Journal of Drug Metabolism & Toxicology
Open Access
ISSN: 2157-7609
ISSN: 2157-7609
Research Article - (2020)Volume 11, Issue 2
A simple, rapid, accurate, efficient and reproducible reverse phase high performance liquid chromatography (RPHPLC) method was developed and subsequently validated for simultaneous estimation of silodosin and dutasteride capsules. The analysis was carried out using Unison C8 column (250 mm x 4.6, 5 μm particle size) at 40°C temperature using mobile phase buffer:Methanol:Acetonitrile (20%:40%:40% v/v/v) at flow rate 1.0 ml/min. Quantification was achieved with PDA detector at a wavelength of 275nm. The silodosin peak retention time was 2.4 mins and the dutasteride retention was 10.9 mins, so the total run time was set to 14mins. The linearity range of Silodosin and Dutasteride is 20-120 μg/ml and 1.25-7.5 μg/ml respectively. Linear regression found to be 0.999. The proposed method was validated according to International Conference on Harmonisation (ICH) guidelines. The results shown in the method was accurate, precise, sensitive and economical. Thus, the current study showed that the developed reverse-phase liquid chromatography method is sensitive and selective for the estimation of Silodosin and Dutasteride in Capsule Dosage Form
Silodosin; Dutasteride; RP-HPLC; Method development;Validation
Silodosin (Brand Name Rapaflo) Silodosin is a medication for the symptomatic treatment of benign prostatic hyperplasia. It act as α1-adrenoreceptor antagonist with high uroselectivity (Selectivity for the prostate). The IUPAC name of this drug was 1-(3-hydroxypropyl)-5-[(2R)-({2-[2-[2-(2,2,2- trifluoroethoxy)phenoxy]ethyl}amino)propyl]indoline-7- carboxamide. Silodosin has high affinity for the α1A adrenergic receptor;it causes practically no orthostatic hypotension (in contrast to other α1 blockers). On the other side, the high selectivity seems to be the cause of silodosin's typical side effect of loss of seminal emission. Silodosin is a prescription medication used for the treatment of the signs and symptoms of benign prostatic hyperplasia (BPH) (Figure 1).
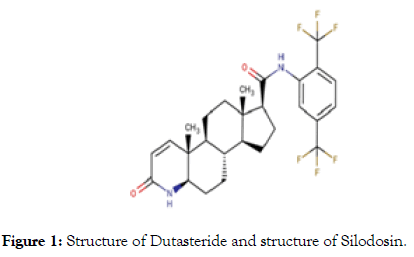
Figure 1: Structure of Dutasteride and structure of Silodosin.
Dutasteride is a Medication used to treat benign prostatic hyperplasia (enlarged prostate) and androgenetic alopecia (pattern hair loss).It was developed by GlaxoSmithKline and is a 5α-reductase inhibitor which prevents the conversion of the androgen sex hormone testosterone into the more potent dihydrotestosterone (DHT). IUPAC name of this drug is (5α, 17β)-N-{2,5-Bis(trifluoromethyl)phenyl}-3-oxo-4-azaandrost-1- ene-17-carboxamide. Dutasteride belongs to a class of drugs called 5α-reductase inhibitors, which block the action of the 5α-reductase enzyme that convert testosterone into DHT. It is an irreversible inhibitor of all three isoforms of 5α-reductase, types I, II, and III.
Instruments
HPLC with the model WATERS e 2695 separation module, PDA WATERS 2998 detector, software Empower. Digital pH meter. Weighing machine. Vacuum filter, Vacuum PR, Ultrasonic bath with the model Lab, Volumetric Flasks which are made of Borosil, Pipettes and Beakers also made of Borosil.
Chemicals and reagents
Silodosin and Dutasteride with the brand name Silo Dart, Potassium hydrogen phosphate with the brand name Merck’s, Methanol for HPLC with the brand name Rankem Acetonitrile for HPLC with the brand name Rankem, Ortho phosphoric acid with the brand name RANKEM and Water with the brand name Milli-Q.
Chromatographic conditions
The chromatographic mode used in this method was RP-HPLC, the Instrument used was Waters e HPLC 2695 with PDA 2998 detector and Empower software. The column temperature was 40°C, Sample temperature was 25°C, and the Column used in this method was Unison C8 (250 mm x 4.6, 5 μm, Make: waters). The pH range was set to be 3.00 and the buffer is set to be 1.8918 g of Dipotassium Hydrogen Phosphate in 1000 ml water, pH 3.00 was adjusted with Ortho phosphoric acid. Buffer Mobile phase is 20% Buffer, 40% Methanol, and 40% Acetonitrile. Flow rate is 1 ml/min, Wavelength is 275 nm, Injection volume 10 μl and the Run time was 12 min.
Preparation of standard
Preparation of Dutasteride Standard Stock Solution: Accurately weighed 50 mg of dutasteride working standard was taken in a 100 ml volumetric flask. Initially add 10 ml of methanol for dissolved dutasteride API and sonication in an ultrasonic water bath for 10 min. Make up to 100 ml volumetric flask with diluent and mixed well.
Preparation of silodosin and dutasteride standard
Accurately weighed 40 mg of silodosin working standard was taken in a 50 ml volumetric flask. Initially add 10 ml of diluent for dissolved silodosin API and sonication in an ultrasonic water bath for 5 min and add 5 ml of Dutasteride standard stock solution. Make up to 50 ml volumetric flask with diluent and mixed well. Further, Pipette out 5 ml of above solution in 50 ml volumetric flask and make up with diluent up to 50 ml mark and mixed well (Table 1).
Table 1: Gradient Program
| Gradient program | |||||
|---|---|---|---|---|---|
| Time(min) | 0 | 6 | 8 | 12 | 13 |
| Mobile phase A (pH 3.0 buffer) | 45 | 45 | 20 | 20 | 45 |
| Mobile phase B (Organic Mixture) | 55 | 55 | 80 | 80 | 55 |
System suitability
System suitability study was performed by initially injecting five successive injections and the results are reported in % RSD. The results were showed in Table 2.
Table 2: System Suitability Results for Silodosin and Dutasteride.
| PARAMETER | SYSTEM SUITABILITY | |
|---|---|---|
| Drug | SILC | DUTC |
| Injection 1 | 129310 | 4756 |
| Injection 2 | 127820 | 4767 |
| Injection 3 | 128184 | 4763 |
| Injection 4 | 129162 | 4740 |
| Injection 5 | 129457 | 4701 |
| Average | 128787 | 4745 |
| SD | 735.145 | 26.876 |
| %RSD | 0.57 | 0.53 |
System precision
The above system suitability solution were used then filtered through 0.45 μ Nylon and then six replicate injections were made under the optimized conditions. Recorded the chromatograms and measured the peak responses. The chromatograms recorded for accuracy test were shown in Figure 2.
Figure 2: Spectrum for Silodosin.
Specificity
Preparation of Blank: Accurately measured 500 ml (50%) of Acetonitrile and 500 ml (50%) of HPLC grade water (Milli-Q water) were mixed and sonication in an ultrasonic water bath for 10 min.
Preparation of placebo
Weighed accurately 91.5 mg of placebo was transferred into 100 ml volumetric flask and then dilute and make up with diluents. These solutions were filtered through 0.45 μ Nylon and then each concentration one replicate injections were made under the optimized conditions. Recorded the chromatograms and measured the peak responses.
The chromatograms recorded for accuracy test were shown in Figure 2.
Accuracy determination, three different concentrations were prepared separately i.e., 50%, 100% and 150% for the analyte and chromatograms are recorded. Results were shown in Table 3.
Table 3: Accuracy results for Silodosin and Dutasteride.
| Drug | % level | Area | Amount added (mg) | Amount found (mg) | % recovery | Mean recovery |
|---|---|---|---|---|---|---|
| SILO | 64415 | 20 | 20.2 | 99.71 | 99.55 | |
| 50% | 64555 | 20 | 20.1 | 99.50 | ||
| 64585 | 20 | 20.3 | 99.45 | |||
| 128750 | 40 | 40.1 | 99.77 | 99.69 | ||
| 100% | 128889 | 40 | 40.2 | 99.67 | ||
| 128921 | 40 | 40.1 | 99.64 | |||
| 193648 | 60 | 60.1 | 99.50 | 99.43 | ||
| 150% | 194256 | 60 | 60.3 | 99.19 | ||
| 193468 | 60 | 60.2 | 99.60 | |||
| DUTA | 50% | 2365 | 25 | 25.3 | 100.11 | 100.12 |
| 2362 | 25 | 25.0 | 100.24 | |||
| 2367 | 25 | 25.1 | 100.03 | |||
| 4731 | 50 | 50.1 | 100.09 | 100.15 | ||
| 100% | 4735 | 50 | 50.3 | 100.21 | ||
| 4728 | 50 | 50.0 | 100.15 | |||
| 7085 | 75 | 75.1 | 100.25 | 100.16 | ||
| 150% | 7092 | 75 | 75.0 | 100.15 | ||
| 7096 | 75 | 75.2 | 100.10 | |||
Preparation of standard solution
Accurately weighed 50 mg of dutasteride and 40 mg of silodosin in methanol and prepare the working standard solutions. Both intra-day and inter-day precision was done by performing five trials at regular intervals on same day and on five alternate days. The results are reported in the form of %RSD.
Preparation of sample solution
Prepare six sample solutions. These solutions were filtered through 0.45 μ Nylon and then each concentration two replicate injections were made under the optimized conditions. Recorded the chromatograms and measured the peak responses. The results are reported in Table 4.
Table 4: Inter day and Intraday Precision Results for Silodosin and Dutasteride.
| PRECISION | INTERDAY | INTRADAY | ||
|---|---|---|---|---|
| Drug | SILC | DUTC | SILC | DUTC |
| njection 1 | 129310 | 4756 | 129462 | 4727 |
| Injection 2 | 127820 | 4767 | 129797 | 4858 |
| Injection 3 | 128184 | 4763 | 130198 | 4815 |
| Injection 4 | 129162 | 4740 | 129802 | 4883 |
| Injection 5 | 129457 | 4701 | 129852 | 4741 |
| Injection 6 | 129085 | 4793 | 130874 | 4754 |
| Average | 128836.3 | 4753.3 | 129997.5 | 4796.3 |
| SD | 668.723 | 309.1 | 488.84 | 65.3 |
| %RSD | 0.52 | 0.65 | 0.38 | 1.4 |
Preparation of stock solution
Accurately weighed 50 mg of dutasteride into 100 ml volumetric flask then add 20 ml of methanol and make up with diluents. Accurately weighed 40 mg of silodosin into 50 ml volumetric flask then add 10 ml of diluents and add 5 ml of above solution and make up with diluents. This was marked and labelled as stock solution. The results were showed in Figure 3 and Table 5.
Figure 3: Spectrum for Dutasteride.
Table 5: Linearity Results for Silodosin and Dutasteride.
| Injection Level | Silodosin | Dutasteride | ||
|---|---|---|---|---|
| Concentration (µg/ml) | Area | Concentration (µg/ml) | Area | |
| njection 1 | 20 | 32122 | 1.25 | 1189 |
| Injection 2 | 40 | 64244 | 2.5 | 2379 |
| Injection 3 | 60 | 96366 | 3.75 | 3569 |
| Injection 4 | 80 | 128489 | 5 | 4758 |
| Injection 5 | 100 | 160611 | 6.25 | 5947 |
| Injection 6 | 120 | 192733 | 7.5 | 7137 |
About 50 mg of dutasteride were weighed and transferred into 100 ml volumetric flask. Initially add 10 ml of methanol for dissolved and sonication in ultrasonic water bath for 10 min. Make up to 100 ml volumetric flask with diluent and mixed well.
Accurately weighed 40 mg of silodosin was taken in a 50 ml volumetric flask. Initially add 10 ml of diluent for dissolved and sonication in ultrasonic water bath for 5 min and add 5 ml of Dutasteride standard stock solution. Make up to 50 ml volumetric flask with diluent and mixed well. The results were showed in Tables 6-10.
Table 6: System Suitability results for filter validation.
| Parameter | System suitability | |
|---|---|---|
| Drug | DUTC | SILC |
| njection 1 | 127830 | 4765 |
| Injection 2 | 129321 | 4740 |
| Injection 3 | 128179 | 4757 |
| Injection 4 | 129162 | 4730 |
| Injection 5 | 129547 | 4762 |
| Average | 128807.8 | 4750.8 |
| SD | 756.097 | 15.123 |
| %RSD | 0.59 | 0.32 |
Table 7: Filter validation results for Silodosin.
| Preparation | Sample area/std avg area | Std wt/50 | 5/50 | Spl wt/200 | Potency/100 | Avg wt/LC | 100 | Percentage |
|---|---|---|---|---|---|---|---|---|
| Unfilter-1 | 119636 | 50.1/50 | 5/50 | 601.10/ | 99.93/100 | 300.55/8 | 100 | 93.03 |
| Unfilter-2 | 119998 | 50.1/50 | 5/50 | 601.10/ | 99.93/100 | 300.55/8 | 100 | 93.31 |
| Nylon filter-1 | 120147 | 50.1/50 | 5/50 | 601.10/ | 99.93/100 | 300.55/8 | 100 | 93.42 |
| Nylon filter-2 | 120059 | 50.1/50 | 5/50 | 601.10/ | 99.93/100 | 300.55/8 | 100 | 93.35 |
| PVDF filter-1 | 119943 | 50.1/50 | 5/50 | 601.10/ | 99.93/100 | 300.55/8 | 100 | 93.26 |
| PVDF filter-2 | 120170 | 50.1/50 | 5/50 | 601.10/ | 99.93/100 | 300.55/8 | 100 | 93.44 |
Table 8: Filter validation results for Dutasteride.
| Preparation | Sample area/std avg area | Std wt/ 100 |
25/ 2500 |
Spl wt/ 200 |
Potency/ 100 |
Avg wt/ LC |
100 | Percentage |
|---|---|---|---|---|---|---|---|---|
| Unfilter-1 | 4761 | 40.1/ 100 |
25/ 2500 |
601.10/ 200 |
99.93/ 100 |
300.55/ 0.5 |
100 | 100.19 |
| Unfilter-2 | 4751 | 40.1/ 100 |
25/ 2500 |
601.10/ 200 |
99.93/ 100 |
300.55/ 0.5 |
100 | 99.98 |
| Nylon filter-1 | 4751 | 40.1/ 100 |
25/ 2500 |
601.10/ 200 |
99.93/ 100 |
300.55/ 0.5 |
100 | 99.98 |
| Nylon filter-2 | 4703 | 40.1/ 100 |
25/ 2500 |
601.10/ 200 |
99.93/ 100 |
300.55/ 0.5 |
100 | 98.97 |
| PVDF filter-1 | 4792 | 40.1/ 100 |
25/ 2500 |
601.10/ 200 |
99.93/ 100 |
300.55/ 0.5 |
100 | 100.85 |
| PVDF filter-2 | 4767 | 40.1/ 100 |
25/ 2500 |
601.10/ 200 |
99.93/ 100 |
300.55/ 0.5 |
100 | 100.32 |
Table 9: Solution stability results for Silodosin.
| Preparation | Sample area | Run Time | Plate count | Tailing | Percentage |
|---|---|---|---|---|---|
| Fresh Sample | 128574 | 2.47 | 3509 | 1.26 | 99.8 |
| Fresh Sample | 127534 | 2.47 | 3515 | 1.26 | 99.0 |
| 24 hrs B.T Sample | 127931 | 2.48 | 3778 | 1.29 | 99.3 |
| 24 hrs B.T Sample | 127752 | 2.48 | 3662 | 1.27 | 99.2 |
| 24 hrs R.F Sample | 127738 | 2.48 | 3625 | 1.27 | 99.2 |
| 24 hrs R.F Sample | 127993 | 2.48 | 3656 | 1.26 | 99.4 |
Table 10: Solution stability Results for Dutasteride.
| Preparation | Sample area | Run Time | Plate count | Tailing | Percentage |
|---|---|---|---|---|---|
| Fresh Sample | 4768 | 10.98 | 148206 | 1.20 | 100.4 |
| Fresh Sample | 4740 | 10.98 | 148841 | 1.17 | 99.8 |
| 24 hrs B.T Sample | 4844 | 11.00 | 142324 | 1.17 | 101.9 |
| 24 hrs B.T Sample | 4824 | 11.00 | 148901 | 1.18 | 101.5 |
| 24 hrs R.F Sample | 4749 | 11.00 | 151301 | 1.14 | 100.0 |
| 24 hrs R.F Sample | 4742 | 11.00 | 140946 | 1.11 | 99.8 |
Prepare a fresh blank solution and fresh standard solution. Used in old mobile phase (Mobile phase Prepared by before two days).The results were showed in Table 11.
Table 11: Mobile phase stability Results for Silodosin and Dutasteride.
| Parameter | Run time of peaks | |
|---|---|---|
| Drug | SILC | DUTC |
| njection 1 | 2.47 | 10.98 |
| Injection 2 | 2.47 | 10.98 |
| Injection 3 | 2.47 | 10.98 |
| Injection 4 | 2.47 | 10.98 |
| Injection 5 | 2.47 | 10.98 |
The analysis was performed in different conditions to find the variability of test results. The following conditions are checked for variation of results.
Effect of variation of temperature
The above prepared sample was analyzed at 35°C and 45°C instead of 40°C remaining conditions are same.10 μl of sample was injected twice and chromatograms were recorded. The chromatograms recorded were shown in Figure 4.
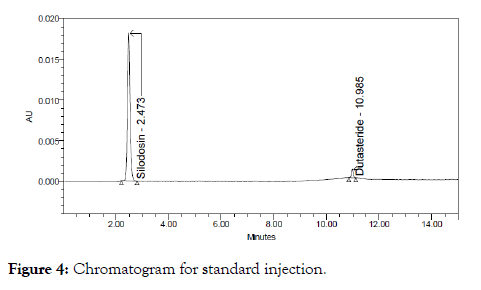
Figure 4: Chromatogram for standard injection.
Effect of variation of flow
The above prepared sample was analyzed at 0.8 ml/min and 1.2 ml/min instead of 1 ml/min, remaining conditions are same. 10 μl of sample was injected twice and chromatograms were recorded. The chromatograms recorded were shown in Figure 5.
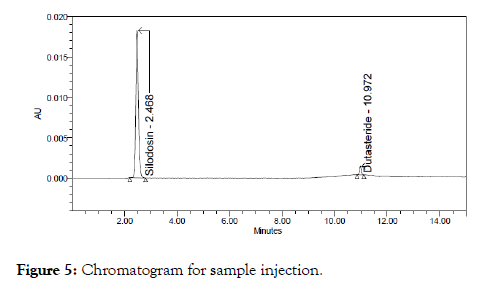
Figure 5: Chromatogram for sample injection.
Method validation
The developed method was validated based on ICH guidelines to detect and quantitate both silodosin and dutasteride in dosage form with use of HPLC system equipped with PDA detector. The chromatograms are showed in Figures 6-9.
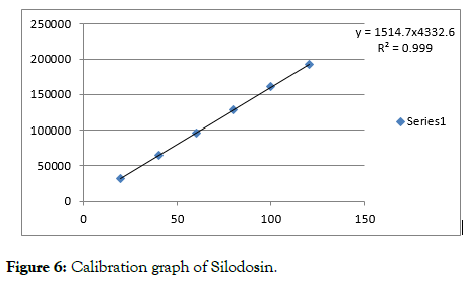
Figure 6: Calibration graph of Silodosin.
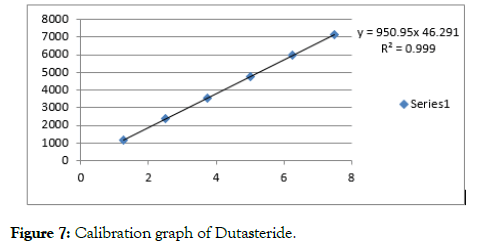
Figure 7: Calibration graph of Dutasteride.
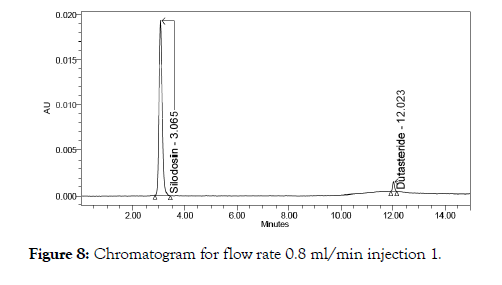
Figure 8: Chromatogram for flow rate 0.8 ml/min injection 1.
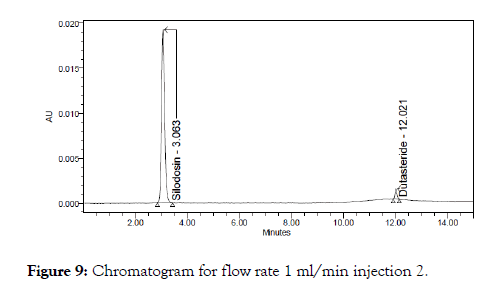
Figure 9: Chromatogram for flow rate 1 ml/min injection 2.
The developed method was validated according to the ICH guidelines as follows:
System suitability
System suitability study was performed by initially injecting five successive injections and the results are reported in % RSD.
System precision
System precision study was performed by injecting six e injections were done and the results are reported in % RSD.
Accuracy
Accuracy study was performed by injecting three different levels i.e., 50%, 100% and 150%. The results are reported in % mean recovery.
Precision
Inter and intra-day precision studies were performed by injecting a sample of 80 ppm of silodosin and 5 ppm of dutasteride six were done in same day and on six alternate days over a period of 10 days and results are reported in % relative standard deviation (%RSD).
Linearity and range
The linearity of proposed method was done by injecting various concentrations of the drug and the results are reported in the form of correlation coefficient. The range was calculated from the calibration graphs constructed by concentration versus peak areas (Figures 6 and 7).
Filter validation
Filter validation study was performed by injecting three different levels i.e., unfiltered centrifuged, nylon filtered and PVDF filtered. Two injections of each level were done and the results are reported in % mean recovery.
Mobile phase stability
Mobile phase stability study was performed by injecting one fresh blank and five fresh standard injections by using previous mobile phase solution. The results are reported in Run time of peaks.
Robustness
Robustness was performed by flow rate variation and temperature variation. Two injections each were done and the acceptance criteria for tailing factor and plate count were determined (Figure 8-10).
Figure 10: Chromatogram for temperature 35°C injection 1.
Method development and validation of Silodosin and Dutasteride was done by RP-HPLC method employing Unison C8 (150 mm x 4.6, 5 μm, Make: waters) using mobile phase as Phosphate Buffer: Acetonitrile: Water in 20:40:40 v/v/v at a flow rate 1 ml/min.
The linearity range of Silodosin and Dutasteride was found to be HPLC 20-120 and 1.25-7.5 μg/ml respectively. Linear regression was not more than 0.999. The values of %RSD were <2% for both the methods. The %recovery varies in the range of 99-100%.
The results show the method was accurate, precise, sensitive, and economic. The HPLC method was more rapid. Method was successfully applied to the pharmaceutical dosage form (Silo Dart capsules).
Citation: Nataraj KS, Rao AS, Harshitha S (2020) Analytical Method Development and Validation for the Estimation of Silodosin and Dutasteride in Capsule Dosage Form by RP-HPLC Method. J Drug Metab Toxicol 11: 245. doi: 10.35248/2157-7609.20.11.245
Received: 05-Mar-2020 Accepted: 18-Mar-2020 Published: 25-Mar-2020 , DOI: 10.35248/2157-7609.20.11.245
Copyright: © 2020 Nataraj KS, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.