Journal of Chromatography & Separation Techniques
Open Access
ISSN: 2157-7064
ISSN: 2157-7064
Research Article - (2023)Volume 14, Issue 3
Erythromycin (ERY) is one of the macrolides used abundantly in veterinary medicines to treat various infections including respiratory, skin and bones. Combination of Sulfadiazine (SFD) and Trimethoprim (TMP) has proven efficacy and is widely used in the treatment of many infectious diseases, due to the efficiency of SFD as a bactericidal and TMP as a bacteriostatic. On the other hand, those residues of antibiotics like ERY, SFD and TMP in animal tissues may have health hazards on humans. A simple and cost effective TLC-densitometric method has been developed to analyse the above mentioned drugs in their dosage form and in spiked chicken muscle and liver samples. Sample preparation was thoroughly studied for extraction and cleaning up trying different extraction methods resulting in using two methanol based extraction steps along with EDTA solution. Moreover, a mixture of chloroform: methanol: ammonia hydroxide solution (33%, v/v) (8.5:1.5:0.1, by volume) was the developing system. In order to obtain the highest possible sensitivity, the separated bands were exposed to iodine vapours in well closed container for 15 minutes and then detection was immediately done at 220 nm. Torsemide was used as internal standard. Linearity was achieved in the ranges of 0.5-10, 0.1-2 µg/band for ERY and SFD, respectively in both spiked muscle and liver samples while for TMP, linearity was proved over the ranges of 0.1-1.8 µg/band for spiked muscle samples and 0.1-1.6 for spiked liver samples. Validation was done in accordance with FDA guidelines for veterinary medicines and all the findings were within the acceptable limits. The method can be utilized to examine the presence of ERY, SFD and TMP in various marketed chicken muscle and liver samples to ensure human safety and maintain public health.
Erythromycin; Sulfadiazine; Trimethoprim; Chromatography; Bioanalysis
Veterinary medicines are classified into two categories: those are used as treatment and preventative measures (such as antibiotics, anthelminthic, and antifungals) and those are used as nutritional supplements (such as growth hormones and stimulants). Today's, farmers and veterinarians employ veterinary drugs as one of the modern essentials for animal treating, prophylaxis, and animal growth stimulants. The misuse of veterinary medicines affects severely either directly or indirectly humans through consuming animal products such as meat, milk and eggs contaminated by significant levels of veterinary drugs residues. This considers veterinary drug residues as a major issue that cannot be neglected [1].
Antibiotics are the most commonly used veterinary drugs, as they work to limit the growth of bacteria and destroy them without harming the host at low concentrations. Macrolides, sulphonamides, tetracycline’s, amprolium, penicillin, streptomycin, tyrosine, aminoglycosides, B-lactams, lincosamides, and quinolones are the most extensively prescribed antibiotics in veterinary field [2]. It has been recently reported that antibiotics are used in high quantities, which leads to their accumulation in the muscles and tissues of animals. The presence of antibiotic residues in chicken meat samples has been demonstrated in several previously reported studies [3-6]. Additionally, consumption of these antibiotic residues in chicken meat will result in the development of health hazards for consumers starting from antibiotic resistance till teratogenicity [2].
To limit these hazards, international communities such as the European Union (EU) and the Food and Drug Administration (FDA) have set restrictions on the use of veterinary drugs and developed the Maximum Residual Levels (MRL), which assure the lowest safe drug residue concentrations [7-8].
In this work, three combined veterinary drugs were analyzed; ERY, SFD and TMP in their pharmaceutical dosage form (Trisin®) and in spiked chicken muscle and liver samples. ERY is a member of the macrolides antibiotics. It is used extensively as a veterinary medicine because of its broad spectrum activity. Macrolides are generally indicated frequently to treat chronic respiratory infections in poultry [9]. They are considered to be bacteriostatic but they are bactericidal at high doses [10]. SFD belongs to sulphonamides antibiotics. It acts as a dihydropteroate synthetase inhibitor. This enzyme is important for Para-Amino benzoic Acid (PABA) synthesis which in turn is vital in the synthesis of folic acid. TMP is an antifolate antimicrobial drug that is frequently used in combination with SFD. They are synergistically act together on stopping folic acid synthesis [11,12]. European Commission (EC) has established the maximum residual level of ERY, SFD and TMP in chicken muscle and liver to be 100, 100 and 50 µg/Kg in order [13].
Higher consumption raises the risk of allergy and bacterial resistance [2]. Referring to the literature review, different analytical methods have been reported for analysis of ERY, SFD and TMP individually or in combined dosage forms and in different matrices. ERY and SFD were concurrently determined by LC-MS-MS methods while for SFD and TMP, they were analyzed together by spectrophotometric and LC-MS-MS [12,14-21]. Also, ERY and TMP were determined by spectrophotometric and LC-MS-MS methods [22-25]. ERY, SFD and TMP were analyzed in different matrices along with other veterinary drugs by different LC-MS-MS methods [26-31]. There was no reported TLC method for analysis of the three drugs in their available marketed veterinary dosage form or in spiked chicken muscle and liver samples.
This study aimed to develop simple, sensitive, rapid and accurate TLC-densitometric method for the simultaneous analysis of ERY, SFD and TMP with the sensitivity required for their analysis in their marketed dosage form and in spiked chicken muscle and liver samples. It has the benefit of being the first developed TLC-densitometric method for the analysis of the proposed drugs in complex matrices in a time and cost-effective manner as well as simple sample preparation steps. Hence, the suggested method can be used as alternative to the money and energy consuming LC-MS-MS methods.
Instrument
For TLC-densitometric method: TLC aluminum plates (20 × 10 cm) coated with 0.25 mm Silica gel 60 F254 (Merck, Darmstadt, Germany) were used as the stationary phase. The samples were applied using a Linomat V applicator and a 100.0 µL syringe. The densitometer (CAMAG, Muttenz, Switzerland) was controlled using the winCATS software (Version 3.15; CAMAG). The slit dimensions were 6 × 0.45 mm thick with a scanning speed of 20 mm/s, the scanning mode was absorbance, and the radiation source was a deuterium lamp. A UV lamp with a short wavelength of 254 nm (Vilber Lourmat, Marne La Vallee, Cedex, France) was utilized till reaching the most appropriate mobile phase.
Other Instruments: Electronic balance (South Carolina, USA); Sonix TV SS-series ultrasonicator (Sartorius, Germany); Rongtai variable volume micropipette instrument Volume: 0.1-100.0 µL (Mainland, Shanghai, China); 80–2C Low-speed 4000 rpm electric centrifuge (Zjmzym, China) with a capacity of 12 × 20 mL and a power supply of 110 V/220 V; 250 VM vortex mixer (Hwashin, Seoul, Korea).
Materials and reagents
1. Erythromycin Thiocyanate (ERY) sample was purchased from Sigma Aldrich, Egypt, with verified purity of 98.91%
2. Sulfadiazine Sodium (SFD) sample was purchased from Sigma Aldrich, and its purity was labelled to be 99.00%
3. Trimethoprim (TMP) sample was bought from Sigma Aldrich, Egypt, with labelled purity of 98.50%
4. Torsemide (TOR) was supplied by Marcyrl, Egypt with purity 98.3%
5. Trisin® water soluble powder, labelled to contain the following amounts of the studied drugs for each 100 gm; 22.90 gm erythromycin thiocyanate (equal to 20 gm erythromycin base); 23.40 gm sulfadiazine sodium (equal to 20 gm sulfadiazine base); 4 gm trimethoprim; Its batch number 190583 and it was manufactured by the Egyptian company ATCO PHARMA and it was obtained from the local market.
6. Chemicals and solvents that were used throughout this study were methanol (Alpha Chemika, India, batch number MNP751), chloroform (Alpha Chemika, India, batch number CF633), ammonium hydroxide solution 33% (PIOCHEM for laboratory chemicals, EDTA and Iodine ADWIC).
Procedure
TLC-densitometric chromatographic conditions: Samples were spotted to the TLC plates as bands of 6.0 mm width by a Camag Linomat V applicator. The bands were spaced 5 mm apart and 10 mm from the plate's bottom edge. The tank was previously saturated with the mobile phase mixture of (chloroform: methanol: ammonium hydroxide solution 33%, v/v) in ratio of (8:2:0.1 by volume) for 15 minutes at room temperature. After development, the separated drugs were exposed to iodine vapours using iodine crystals for 15 minutes and then they were UV scanned at 220 nm.
Stock standard solutions: Solutions of ERY (5000 µg/mL), SFD (1000 µg/mL), TMP (1000 µg/mL) and TOR (5000 µg/mL) were prepared in methanol. They were all prepared in separate 10 mL calibration flasks.
Working standard solutions: Working standard solutions of SFD (100 µg/mL), TMP (100 µg/mL) were prepared separately in 10 mL volumetric flasks in methanol from their previously mentioned stock standard solutions.
Pharmaceutical formulation: Stock solution of Trisin® (water soluble powder) was prepared by transferring 0.107 gm accurately into 25 mL glass volumetric flask then the volume was completed to the mark with methanol to obtain stock solution of 1000, 978.63 and 170.94 µg/mL for SFD, ERY and TMP, respectively. Three different samples were then prepared from sample stock solution by taking accurate separate 1 mL, 1.5 mL and 2 mL into three different 10 mL volumetric flasks and then 1 mL TOR was added from its stock solution and the final dilution was done by methanol.
Linearity and calibration curves
For pure samples: Calibration curves were constructed after the preparation of serial dilutions each of ERY, SFD and TMP in the ranges of 50-1000 µg/mL, 10-200 µg/mL and 10-180 µg/mL, in order from their respective stock solutions (5000 for ERY and 1000 µg/mL for SFD and TMP) in three separate sets of 10 mL volumetric flasks. To each sample, 1 mL TOR was added from its stock solution (5000 µg/mL), the volume was then adjusted with methanol to 10 mL and then 10 µL of each sample was spotted in triplicates to the TLC plates and then chromatographic separation was carried out as was explained before. For data analysis, peak area ratios (peak area of the analyte/peak area of IS) were recorded for each component. After that, calibration curves were created relating the determined peak area ratio to the corresponding concentration, and regression equations were calculated.
For spiked muscle and liver samples: Calibration standards were prepared within the same concentration ranges previously mentioned under calibrations for pure samples except for TMP for spiked chicken muscles which ranged from 0.1-1.6 µg/mL. For each sample preparation, 2 gm chicken muscle or liver was homogenized well in mortars and then the homogenized tissues were transferred to series of tubes and then spiked with the calculated amount of each drug, separately. 200 µL of 0.1 N EDTA was added to each sample followed by 1 mL TOR (500 µg/mL) and the volume was completed to 5 mL with methanol. Samples were then thoroughly vortex for 5 minutes and centrifuged for 15 min at 3500 rpm to remove the precipitated proteins and fats. The clear supernatant was then transferred to another clean series of 10 mL test tubes. Additional, 4 mL of methanol was added to the treated tissue and then shaked well for another 5 minutes and centrifuged again for 15 minutes at 3500 rpm to ensure full extraction of the drugs. The supernatant was then added to the first one. The volume was then reached to 10 mL with methanol. After that, samples were applied to TLC plates (10 µL) and the chromatographic conditions were then followed. Peak area ratios were recorded and the calibration curves were constructed.
Quality control samples: Quality Control Samples (QCS) of (2.00, 6.00, and 8.00 µg/band of ERY, 0.40, 1.20, and 1.80 µg/band of SFD and 0.40, 1.20, and 1.60 µg/band of TMP), were prepared in the same manner as calibration spiked tissues samples and then were used for the validity of the developed method following the directions outlined by FDA guidelines [32].
Application of pharmaceutical formulation: Three distinct dosage form concentrations were employed for application (0.97:1:0.17 µg/band), (1.43:1.46:0.25 µg/band), and (1.95:1.99:0.34 g/band) for ERY, SFD, and TMP, respectively. Each sample contained 500 µg/mL IS. 10 µL of each was applied to TLC plates in triplicates. Peak area ratios were computed for each drug, and the regression equation was used to calculate the corresponding concentrations in the prepared pharmaceutical dosage form solutions. Furthermore, the standard addition approach has been applied on three different levels for each drug.
Poultry meat is considered as high nutritional food that is highly consumed by all ages. Chicken meat is rich in proteins, which helps to maintain muscle mass and their development. It also works with calcium to protect and build bones. Moreover, chicken meat contains high level of nutrients for brain health, nervous and immune system such as riboflavin, niacin, biotin, pantothenic acid, B6, B12, potassium, selenium magnesium and zinc. This drew the attention for the drug residues analysis in poultry meat as chicken for their healthy body building benefits [33-34]. This study included one of the most commonly used pharmaceutical dosage forms in the treatment of poultry in the Egyptian market, which is Trisin®. This formulation contains a combination of three antibiotics: ERY, SFD, and TMP. As mentioned before [13], exceeding MRL of ERY (100 µg/kg), SFD (100 µg/kg) and TMP (50 µg/kg) may rise health risks for consumers. In this research, FDA validated TLC method was developed for the analysis of the drugs of interest in their pharmaceutical dosage form and also in spiked chicken muscle and liver samples [32]. The method was characterized by the ability to clearly separate the drugs and the highly precise ability to extract them from their matrices. Furthermore, the method also exhibited good sensitivity and resolution for quantifying the intended analytes in spiked samples.
Method optimization
Sample extraction: Chicken meat is mainly composed of 70% water, 20% proteins, 5% lipids, 5% minerals and vitamins. Extraction process involved EDTA and methanol as extraction solvents. EDTA is considered as a strong chelating agent that form complexes with minerals found in muscles and liver (metalloprotiens) [35]. Following the previously published methods, 200 µL of 0.1 M EDTA was sufficient for metals chelation [36-37]. On the other hand, organic solvents as acetonitrile and methanol were used for protein and fats precipitation [38]. They were tested either individually or in combined mixtures. It was found that the clearest densitogram was observed upon using methanol alone. The added volume of methanol was then optimized and finally two step method was used for optimum recovery. The first step included completing the sample volume to 5 mL with methanol then centrifuging at 3500 rpm for 15 minutes. The second step included the addition of another 5 mL of methanol again to the residue to ensure higher extraction recovery of the analyzed drugs and then centrifuging at 3500 rpm for 15 minutes. The resulted clear supernatants were combined and then introduced for analysis by the proposed TLC densitometric method. The extraction process was characterized by the use of few numbers of solvents and steps with high extraction efficiency.
Optimization of the new TLC-densitometric method: TLC separation was carried out using Silica gel 60 F254 plates. Several developing systems were tested to obtain the required resolution, including (ethyl acetate: methanol), (methylene chloride: methanol), and (chloroform: methanol) in the ratio of (5:5, v/v). Chloroform: methanol mixture proved to be a promising mobile phase; therefore method optimization was carried out using that solvents mixture. Different ratios ranged from (9:1, v/v) to (5:5, v/v) of chloroform: methanol was tested. It was found that the ratio (8.5:1.5, v/v) was the best one regarding Rf value and separation of SFD and TMP. On the other hand, tailed a symmetric peaks were resulted for both ERY and TMP. Additionally, the small Rf value of ERY led to significant interference from muscle and liver. Different pH values were tested (acidic and basic) in order to enhance the shape of the separated peaks along with chromatographic separation where different amounts of ammonium hydroxide solution (33%) and glacial acetic acid individually (0.05, 0.10 and 0.20 mL) were tested. ERY was found to be unstable in acidic medium leading to its degradation [39]. On using ammonium hydroxide solution (0.1 mL), acceptable peaks for ERY and TMP were obtained without affecting the Rf value of ERY.
Hence, plates with different lengths were tested (10 cm to 13 cm). Good separation of ERY from the muscle and liver matrices with suitable Rf value was obtained upon separation using 12 cm length TLC plates. Finally, the optimum developing system was (chloroform: methanol: ammonium hydroxide solution 33%) in ratio of (8.5:1.5:0.1, by volume). Additionally, 15 minutes for mobile phase saturation was sufficient to obtain optimum chromatographic separation. Regarding detection wavelength, first trials began with scanning at different wavelengths; 210, 220 and 254 nm. It was observed that ERY had very low sensitivity and cannot be determined with the sensitivity required for its analysis in MRL limits.it was reported that iodine can be used as staining reagent for TLC detection in different reported studies [40,41].Hence, the chromatographically developed plates were exposed to iodine vapours in well closed tanks. It was observed that the exposure time of TLC plates to iodine had significant effect on signal to noise ratio. Different exposure time intervals (10, 15 and 20 minutes) were tested. Time lower than 15 minutes resulted in un complete saturation for the double bond in the studied components, while higher than 20 minutes led to dark yellow background of the plates resulting in a decrease in signal to noise ratio. Hence, optimum exposure time was found to be 15 minutes.
Choosing of suitable Internal Standard (IS): It is reported that using an internal standard helps in improving the accuracy and the precision of the chromatographic analysis [42]. It permits the use of dependable data as it eliminates any variations in the instrument that occur from one sample to another. Many compounds were tested such as diclofenac sodium, chymotrypsin, domperidone, famotidine, hyoscine butylbromide and Torsemide (TOR). TOR was the chosen internal standard at a constant concentration of (5 µg/band). Complete separation between ERY, SFD, TMP and TOR are displayed in the final chromatograms given in Figures 1a-1c where the Rf values were 0.07, 0.23, 0.42 and 0.68 for ERY, TOR, SFD and TMP, respectively.
Method validation: The suggested TLC-densitometric method was validated in accordance with FDA center [32] for veterinary drugs guidelines by employing results of QCs samples to establish the method's validity and acceptability.
Linearity and calibration curves: Calibration curves were developed for standard samples using eight concentrations for each of the studied drugs ranging from 0.5-10 µg/band, 0.1-2 µg/band and 0.1-1.8 µg/band with correlation coefficients of 0.9998, 0.9998 and 0.9997 for ERY, SFD and TMP, respectively. On other hand, linearity was established for the samples of spiked muscle (n=8) and it was investigated in the ranges of 0.5-10 µg/band, 0.1-2 µg/band and 0.1-1.8 µg/band resulting in correlation coefficients of 0.9995 for ERY and SFD and 0.9996 for TMP. For spiked liver samples, linearity was achieved using 8 concentrations for ERY and SFD while for TMP, seven concentrations were used in ranges of 0.5-10 µg/band, 0.1-2 µg/band and 0.1-1.8 µg/band for ERY, SFD and TMP, consequently resulting in correlation coefficients of 0.9994, 0.9996 and 0.9997, in order. Detailed linear regression equations are presented in Table 1. All of the regression equation parameters obtained confirmed the methods linearity within the tested ranges.
Accuracy and precision: Accuracy and precision were assessed by evaluating the three QC samples five times each. Bias was used to describe accuracy. Intraday accuracy ranged from (-) 9.26 to (+) 6.17 for spiked muscle samples and from (-) 6.96 to (+) 2.60 for spiked liver samples while for interday accuracy, bias ranged from (-) 7.68 to (+) 4.24 for spiked muscle samples while for spiked liver samples, it ranged from (-) 5.46 to (+) 1.60 (Table 2). Contrarily, precision (intraday and interday precision) was represented as %RSD. Intraday precision ranged from 0.93 to 3.64 for spiked muscle samples and from 0.69 to 2.64 for spiked liver while interday precision ranged from 1.60 to 7.49 for spiked muscle samples and from 1.67 to 6.25 for spiked liver samples (Table 2). All these findings confirmed that the developed method is of high accuracy and precision.
Limit of detection and limit of quantitation: LOD and LOQ are used to evaluate the method's analytical sensitivity. They were calculated using the slope of the calibration curves and the standard deviations of the intercept using the equation for LOD (3.3 SD/slope) and for LOQ (10 SD/slope). For standard samples, LOD and LOQ were resulted in 0.16, 0.47 µg/band for ERY, 0.03, 0.09 µg/band for SFD and 0.02, 0.08 µg/band for TMP, respectively. Regarding the spiked muscle samples, LOD and LOQ for ERY were 0.16, 0.48 µg/band, 0.02 and 0.06 µg/band for SFD and TMP. For spiked liver samples, the LOD and LOQ for the ERY were 0.16 and 0.48, 0.02 and 0.06 for SFD and 0.03, 0.09 for TMP, respectfully. The obtained values proved that the suggested method had high sensitivity that is required to quantify the studied components even when they are found in the tested tissues with the concentrations corresponding to their MRL. The findings ensured that the method is accurate and can be used to quantify the studied drugs in their available dosage form.
Specificity and selectivity: Selectivity was ensured by visual inspection of the chromatograms of blank muscle or liver, as well as those spiked with drugs and internal standards. As shown in Figures 1c-1e, there was no interference from muscle or liver components with the spiked drugs and internal standards. Additionally, the method was applied to Trisin® (water soluble powder) where good percentage recoveries (99.88, 100.34 and 99.00 for ERY, SFD and TMP in order) were resulted, indicating no interference between the excipients and the separated drugs (Table 1). Standard addition technique was also carried out to evaluate accuracy of the method and the percentage recoveries obtained has revealed no interference between the studied drugs and pharmaceutical excipients.
| Method | Drug | Range (µg/band) | Slope | Intercept | Correlation coefficient (r) | LOD | LOQ | |
|---|---|---|---|---|---|---|---|---|
| Coefficient 1 | Coefficient 2 | |||||||
| Pure standard calibration | Erythromycin | 0.5-10 | -0.0049 | 0.3065 | 0.1848 | 0.9998 | 0.16 | 0.48 |
| Sulfadiazine | 0.1-2 | -0.0186 | 1.2058 | 0.1178 | 0.9998 | 0.03 | 0.09 | |
| Trimethoprim | 0.1-1.8 | 0.0532 | 1.3421 | 0.1049 | 0.9997 | 0.02 | 0.08 | |
| Spiked Muscle standard calibration | Erythromycin | 0.5-10 | -0.0229 | 0.5009 | 0.1621 | 0.9995 | 0.16 | 0.48 |
| Sulfadiazine | 0.1-2 | -0.1289 | 1.2445 | 0.0687 | 0.9995 | 0.02 | 0.06 | |
| Trimethoprim | 0.1-1.6 | -0.6856 | 2.1276 | 0.1267 | 0.9996 | 0.02 | 0.06 | |
| Spiked Liver standard calibration | Erythromycin | 0.5-10 | -0.0302 | 0.6675 | 0.1003 | 0.9994 | 0.16 | 0.48 |
| Sulfadiazine | 0.1-2 | -0.3282 | 1.5999 | 0.0181 | 0.9996 | 0.02 | 0.06 | |
| Trimethoprim | 0.1-1.8 | 0.1114 | 1.4403 | 0.1151 | 0.9997 | 0.03 | 0.09 | |
| Pharmaceutical formulation a (Mean ± STD) |
Erythromycin | 99.88 ± 2.05 | ||||||
| Sulfadiazine | 100.34 ± 2.43 | |||||||
| Trimethoprim | 99.00 ± 1.70 | |||||||
| Standard addition b (Mean ± STD) | Erythromycin | 100.44 ± 2.18 | ||||||
| Sulfadiazine | 96.65 ± 0.48 | |||||||
| Trimethoprim | 98.65 ± 0.80 | |||||||
Note: a) average of 6 determinations (proposed concentrations were 0.978/1/0.17, 1.467/1.5/0.255, 1.96, 2/0.34 for ERY, SFD and TMP respectively); b) average of 4 determinations standard addition samples (the added concentration to DF were 1, 2 and 4 µg band for ERY 0.2, 0.4 and 0.8 µg band for both SFD and TMP ).
Table 1: Regression parameters of the proposed method for determination of erythromycin, sulfadiazine and trimethoprim.
| (Spiked Muscle) | |||||||
| Erythromycin | Concentration (µg band) | Intraday | Interday | ||||
| %Recovery* | %RSD | %Bias | %Recovery | %RSD | %Bias | ||
| 2.00 (LQC) | 106.17 | 0.99 | 6.17 | 104.24 | 2.78 | 4.24 | |
| 6.00 (MQC) | 99.13 | 2.19 | -0.87 | 97.47 | 2.05 | -2.53 | |
| 8.00 (HQC) | 96.39 | 2.05 | -3.61 | 95.06 | 1.6 | -4.94 | |
| Sulfadiazine | Concentration (µg band) | %Recovery | %RSD | %Bias | %Recovery | %RSD | %Bias |
| 0.40 (LQC) | 101.1 | 1.76 | 1.1 | 96.53 | 7.49 | -3.47 | |
| 1.20 (MQC) | 95.63 | 0.93 | -4.37 | 94.02 | 2.56 | -5.98 | |
| 1.60 (HQC) | 90.74 | 1.95 | -9.26 | 92.32 | 3.94 | -7.68 | |
| Trimethoprim | Concentration (µg band) | %Recovery | %RSD | %Bias | %Recovery | %RSD | %Bias |
| 0.40 (LQC) | 99.86 | 3.64 | -0.14 | 98.2 | 4.48 | -1.8 | |
| 1.20 (MQC) | 96.68 | 0.97 | -3.32 | 95.1 | 2.54 | -4.9 | |
| 1.60 (HQC) | 102.2 | 2.96 | 2.2 | 100.01 | 1.94 | 0.01 | |
| (Spiked Liver) | |||||||
| Erythromycin | Concentration (µg band) | Intraday | Interday | ||||
| %Recovery | %RSD | %Bias | %Recovery | %RSD | %Bias | ||
| 2.00 (LQC) | 93.04 | 0.72 | -6.96 | 94.54 | 3.05 | -5.46 | |
| 6.00 (MQC) | 95.57 | 0.69 | -4.43 | 97.63 | 3.94 | -2.37 | |
| 8.00 (HQC) | 99.76 | 1.37 | -0.24 | 101.08 | 2.87 | 1.08 | |
| Sulfadiazine | Concentration (µg band) | %Recovery | %RSD | %Bias | %Recovery | %RSD | %Bias |
| 0.40 (LQC) | 96.04 | 1.07 | -3.96 | 97.64 | 1.67 | -2.36 | |
| 1.20 (MQC) | 94.49 | 0.93 | -5.51 | 99.4 | 5.64 | -0.6 | |
| 1.60 (HQC) | 102.6 | 1.35 | 2.6 | 101.6 | 4.08 | 1.6 | |
| Trimethoprim | Concentration (µg band) | %Recovery | %RSD | %Bias | %Recovery | %RSD | %Bias |
| 0.40 (LQC) | 95.78 | 1.58 | -4.22 | 96.13 | 2.15 | -3.87 | |
| 1.20 (MQC) | 99.06 | 2.64 | -0.94 | 99.13 | 2.91 | -0.87 | |
| 1.60 (HQC) | 99.49 | 2.35 | -0.51 | 96.95 | 6.25 | -3.05 | |
Table 2: Precision (Intraday, Interday) and accuracy of TLC-densitometric method in spiked chicken muscle and liver samples.
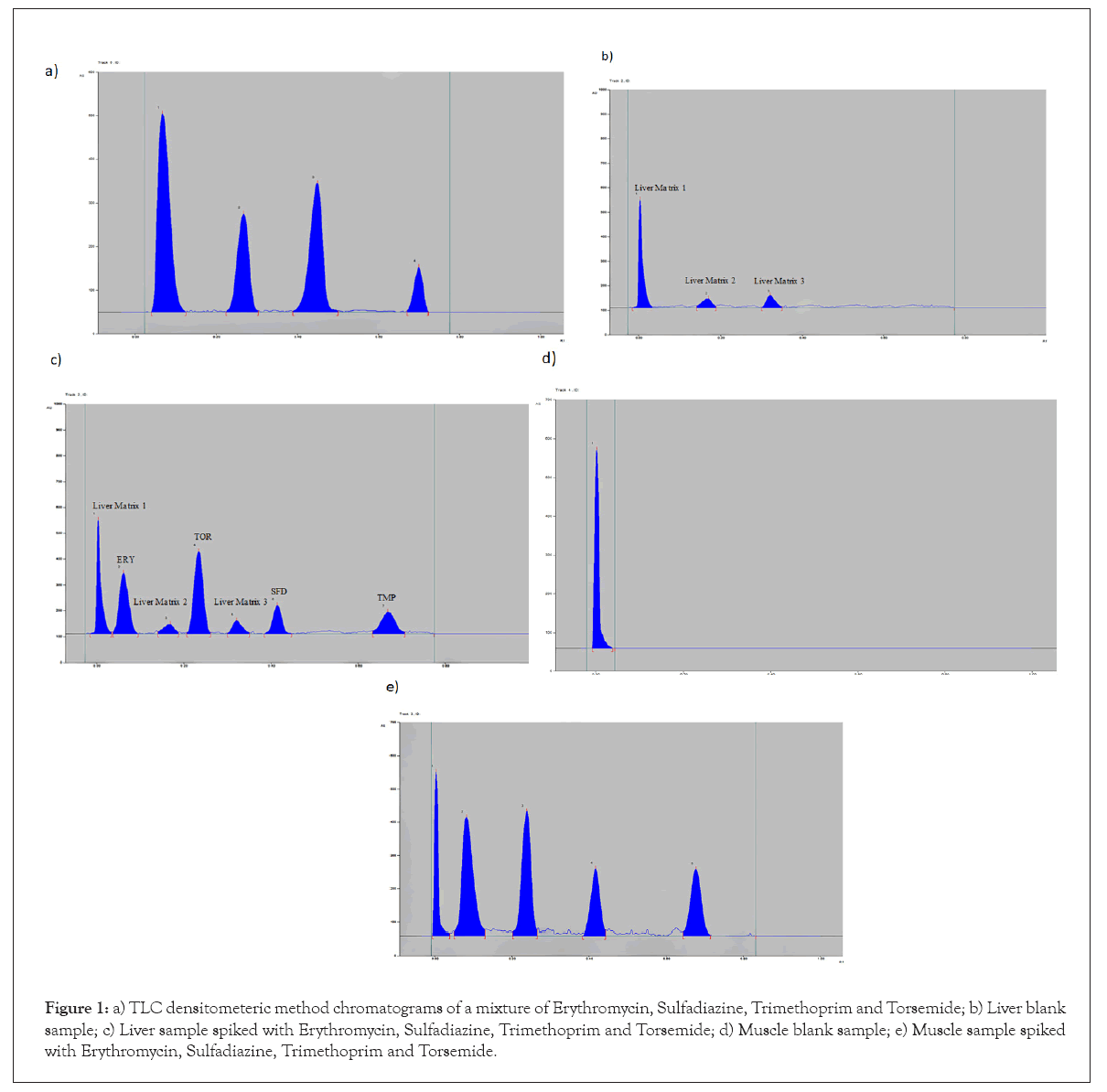
Figure 1: a) TLC densitometeric method chromatograms of a mixture of Erythromycin, Sulfadiazine, Trimethoprim and Torsemide; b) Liver blank sample; c) Liver sample spiked with Erythromycin, Sulfadiazine, Trimethoprim and Torsemide; d) Muscle blank sample; e) Muscle sample spiked with Erythromycin, Sulfadiazine, Trimethoprim and Torsemide.
Extraction recovery: The extraction recovery was determined by comparing the peak area of the drugs obtained from spiked muscle and liver samples to that of pure standards. Extraction recovery was evaluated using three different concentrations for each drug in and it was represented as percentage recovery ± %RSD. The resulting percentage recoveries for spiked muscle samples ranged from 96.95 ± 11.16 to 103.44 ± 4.64 for ERY, 103.13 ± 6.36 to 105.82 ± 3.85 for SFD and from 96.49 ± 3.80 to 100.14 ± 10.84 for TMP while for spiked liver samples, extraction recoveries ranged from 96.87 ± 2.87 to 103.00 ± 3.78 for ERY, for 93.49 ± 6.56 to 96.12 ± 2.30 SDF and from 94.34 ± 7.36 to 104.44 ± 5.56 for TMP. Results in Table 3 ensured the reproducibility and efficiency of extraction process.
| (Spiked Muscle) | ||
| Drug | Concentration (µg band) | Extraction recovery (%Recovery ± %RSD) |
| Erythromycin | 2 | 96.95 ± 11.16 |
| 6 | 103.44 ± 4.64 | |
| 8 | 98.19 ± 2.54 | |
| Mean Recovery ± %RSD | 99.53 ± 4.49 | |
| Sulfadiazine | 0.4 | 103.13 ± 6.36 |
| 1.2 | 105.82 ± 3.85 | |
| 1.8 | 103.36 ± 2.27 | |
| Mean Recovery ± %RSD | 104.10 ± 2.06 | |
| Trimethoprim | 0.1 | 96.49 ± 3.80 |
| 1.2 | 98.22 ± 7.63 | |
| 1.6 | 100.14 ± 10.84 | |
| Mean Recovery ± %RSD | 98.30 ± 3.52 | |
| (Spiked Liver) | ||
| Drug | Concentration (µg band) | Extraction recovery (%Recovery ± %RSD) |
| Erythromycin | 2 | 103.00 ± 3.78 |
| 6 | 96.87 ± 2.87 | |
| 8 | 98.79 ± 5.20 | |
| Mean Recovery ± %RSD | 99.55 ± 1.17 | |
| Sulfadiazine | 0.4 | 96.12 ± 2.30 |
| 1.2 | 93.49 ± 6.56 | |
| 1.8 | 94.37 ± 2.78 | |
| Mean Recovery ± %RSD | 94.66 ± 2.33 | |
| Trimethoprim | 0.1 | 104.44 ± 5.56 |
| 1.2 | 102.84 ± 3.65 | |
| 1.6 | 94.34 ± 7.36 | |
| Mean Recovery ± %RSD | 100.54 ± 1.85 | |
Table 3: Extraction recoveries of the studied drugs by TLC densitometric method.
Stability studies: Measuring of drug stability in the liver and muscle matrices under several storage conditions is regarded as a key factor. QCs samples were initially subjected to bench-top stability (8 hours at room temperature), followed by three freeze-thaw cycles, freezing at -20°C for 12 hours and then thawing to room temperature (Freeze and Thaw Stability). Results in Tables confirmed that muscle and liver matrices had no effect on the stability of the examined drugs under the tested different storage conditions.
Robustness: Robustness was evaluated by carrying out the proposed method after making minor chromatographic modifications in method parameters. Two conditions were slightly changed; ammonium hydroxide amount and the saturation time. Regarding ammonium hydroxide, it was added in three different amounts (0.08, 0.10, and 0.12 mL) while for the mobile phase's saturation time, chromatographic development was assessed after 15, 20, and 25 minutes. Rf values were recorded after the tested changes, and %RSD values were computed. The results showed that the slightly tested conditions had no significant effect on the Rf of the separated analytes, verifying the methods' robustness Table 4.
| Method | TLC-densitometeric Method | |
|---|---|---|
| Ammonia ratio ± 0.02 Ml | Mobile phase saturation time ± 5 min | |
| Erythromycin (%RSD) | 0.25 | 0.12 |
| Sulfadiazine (%RSD) | 0.11 | 0.05 |
| Trimethoprim (%RSD) | 0.06 | 0.07 |
Table 4: Results of robustness of the developed TLC densitometeric method.
System suitability: It assesses the effectiveness of the drug separation and the chromatographic system's performance. Densitogram obtained from application to liver sample was used to calculate these parameters due to its higher number of interfering peaks. The results of calculated parameters like selectivity, resolution, capacity and tailing factors proved the perfect separation between the investigated components and different interfering components from liver and muscle [43] (Table 5).
| TLC-densitometeric Method (Liver) | ||
|---|---|---|
| Parameters | Reference range | |
| Resolution (RS) | Rs (Liver Matrix 1-ERY)=1.5 | ≥ 1.5 |
| Rs (ERY-Liver matrix 2)=2.23 | ||
| Rs (Liver matrix 2-TOR)=1.51 | ||
| Rs (TOR- Liver matrix 3)=2.17 | ||
| Rs (Liver matrix 3-SFD)=2.18 | ||
| Rs (SFD-TMP)=5.53 | ||
| Selectivity (α) | Rs (Liver Matrix 1-ERY)=4.9 | ≥ 1 |
| Rs (ERY-Liver matrix 2)=2.16 | ||
| Rs (Liver matrix 2-TOR)=1.33 | ||
| Rs (TOR- Liver matrix 3)=1.36 | ||
| Rs (Liver matrix 3-SFD)=1.24 | ||
| Rs (SFD-TMP)=1.59 | ||
| Capacity Factor (K) | Rs (ERY)=13.2 | >0.1 |
| Rs (TOR)=3.34 | ||
| Rs (SFD)=1.38 | ||
| Rs (TMP)=0.51 | ||
| Tailing Factor (T) | Rs (ERY)=1.08 | >1.5 |
| Rs (TOR)=1.07 | ||
| Rs (SFD)=1.00 | ||
| Rs (TMP)=1.14 | ||
Table 5: System suitability parameters for TLC-densitometeric method.
Statistical comparison with the reported methods
Statistical comparison was established between the proposed and the reported methods [44,45] (Table 6). The computed t-values and F-values were lower than those of the reported methods indicating that there was no significant difference between the suggested and reported methods.
| Method | TLC-densitometeric method | Reported methods | ||||
|---|---|---|---|---|---|---|
| ERY | SFD | TMP | ERY | SFD | TMP | |
| Mean | 100.22 | 99.96 | 99.99 | 100.02 | 99.94 | 99.54 |
| SD | 0.85 | 1.15 | 1.19 | 1.47 | 0.94 | 0.86 |
| Variance | 0.72 | 1.32 | 1.42 | 2.16 | 0.87 | 0.74 |
| N | 5 | 5 | 5 | 5 | 5 | 5 |
| Student's t-test (2.306) | 0.8 | 0.97 | 0.51 | - | - | - |
| F-test (6.388) | 2.99 | 1.52 | 1.93 | - | - | - |
Table 6: Statistical comparison between the developed method and the reported ones.
A novel TLC-densitometric method was developed for simultaneous determination of ERY, SFD and TMP in spiked muscle and liver samples using Torsemide as an internal standard. Method validation was done according to FDA center for veterinary medicines guidelines and all results were within the acceptable limits. In addition, the proposed method has shown to be efficient and accurate for estimating the combined ERY, SFD, and TMP in Trisin® water soluble powder. The proposed method was the first developed TLC-densitometric method for analysis of the studied mixture with high sensitivity and simple preparation procedure. Additionally, the method is time and cost effective, so it can be used as alternative to other money consuming chromatographic methods.
The authors declare no conflict of interest.
s[CrossRef][Google Scholar][PubMed]
Citation: Sharkawi M, Safwat M, Abdelaleem E, Abdelwahab N (2023) Chromatographic Analysis of Triple Antibiotic Therapy; Erythromycin, Sulfadiazine and Trimethoprim in Different Edible Chicken Tissues, J Chromatogr Sep Tech. 14:514
Received: 07-Apr-2023, Manuscript No. JCGST-23-23363; Editor assigned: 11-Apr-2023, Pre QC No. JCGST-23-23363(PQ); Reviewed: 28-Apr-2023, QC No. JCGST-23-23363; Revised: 08-May-2023, Manuscript No. JCGST-23-23363(R); Published: 17-May-2023 , DOI: 10.35248/2157-7064.23.14.514
Copyright: © 2023 Sharkawi M, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.