes cultivated in the medicinal plant garden, the identification of curcuminoids was necessary.
The measured mass was compared with the calculated mass of the composition formula of compounds previously reported as components of Curcuma rhizomes [7,22,23]. Proposed components of Curcuma rhizomes from the garden, which were assumed from the difference between the measured mass and calculated mass, are shown in Table 2.
| (a) Curcuma longa | |||||
| Compounds | [M+H] | Mass | (mmu) mass difference | ||
| Calcd. | Measd. | ||||
| Zingiberene | C15H25 | 205.1956 | 205.1944 | 1.2 | |
| ar-turmerone | C15H21O | 217.1592 | 217.1589 | 0.3 | |
| germacrone | C15H23O | 219.1749 | 219.1738 | 1.1 | |
| Curcumenol isocurcumenol | C15H23O2 | 235.1698 | 235.1684 | 1.4 | |
| demethoxycurcumin | C20H19O5 | 339.1233 | 339.1251 | 1.8 | |
| curcumin | C21H21O6 | 369.1338 | 369.1346 | 0.8 | |
| (b) C. xanthorrhiza | |||||
| Compounds | [M+H] | mass | (mmu) mass difference | ||
| Calcd. | Measd. | ||||
| ar-curcumene | C15H23 | 203.18 | 203.1801 | 0.1 | |
| Zingiberene | C15H25 | 205.1956 | 295.1966 | 1 | |
| ar-turmerone | C15H21O | 217.1592 | 217.1599 | 0.7 | |
| Xanthorrhizol | C15H23O | 219.1749 | 219.1763 | 1.4 | |
| Demethoxycurcumin | C20H19O5 | 339.1233 | 339.1208 | 2.5 | |
| Curcumin | C21H21O6 | 369.1338 | 369.1362 | 2.4 | |
| (c) C. aromatica | |||||
| Compounds | [M+H] | mass | (mmu) mass difference | ||
| Calcd. | Measd. | ||||
| ar-curcumene | C15H23 | 203.18 | 203.1776 | 0.4 | |
| Germacrone a-Turmerone | C15H23O | 219.1749 | 219.1722 | 2.7 | |
| (d) C. zedoaria | |||||
| Compounds | [M+H] | mass | (mmu) mass difference | ||
| Calcd. | Measd. | ||||
| Furanodiene | C15H21O | 217.1592 | 217.1572 | 2 | |
| Curzerenone | C15H23O | 231.1385 | 231.137 | 1.5 | |
Table 2: Calculated monoisotopic mass and measured mass of compounds originating from Curcuma species rhizomes harvested from the garden using DART-TOFMS.
Using this simple procedure, molecular ions of curcumine (1) and demethoxycurcumine (2) were identified only from C. longa and C. xanthorrhiza, and curcumin (1) was not detected from rhizomes of C. aromatica and C. zedoaria harvested from the garden. Bisdemethoxycurcumin was not detected in Curcuma plants species from the garden. Sesquiterpenoids, e.g. xanthorrhizol (3) and arturmerone (4), were also detected using this procedure.
Superoxide anion radical (O2∙ˉ) scavenging activity of rhizome extracts
The rhizomes harvested from the garden were extracted with both PB and MeOH, and then each SOSA was measured using ESR. PB or MeOH solutions without sample were used as blanks when the relative percentage of SOSA was calculated. The median inhibitory concentration (IC50) on SOSA was obtained from the relationship between SOSA and concentration of extract.
The results showed that all PB extracts and MeOH extracts from the Curcuma rhizomes species cultivated in the medicinal plant garden exhibited antioxidant activity. ESR spectra of DMPO-OO– observed upon the addition of various concentrations of MeOH extract from C. xanthorrhiza rhizome are shown in Figure 2. A reduction in the signal intensity was observed when a concentration of a sample extract increased. All extracts from the Curcuma species rhizomes exhibited the same tendency, thus were confirmed as a superoxide anion radical (O2∙ˉ) scavenger.
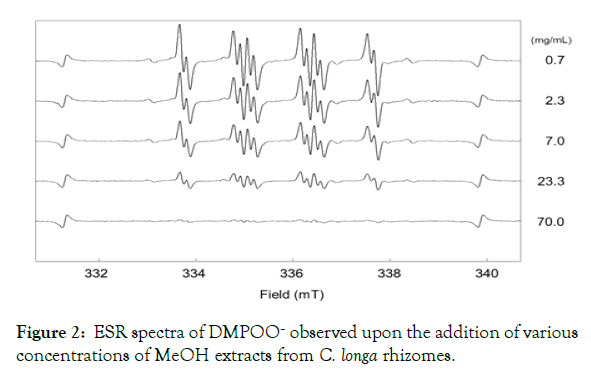
Figure 2: ESR spectra of DMPOO– observed upon the addition of various concentrations of MeOH extracts from C. longa rhizomes.
Figure 3 shows the relationship between the relative inhibitory effects of the formation of DMPO-OOˉ and logarithm of various concentrations of PB extract and MeOH extract from C. xanthorrhiza rhizomes. The relative inhibition of the formation of DMPO-OOˉ increased with the increasing concentration of each extract. The linearity was expressed as y=16.111ln(x)+40.414; R2=0.9704 of MeOH extract, and as y=16.093ln(x)+20.819; R2=0.9878 of PB extract. The MeOH extract tended to exhibit greater scavenging activity against O2∙ˉ than the PB extract. Extracts of C. longa exhibited the same tendency. The IC50 values of the PB extract and MeOH extract were obtained. It is known that the lower the IC50 value, the higher the antioxidant activity.
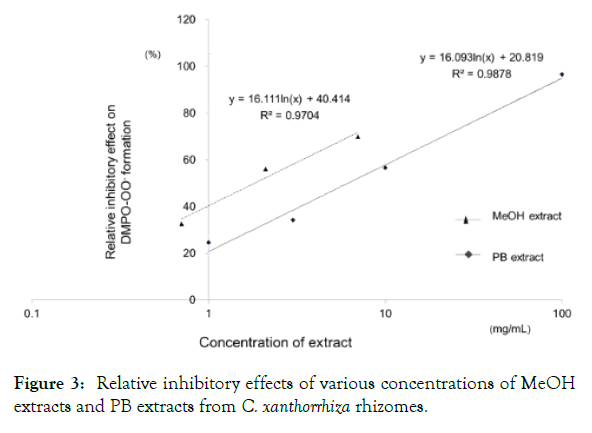
Figure 3: Relative inhibitory effects of various concentrations of MeOH extracts and PB extracts from C. xanthorrhiza rhizomes.
Subsequently, extracts from rhizomes of C. zedoaria and C. aromatica were analyzed using the same method. Figure 4 shows the relationship between the relative inhibitory effects of the formation of DMPO-OOˉ and logarithm of various concentrations of PB extract and MeOH extract from C. zedoaria rhizomes. The linearity was expressed as y=14.398ln(x) + 15.766; R2=0.9863 of PB extract, and as y=18.601ln(x) - 17.878; R2=0.9994 of MeOH extract. Extracts of C. aromatica exhibited the same tendency. Although both of these species exert anti-oxidant activity, their activities were not as strong as those of C. longa and C. xanthorrhiza.
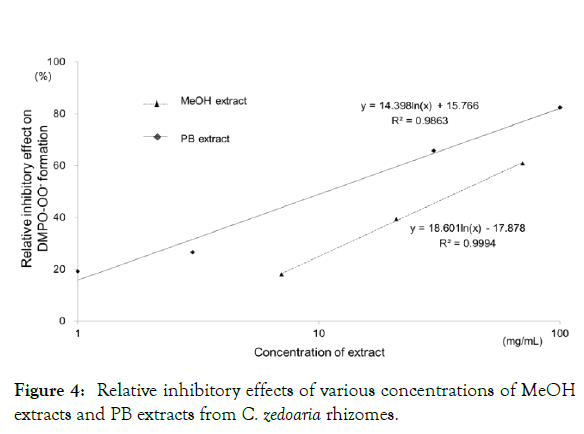
Figure 4: Relative inhibitory effects of various concentrations of MeOH extracts and PB extracts from C. zedoaria rhizomes.
SOD was used as a standard, and the activity of each rhizome was shown as the SOD equivalent (U/mg) for comparison. The results are shown in Table 3. Although all extracts exhibited antioxidant activity, the MeOH extract of C. longa exhibited the greatest activity among the extracts investigated.
| Extract | IC50 of extract(mg/mL) | IC50 of SOD (U/mL) | antioxidant unit of extract (U/mg) |
|---|---|---|---|
| C. longa in PB | 8.5 | 16.7 | 1.96 |
| C. aromatica in PB | 5.77 | 4.15 | 0.72 |
| C. zedoaria in PB | 10.78 | 4.15 | 0.38 |
| C. xanthorrhiza in PB | 6.13 | 4.15 | 0.68 |
| C. longa in MeOH | 2.9 | 19.2 | 6.62 |
| C. aromatica in MeOH | 35.41 | 8.71 | 0.25 |
| C. zedoaria in MeOH | 40.17 | 8.71 | 0.22 |
| C. xanthorrhiza in MeOH | 2.19 | 8.71 | 3.98 |
Table 3: IC50 values of Curcuma species rhizome extracts and standard SOD on superoxide anion radical (O2.–) scavenging activity and SOD equivalent values of the extracts.
The potency order of the MeOH extract was C. longa>C. xanthorrhiza >C. aromatica >C. zedoaria. The results indicate the potent anti-oxidant activity of the MeOH extracts of C. longa and C. xanthorrhiza. The total phenolic and flavonoid contents present in the rhizome are correlated with the anti-oxidant activity. High levels of these compounds in the MeOH extracts indicated strong anti-oxidant activity. The phenolic hydroxyl group, methoxyl group, and the 1,3-diketone are important structural features for the activity. Curcumin, demethoxycurcumin, bisdemethoxycurcumin, 5-hydroxy-1,7-bis(4-hydroxy-3-methoxyphenyl)-1-hepten-3-one, and 3-hydroxy-1,7-bis(4-hydroxyphenyl)-6-hepten-1,5-dione present in C. longa contributed to the potent anti-oxidant activity [3]. Some sesquiterpenoids, e.g. xanthorrhizol (3) in C. xanthorrhiza and arturmerone (4), also exhibited anti-oxidant activity. The phenolic hydroxyl group on the bisabolene skeleton as seen in xanthorrhizol (3) is also an important structural feature. ar-Turmerone (4) has anti-oxidant activity, although its activity is weaker than those of the compounds mentioned previously.
As shown in Table 2, curcumin (1), demethoxycurcumin (2), xanthorrhizol (3), and ar-turmerone (4) were detected from the rhizomes harvested from the garden using DART-TOFMS. The order of antioxidant activity of the MeOH extracts, i.e., the strongest C. longa followed by C. xanthorrhiza, could be due to the high levels of curcuminoids, ar-turmerone, and xanthorrhizol present.
Lower antioxidant activities of C. zedoaria and C. aromatica may depend on the lower levels of phenolic and flavonoid contents in both of them compared with C. longa and C. xanthorrhiz [3]. Using DART-TOFMS, curcumin (1) was not detected from the rhizomes of C. zedoaria and C. aromatica cultivated in the medicinal plant garden. Its absence resulted in less potent antioxidant activity of the MeOH extracts of both species.
The rhizomes of Curcuma species cultivated in the garden exhibited antioxidant activity. Curcumin (1), demethoxycurcumin (2), and ar-turmerone (4) were identified from C. longa and C. xanthorrhiza, and xanthorizol (3) was identified from C. xanthorrhiza using DART-TOFMS. Phenolic compounds extracted with MeOH play an important role in antioxidant activity [24,25]. The presence of high levels of curcuminoids and other compounds in MeOH extracts reflect the potency of antioxidant activity.
Curcuma rhizomes, i.e., Curcuma longa, Curcuma aromatica, Curcuma zedoaria, Curcuma xanthorrhiza, cultivated in the medicinal plant garden of Hoshi University were evaluated. Volatile compounds were identified using TD-GC-MS with SPME fiber as an adsorption device, and comparative results between curcuma species were presented and the results were compared with those previous reported. Curcuminoids, which are main active compound in C. longa rhizome and were not detected by TD-GC-MS, were detected using DART-TOFMS. Curcumin and demethoxycurcumin were identified from C. longa and C. xanthorrhiza without complicated preparation prior to measurement. Antioxidant activities of extracts from the rhizomes were investigated using ESR spin-trapping method. The MeOH extracts of C. longa and C. xanthorrhiza rhizomes exhibited greater antioxidant activity than others. The presence of high levels of curcuminoids and other compounds in MeOH extracts reflect the potency of antioxidant activity.
The authors thank Professor emeritus S. Nagumo for developing the medicinal plant garden of Hoshi University and Mr. Y. Edano for cultivating the plants and are grateful to Prof. K. Saito of the Laboratory of Analytical Chemistry of Hoshi University for lending the ESR instruments used.
Citation: Kasai H, Yamane Y, Ikegami-Kawai M, Sudo H (2019) Analysis of Compounds of Curcuma Rhizome Using Mass Spectrometry and Investigation of the Antioxidant Activity of Rhizome Extracts. Med Aromat Plants (Los Angeles) 8.336. doi: 10.35248/2167-0412.19.8.336
Received: 27-Jul-2019 Accepted: 07-Aug-2019 Published: 14-Aug-2019 , DOI: 10.35248/2167-0412.19.8.336
Copyright: © 2019 Kasai H, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.