Andrology-Open Access
Open Access
ISSN: 2167-0250
ISSN: 2167-0250
Review Article - (2025)Volume 14, Issue 2
Background: Prostate cancer is one the most common cancers of men worldwide and a leading curse of death from male malignant diseases. Androgen Receptor (AR) and Fatty Acid-Binding Protein 5 (FABP5) play important roles in prostate cancer development, expansion, and malignant progression.
Objectives: In this short review, we intend to provide an overview on roles of AR and FABP5 play in development, progression of prostate cancer and the development of resistance to current treatment. We also intend to discuss the possible future effective treatment for Castration-Resistant Prostate Cancer (CRPC) which is currently an incurable disease.
Methods: The literature survey in this study was conducted by searching databases, including EBSCO Host information services, National Library of Medicine, Oxford Academic, Acta Pharmacologica Sinica, Elsevier Database, National Cancer Institute, Harvard Health Publishing, and Memorial Sloan Kettering Cancer Center.
Results and discussion: Androgens or male hormone is essential for normal prostate growth. AR responds to androgen binding, which in turn activates a cascade of conformational changes, that enables androgen to reach prostate cells to initiate transcriptional activity, and to alter the gene expressions. Over-activation of androgen through AR leads to the development of prostate cancer. Drugs developed to control the interactions between androgens and their receptor to inhibit the growth of the cancer cells. However, as the prolonged Androgen-Deprivation Therapy (ADT), the resistance to the treatment is gradually developed: Androgen-dependent prostate cancer gradually become CRPC. The promotive role of androgen-initiated signal pathway was gradually replaced by FABP5-initiated signal transduction pathway, which eventually become dominant in CRPC cells.
Conclusion: For a future effective CRPC treatment, ADT should be combined with inhibition of FABP5. The suppression of both AR and FABP5-expression (including gene knockout) in the cancer cells may provide a new way for an effective treatment of CRPC.
Prostate cancer; Androgen; AR; FABP5; CRPC; ADT; AR splice variants
Recent worldwide increase in prostate cancer is noticed with concerns on its potential economic and health impact, and this concern is exacerbated with a proportional increase in ageing population. Studies showed that approximately 1.4 million cases of prostate cancer were diagnosed in 2020 globally, with a consensus to be in an increasing trend, and with an incidence rate of 30.7 per 100,000 males [1]. Recent studies also identified prostate cancer as the 5th most common cancer in 112 countries, and the leading cause of cancer-related deaths in 48 countries [1-3], particularly the developed countries.
Family history, age, and race are some of the well-known risk factors for prostate cancer. Several additional risk factors related to lifestyle and dietary behavior were introduced, these include obesity, fitness, diabetes mellitus, dietary pattern and supplementation with vitamin E. Moreover, dwelling into the Human Development Index, a measure of average achievement in key dimensions of human development, including life expectancy, birth, education index, and gross national income per capita, demonstrated a significant effect on the morbidity and mortality of prostate cancer patients [1,4-8].
Androgens, through interactions with their receptor AR, are essential for prostatic development and normal function. Prostate cancer developed only when this normal mechanism is persistently over functioning. Building upon this point of view, this review aims to delve into the actions of AR and FABP5 in malignant progression of prostate cancer. It seeks to elucidate the roles that androgens play in the normal morphology and functioning of the prostate and how AR and FABP5 play the important promotive roles in prostate cancer development and progression. It also seeks to look on how a targeted approach to AR or FABP5 may effectively suppress the highly malignant CRPC.
Databases searched in this study include EBSCO Host Information Services, National Library of Medicine, Oxford Academic, Acta Pharmacologica Sinica, Elsevier Database, National Cancer Institute, Harvard Health Publishing, and Memorial Sloan Kettering Cancer Center. Studies included in the review were selected using inclusion and exclusion criteria to reduce the risk of bias on results. Inclusion criteria were:
• Full English language papers.
• Peer-reviewed papers.
• Studies conducted on population samples of broad
community-based males, with or without, or with a known
risk for prostate cancer.
• Studies promoting innovation and further research for
prostate cancer, but not supported or funded by
pharmaceutical companies.
Exclusion criteria used in this study were:
• Studies that were not published in full English language.
• Studies that was not relevant to AR, prostate cancer
development and treatment.
• Studies that was not peer-reviewed.
• Studies that were dated more than 15 years from the present.
• Studies that were not conducted from a broad communitybased
sample of males with or without, or with a known risk
for prostate cancer.
Androgen's effect on prostate gland is controlled by the androgen signaling axis and is of importance in development of prostate cancer and progression to metastatic equivalent. The axis involves testicular synthesis of testosterone, its transport, and conversion to more active metabolite 5α-Dihydrotestosterone (DHT) by 5α-reductase. Through binding to testosterone and DHT, AR induces transcriptional activity (Figure 1), which is modulated with AR-coregulators and by phosphorylation in response to the sudden burst of growth factors [9].
The main recommendations for prostate cancer treatment can be found in numerous Clinical Practice Guidelines (CPG) published by prestigious organizations such as European Society for Medical Oncology (ESMO) and National Comprehensive Cancer Network (NCCN) [1]. However, current CPG lace consensus for the optimal treatments of both localized and metastatic prostate cancer. The recommended first-line treatments after prostatectomy are radiation therapy for localized prostate cancer and ADT for metastatic forms of the disease. ADT has a high risk of failure and the cancer cells often progress to a hormone-refractory state or CRPC [3].
Recent investigations significantly advanced our understanding of the complexities inherent in prostate cancer pathogenesis, particularly on the deviations in conventional androgen signaling axis. Central to these findings was the dysregulation of AR activity, a critical factor in the development of treatment-resistant phenotypes in prostate cancer. This dysregulation unfolded through a series of intricate mechanisms, encompassed aberrations in transduction cascades, altered expression of AR coregulators, and mutations directly impacting the AR gene. Collectively, these alterations were instrumental in the high incidence of therapeutic failures and the emergence of refractory cancer subtypes. In this context, we conducted a study which demonstrated that AR-knockout resulted in a marked attenuation of malignant characteristics of prostate cancer cells. This includes a significant reduction in cell proliferation, invasion, wound healing, and colony formation. Our study also found that FABP5 substitute AR to perform a promotive role in highly malignant CRPC cells [10]. Previous relevant studies fully demonstrated the AR's important role in prostate cancer pathophysiology and its relation with FABP5 in malignant progression, and hence laid down the scientific basis for new targeted therapeutic interventions by interfering the biological activities of AR and FABP5 [3,11-14]. The biological function of AR in prostate gland is shown in Figure 1.
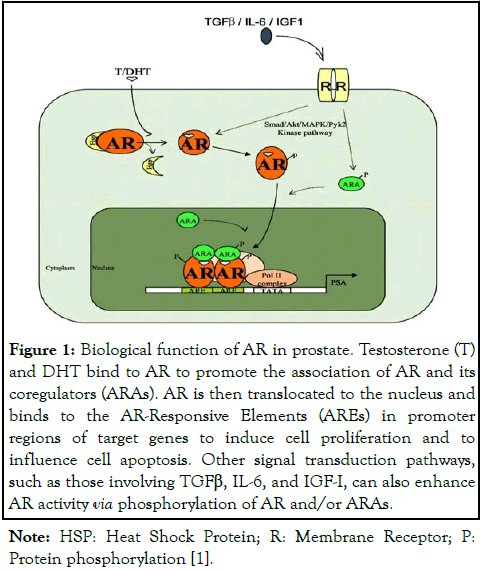
Figure 1: Biological function of AR in prostate. Testosterone (T) and DHT bind to AR to promote the association of AR and its coregulators (ARAs). AR is then translocated to the nucleus and binds to the AR-Responsive Elements (AREs) in promoter regions of target genes to induce cell proliferation and to influence cell apoptosis. Other signal transduction pathways, such as those involving TGFβ, IL-6, and IGF-I, can also enhance AR activity via phosphorylation of AR and/or ARAs.
Note: HSP: Heat Shock Protein; R: Membrane Receptor; P: Protein phosphorylation [1].
AR is a member of the nuclear receptor superfamily, which includes estrogen receptor, progesterone receptor, glucocorticoid receptor, and thyroid hormone receptor. As illustrated in Figure 2, the AR gene is located in chromosome X (Xq11-12) and consists of 8 exons coding for a protein about 110 kDa in size. As shown in Figure 2, AR has four regions: An N-terminal, an Nh2 Terminal Transactivation Domain (NTD) encoded by exon 1, a DNA-Binding Domain (DBD) encoded by exons 2-3, a hinge region encoded by exon 4, and a Ligand Binding Domain (LBD) encoded by exons 5-6. The NTD domain contains glutamine repeats, and these glutamine repeats vary among different men, which then results in a variation of amino acid transcriptions in AR. Currently, there are 919 identified amino acids in AR and all are registered in the AR gene mutation database. Variations in glutamine repeat sequences often result in an increased transcriptional AR activity associated with shorter repeats when compared to that associated with normal-sized glutamine repeats. Increased AR transcriptional activity is associated with an increased risk for prostate pleomorphism, some of which eventually developed to prostate cancer. In contrast to this, men with longer glutamine repeats are often associated with testicular atrophy, which is associated with gynecomastia, erectile dysfunction, and muscular atrophy, due to a suppressed action of testosterone. Testosterone and its metabolite DHT bind to the LBD, followed by conformational changes in AR. After binding, AR is translocated into the nucleolus, forming a dimer, and binds to the ARE in the promoter region of the enhancer genes through the zinc-finger of the DBD, enabling the effects of testosterone and DHT to be expressed. The NTD contains a transcriptional regulatory region or Activation Function-1 (AF-1), and the LBD contains Activation Function-2 (AF-2). Upon DNA binding, the AR dimer forms a complex with coactivator and coregulatory proteins at AF-1 and AF-2 regions. These coregulatory proteins include SRC1, SRC2, SRC3, p300/CBP, and AEA54, among many others. AR regulates the gene expressions with diverse functions located downstream of the ARE, including secreted proteins (KLK3, KLK2), fusion gene product (TMPRSS2-ERG), growth stimulators (IGF1R, APP), PI3K modulation (FKBP5), transcription factors (NKX3.1, FOXP1), metabolic enzyme (CAMKK2), cell cycle regulators (UBE2C, TACC2), and glucuronidation (UGT1A1) [9,11,12,15-21]. Except the full-length AR protein, several truncated forms or variants were detected in cells. These variants are truncated at the DNA-Binding Domain (DBD) or Ligand-Binding Domain (LBD).
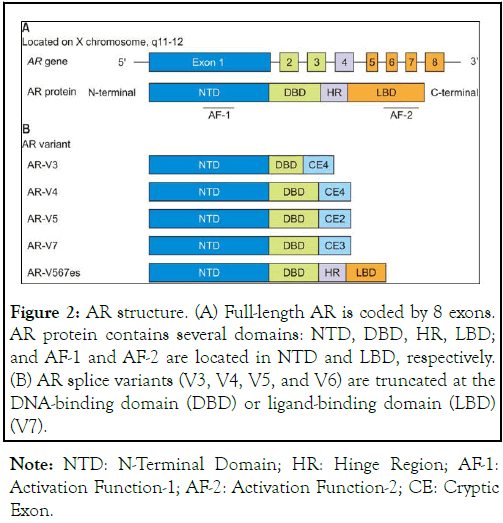
Figure 2: AR structure. (A) Full-length AR is coded by 8 exons. AR protein contains several domains: NTD, DBD, HR, LBD; and AF-1 and AF-2 are located in NTD and LBD, respectively. (B) AR splice variants (V3, V4, V5, and V6) are truncated at the DNA-binding domain (DBD) or ligand-binding domain (LBD) (V7).
Note: NTD: N-Terminal Domain; HR: Hinge Region; AF-1: Activation Function-1; AF-2: Activation Function-2; CE: Cryptic Exon.
Androgens include testosterone, androstenedione and Dehydroepiandrosterone (DHEA). Testosterone is synthesized in Leydig cells of the testis. Androstenedione and DHEA are synthesized from the adrenal glands. Adrenal androgen is converted to testosterone by 17-beta-hydroxysteroid dehydrogenase in the cytoplasm of prostate glandular cells. Immunohistochemical staining of normal prostate cells reveals strong staining for AR in luminal cells, fibromuscular stromal cells, and endothelial cells, but only with weak staining in basal cells. Luminal cells in prostate are associated with the production of Prostate-Specific Antigen (PSA), and stromal cells are implicated in normal prostate growth. Recent studies showed that prostate cancer had a predominant luminal phenotype with loss of basal cells due to their weak staining to AR. It was concluded that luminal cells and Luminal Progenitor (LP) cells may be the origin of prostate cancer cells and that LP cells are the driving force for prostate tumorigenesis and cancer development. Loss of the basal cells of the prostate is often associated with the local invasiveness of the disease and the potential for distant metastasis. In prostate cancer, the actions of AR include: 1) PSA synthesis; 2) Regulation of lipid metabolism; 3) Promotion of prostate growth; and some other functions. In 2005, it was reported that AR was also implicated in the gene fusion between The Transmembrane Protease Serine 2 gene (TMPRSS2) and E Twenty-Six (ETS), with the ERG gene as the most prevalent member of the ETS family fusing with TMPRSS2. In several mice models, selective knock-out of stromal AR resulted in inhibition of prostate development. The prevalence of TMPRSS2-ERG gene fusion is found to be at 30-50% of all localized prostate cancer cases. Stromal AR-producing cells play a critical role in the transcription of the TMPRSS2 gene. Mice models with specific knock-out of stromal AR presented with suppressed development of prostatic intraepithelial neoplasia, prostate cancer precursors, through the modulation of pro-inflammatory cytokines [9,11,13,22-24].
Using androgen antagonists to suppress the biological activity of androgen is the main treatment approach for prostate cancer. Drugs such as bicalutamide and flutamide antagonize androgens by binding to the LBD region of AR to competitively inhibit the binding of androgens to LBD [9,25].
Enzalutamide, a second-generation non-steroidal androgen antagonist, with known greater affinity to the LBD region of AR, with a confirmed prolongation of overall patient survival of 4.8 months by the AFFIRM trials [9,26].
In cases with low levels of serum androgen, as seen in patients undergoing ADT, the enzymes CYP11A1 and CYP17A1 convert DHEA and androstenedione into testosterone and DHT. Though weak adrenal androgens, prostate cancer often overexpresses these enzymes, which lead to increased precastration androgen level to promote the function of prostate stromal cells and to facilitate the progression of prostate cancer to CRPC [9]. CRPC cells also expresses 3β-Hydroxysteroid Dehydrogenase type 1 (HSD3β1), which catalyzes a rate-limiting step in the conversion of DHEA to DHT from the adrenal glands. HSD3β1 was found to produce sufficient quantity of DHT to activate AR. Abiraterone, a CYP17A1 inhibitor, was used to suppress androgen production from the conversion of weak androgen to testosterone and DHT from the adrenal glands. Studies confirmed the efficacy of using enzalutamide and abiraterone in patients with metastatic CRPC, however, due to the persistence of non-adrenal production of androgens (de novo synthesis from cholesterol to become independent sources of non-adrenal androgens), patients being treated with the combination medications eventually developed resistance to either, or both medications [9,27].
Apalutamide, a drug introduced in 2018, showed a greater efficacy compared to enzalutamide. It worked by binding to the LBD in AR with the same mechanism as bicalutamide, but with no agonistic activity. By binding to LBD, apalutamide inhibits the nuclear localization and DNA binding of AR in prostate cancer cells, leading to an inhibition of prostate cancer cell growth [9,28].
Most of the accepted approaches for prostate cancer management mainly involve in targeting the LBD region of the AR. However, a new experimental drug, EPI, aimed to concentrate on targeting AF-1 within NTD is important in AR transcription [9]. EPI 506, a prodrug of EPI-002 with antagonistic activity to AF-1, was shown to have excellent inhibitory activity to the growth of prostate cancer cells with aberrant AR activity and with AR-mutations which resulted in reducing AR functioning that was commonly seen in patients with CRPC [29].
More recently, our team studied the effect of AR-knockout in prostate cancer cells and confirmed that knocking out AR in CRPC cells significantly inhibited their malignant characteristics [13]. Further investigations revealed that the suppression of CRPC cells by AR-knockout was achieved by interfering the AR/FABP5-P-PPARγ-VEGF signaling axis [30,31]. Our study suggested that this axis could be a crucial factor in developing prostate cancer drug resistance through the modulation of the AR Variant 7 (ARV7) [13,32] and notably, disrupting this pathway resulted in the attenuation of ARV7's influence on drug resistance. Intriguingly, the interplay between FABP5 and AR emerges as a pivotal mechanism in regulating this pathway. FABP5 exerts control over the dominant AR splicing variant 7 (ARV7), while AR reciprocally regulates the expression of FABP5. This bidirectional interaction underscored a sophisticated regulatory network with significant implications for prostate cancer biology [10,30,33,34]. RNA profiling studies further elaborated this complex interplay by identifying the top six Differentially Expressed Genes (DEGs) in 22RV1 cells consequent to either FABP5 or AR knockout. These genes include CRIP2, ERG3, FOSB, GRPR, CAV1, and NR1H4. GO-enriched pathway analysis of the three most significantly upregulated DEGs (CRIP2, ERG3, and FOSB) disclosed their involvement in the regulation of cellular responses to the type I interferon pathway, the progesterone signaling pathway, and the development of the peripheral nervous system. Conversely, the analysis of the three most significantly downregulated DEGs (GRPR, CAV1, and NR1H4) highlighted their roles in pathways associated with fatty acid and lipid transport, as well as in steroid and cholesterol metabolic processes [13,35-40].
FABP5 is known to play a significant role in the progression and metastasis of prostate cancer. FABP5 facilitates the uptake and intracellular trafficking of fatty acids, which are essential for the energy metabolism and growth of cancer cells.
By binding to and activating peroxisome proliferator-activated receptor gamma (PPARγ), FABP5 promotes the expression of genes involved in lipid metabolism, inflammation, and cell proliferation [13,41]. In vitro studies demonstrated that overexpression of FABP5 in prostate cancer cells led to an enhanced cell proliferation, migration, and invasion, indicating its contribution to the aggressiveness of the cancer [30,31]. Additionally, FABP5 was linked to the increased resistance to apoptosis, further supporting its role in cancer cell survival and growth [42]. Recent studies in vivo reinforced the critical involvement of FABP5 in prostate cancer. Knockdown or inhibition of FABP5 of prostate cancer cells produced highly significant reductions in both the size of primary tumor and the rate of metastasis in mouse models. This is partly due to the decreased activation of PPARγ, which otherwise promotes the transcription of pro-tumorigenic genes [43,44]. Vascular Endothelial Growth Factor (VEGF), another key player in prostate cancer, is influenced by the FABP5-PPARγ axis. Modulating FABP5 expression levels was shown to affect VEGF expression, thereby impacting angiogenesis and tumor vascularization [14,30,31].
The introduction of a dominantly-negative mutant of FABP5 (dmrFABP5) was explored as a therapeutic strategy. This dmrFABP5 was generated by mutating 2 of 3 key amino acids in the fatty acid-bind motif of the FABP5 cDNA. DmrFABP5 may bind to PPARγ to prevent it from binding FABP5, but it is not capable of binding to fatty acids which are legends to activate PPARγ. By interfering fatty acid transportation, dmrFABP5 blocked the pro-tumorigenic pathways and exhibited a tumor-suppressing effect, like that obtained by FABP5-inhibition [43]. Both in vitro and in vivo studies showed that dmrFABP5 significantly suppressed prostate cancer cell growth, migration, and angiogenesis; highlighting its potential as a new therapeutic target [30,41,43].
The over activation of fatty acid-initiated signal transduction pathways was implicated in the development and progression of prostate and other cancer types [13,35-40]. These recent studies not only enhanced our understanding of the molecular underpinnings of prostate cancer but also suggested potential therapeutic options for intervention, particularly in the context of drug-resistant CRPC.
Binding of the legends to LBD leads to conformational changes of AR which resulted in malignant changes of prostate cells. Modern approach in management of prostate cancer is to inhibit the function of AR through targeting the LBD by drugs that competitively suppress the binding of LBD to legends such as testosterone and DHT [45]. A recently reported experimental drug EPI, which is still undergoing the process of testing, is trying to target AF-1, which involves in coregulatory and coactivator proteins that promote prostate cell growth [46]. A possible combination of LBD inhibitor and AF-1 inhibitor could be beneficial in suppressing prostate cancer growth and proliferation. Another possible approach to prostate cancer management is the ablation of AR receptors to decrease or minimize its exposure to androgens. For drug treatment, resistance is often observed even with combination therapy due to de novo synthesis of androgens from cholesterol and other non-adrenal sources.
The other potential therapy is the strategic disruption of AR signaling through AR-knockout, which emerges as a potentially transformative approach in mitigating the risk of treatment remission in prostate cancer [13], particularly under the shadow of persistent androgen production despite androgen deprivation therapy. This novel therapeutic avenue heralds a shift of paradigm in of prostate cancer management, offering an alternative to the conventional treatment regime currently in practice.
Furthermore, recent research has uncovered a critical role of FABP5 in the malignant progression of prostate cancer [13]. Naeem et al., demonstrated that FABP5 can act as a substitute for AR in promoting cancer cell growth, particularly in CRPC cells. The study highlighted that knocking out FABP5 significantly suppressed malignancy by interfering with the FABP5–PPARγ–VEGF signaling axis. This finding emphasizes the therapeutic potential of FABP5-knockout in conjunction with AR-targeted approaches to address androgen-independent pathways in CRPC management [13].
A more profound comprehension of the implications of AR knockout unveils its prospective role in preempting the transition of prostate cancer into a castration-resistant state, a critical juncture in the disease’s progression. Furthermore, this approach bears the potential to substantially reduce the incidence of metastasis, curtailing the spread of cancerous cells to distant parts of the body. Such advancements underscore a pivotal shift in our approach to prostate cancer treatment, steering away from traditional methods and embracing more targeted, molecularly informed strategies. The exploration of AR or FABP5 knockout not only enriches our understanding of prostate cancer biology but also opens up new horizons for clinical intervention. By targeting the very mechanisms that drive the progression and resistance of prostate cancer, we stand at the cusp of a new era in oncological therapeutics, one that promises enhanced efficacy, reduced recurrence, and improved patient outcomes.
In conclusion, both AR and FABP5 play important roles in prostate cancer development and malignant progression. The increased FABP5 activity is crucial to the conversion of the cancer cells from into androgen independent CRPC cells. The incorporation of FABP5 inhibition including FABP5-knockout into therapeutic strategies provides a dual-targeted approach that holds great promise in mitigating malignancy and resistance mechanisms, may mark a significant milestone in the ongoing quest to conquer one of the most prevalent and challenging malignancies.
None.
The authors have no conflict of interest to disclose.
Some work described in this study came from the results of a project grant (Y Ke) supported by Welcome Trust (Grant Number: 062991) and several 3-year project grants (Y Ke) from North West Cancer Research Fund.
Abdulghani A Naeem and Saud A Abdulsamad contributed equally to this work.
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
Citation: Naeem AA, Abdulsamad SA, Zhang J, Guo W, He G, Ke Y (2025) Androgen Receptor and the Fatty Acid-Binding Protein 5 in Malignant Progression of Prostate Cancer and in Development of Resistance to Treatment Populations. Andrology. 14:340.
Received: 21-Dec-2024, Manuscript No. ANO-24-36073; Editor assigned: 24-Dec-2024, Pre QC No. ANO-24-36073 (PQ); Reviewed: 07-Jan-2025, QC No. ANO-24-36073; Revised: 13-Feb-2025, Manuscript No. ANO-24-36073 (R); Published: 20-Feb-2025 , DOI: 10.35248/2167-0250.25.14.345
Copyright: © 2025 Naeem AA, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.