Journal of Clinical & Experimental Dermatology Research
Open Access
ISSN: 2155-9554
ISSN: 2155-9554
Original Research Article - (2019)Volume 10, Issue 4
In this study, topical water in oil W/O emulsion containing 3% grape seed extract (GSE) was developed and evaluated its anti-aging effects in comparison with its vehicle (base) as control. Both base and selected formulation were stored at 8°C ± 0.1°C, 25°C ± 0.1°C, 40°C ± 0.1°C, 40°C ± 0.1°C with 75% relative humidity (RH) for time duration of 12 weeks in order to evaluate their physiochemical stability. With an objective to determine its anti-aging potential & safety of the formulation containing GSE, various skin parameters such as moisture level, pH value, sebum content, mean pore size, elasticity and roughness were evaluated by using non-invasive biophysical techniques. Both base and formulation were applied on human cheeks of 20 healthy female between ages of 28-58 for a period of 12 weeks. Panelists also answered a preformed questionnaire to assess performance of the product. The results of the non-invasive biophysical test have shown that the product increases skin moisture, elasticity, and smoothness significantly (p<0.05). In addition, product also decreases skin roughness and mean pore sizes significantly (p<0.05) while the base shows insignificant result (p>0.05).
Anti-ageing; Emulsion; Formulation
GSE: Grape Seed Extract; ANOVA: Analysis of Variance; ROS: Reactive Oxygen Species; W/O: Water in Oil; UV: Ultra Violet
Skin provides protection against the external environment [1]. It helps to maintain the fluid balance, regulates body temperature, safeguards body against the detrimental effects of sunlight, microbes and chemicals [2,3]. Skin structure can be broadly classified into three layers: epidermis, dermis and hypodermis [4]. The first two layers have a thickness of around 0.07-0.12 mm and 1-4 mm respectively [5]. Reticular dermis, lower region of dermis consists of collagen and elastin fibers (0.3 -3 μm in diameter) [6].
Owing to external and internal causes, collagen fibers and elastic fibers present in dermal tissue of skin are modified or damaged which lead to formation of wrinkles and skin-sagging, because the reduction of elasticity of skin is one of the major causes of the skin-aging [7].
Skin-aging is one of complex biological processes. Skin-aging is classified into intrinsic and extrinsic skin-aging. Intrinsic-aging is also recognized as natural aging and it is chronologically emerging and inevitable process accompanied by profound genetic changes [8]. It is also characterized by fine wrinkles, roughness, dryness, sagging and epidermal thinning [9]. Extrinsic aging (often referred as photo-ageing) is caused due to exposure of skin to environmental factors which cause oxidative damage in skin [10]. Hyper pigmentation is characterized by dry and rough skin as well as deep wrinkles. Environmental factors such as cigarette smoke, air pollution and ozone contribute to premature skin aging [11]. However, 90% of skin-aging is due to exposure of skin to UV-radiations [12]. Thus, sun protection is considered as one of important factors to delay skin-aging. UVlight is artificially divided into three ranges: (i) UV-A is radiation in range of 320-400 nm, (ii) UV-B is radiation in range of 290-320 nm, (iii) UV-C is radiation in range of 100-290 nm.
Normally, UV-B has been found causing sun-burn and some other studies also indicate that UV-A may also cause skin damage [13]. Exposure of skin to UV-radiations induces photooxidative reactions which weaken the anti-oxidant defense system and increase the reaction oxygen species (ROS) at cellular level [14].
Ability of natural compounds having anti-oxidants when used in skin protection topical application, indicates that it may significantly slow down the process of skin-aging [15].Antioxidant of GSE fights against the harmful effects of UVradiations through the following mechanism [14]:
i) By reducing sun burns and inflammation induced through UV
ii) By eliminating the ROS and free-radicals which are harmful to the skin
iii) By adjusting the change signal path due to UV-radiation
Grape (Vitis vinifera) fruit and its seeds have been used for many years because of its nutritional and medicinal benefits. Grape seed extract has been found rich in sugar, polyphenols (i.e. flavonoids, anthocyanin, proanthocyndins & tannins), organic acids, mineral salts and vitamins [16,17]. GSE also contains high amount of resveratrol, stillben derivatives which are considered as one of the most effective anti-oxidant [18,19].
In this study, a stable dermatological formulation having GSE as a main ingredient, was formulated and applied on human cheeks and compared with controlled formulation (without GSE) and the results were investigated. Different parameters related to skin-aging were also evaluated.
Methyl paraben (sigma chemicals USA), Glycerin (Merck, Germany), Propyl paraben (Merck Germany), Grape seeds (purchased from local market), Abil EM 90 (frank chemical, Germany), Paraffin oil (Merck, Germany), Distilled water (prepared from distillation plant, IM 100-0.43.IRMECOGMBH, Germany) Triethanolamine (Merck, Germany), 2,2-diphenyl 1- picrylhydrazyl (DPPH) (Sigma chemical Co., USA).
Corneometer CM825® (courage & khazaka electronic Gmbh, Cologne, Germany), Sebum meter® SM 815 (courage & khazaka electronic Gmbh, Cologne, Germany), Skin pH meter 900® (courage & khazaka electronic Gmbh, Cologne, Germany), Aramo Ts skin diagnosis system (Aramhuvis co., Ltd, Korea). Cutometer (courage & khazaka electronic Germany), Conductivity meter (WTW, Germany).
Measurement of anti-oxidant activity
Anti-oxidant activity of GSE was measured by employing Marsden S. Blois method using DPPH (1, 1- diphenil-2- picryhydrazyl) which is also a stable free radical. Equal volume of diluted extract was mixed with equal volume of DPPH 0.5 μl in analytical grade ethanol, and the final mixture was kept at room temperature for 30 minutes. After that, absorption of the mixtures at 517 nm was taken, in comparison with the control solution (maximum absorption). Vitamin-C was used as a standard. Action of free radical was calculated in percentage inhibition according to the following equation:
% Inhibition=[(A control – A test)/A control] × 100
Preparation of formulation
Hydrophilic-Lipophilic Balance, HLB, is the ratio of oil soluble portion to water soluble portion of the molecule and it was first developed by Griffin. Griffin has directed his activities to select optimal non-ionic emulsifier ensuring stability of emulsion. ABIL-EM 90 (cetyl dimethicone copolyol) is an oil soluble emulsifier having HLB value 5.
In this study, W/O emulsion was formulated by addition of an aqueous phase to an oily phase with continuous stirring. Oily phase consists of surfactant ABIL-EM 90 and paraffin oil. Both paraffin oil and surfactant ABIL EM 90 (Oily phase) were heated up to the temperature of 80 ± 0.1°C. During the same time period, the aqueous phase consisting of distilled water was also heated on the same above mentioned temperature and then GSE was mixed into it. Methyl paraben and glycerin were also added to aqueous phase. After that, aqueous phase was poured into the oily phase drop by drop. Stirring was continued at 2000 rpm by the mechanical mixer for 15 minutes until complete aqueous phase was added to it. To provide good fragrance to the formulation, added 3-4 drops of lemon oil during the period of stirring. After the complete addition of aqueous phase to oily phase, mixer speed was reduced to 1000 rpm for good homogenization for a time period of 10 minutes and then mixer speed further reduced to 500 rpm for 5 minutes in order to get complete homogenization. The emulsion was gradually cooled to room temperature. pH adjustment was made with 10% W/W of Triethanolamine solution. The base was also prepared with the same method and with the same constituents but without GSE.
Determination of emulsion type
Emulsion type was determined through utilization of dilution test based on solubility of the emulsion’s external phase in water or oil. Briefly, a few drops of prepared emulsion were added to the test tube containing a small amount of water. If the outer phase of the emulsion distributed evenly in water, it would be determined as O/W type and if it was separated as a layer, it would be determined as W/O type.
Pharmaceutical stability test
Stability test for base and formulation were taken at (8 ± 0.1°C), refrigerator (25 ± 0.1 °C ), room temperature (40 ± 0.1 °C ), accelerated temperature and (40 ± 0.1°C) with relative humidity (RH) 75%. Physical characteristics of emulsion i.e. liquefaction, coalescence, partial phase separation and complete phase separation were observed at various time intervals for a period of 12 weeks.
Viscosity of an emulsion plays a significant part in its flow characteristics and is considered as one of valuable process indicators of emulsion quality [20]. After the preparation of emulsion, temperature and time dependent process occurred which resulted in an increased liquefaction and decreased viscosity [21]? Liquefaction did not occur in any of the samples of formulation stored at 8 ± 0.1°C (refrigerator) and 25 ± 0.1°C (room temperature), 40 ± 0.1°C (accelerated temperature) and 40 ± 0.1°C with RH 75% during the whole observation period of 12 weeks. However, the base remained stable at 8 ± 0.1°C (refrigerator) and 25 ± 0.1°C (room temperature) whereas slight liquefaction occurred at 40 ± 0.1°C (accelerated temperature) during the time interval of 8 and 10 weeks. It is pertinent to mention here that coalescence was observed at 40 ± 0.1°C (accelerated temperature) during 12th week of observation. At 40 ± 0.1°C with RH 75%, liquefaction, coalescence, partial phase separation and complete phase separation occurred during 2nd, 4th, 8th and 12th week of study respectively (Table 1).
| Time | 8 ± 0.1 | 25 ± 0.1 | 40 ± 0.1 | 40 ± 0.1 with 75 % RH | ||||
|---|---|---|---|---|---|---|---|---|
| B | F | B | F | B | F | B | F | |
| 0 hour | S | S | S | S | S | S | S | S |
| 1 Day | S | S | S | S | S | S | S | S |
| 1 week | S | S | S | S | S | S | S | S |
| 2 weeks | S | S | S | S | S | S | + | S |
| 4 weeks | S | S | S | S | S | S | ++ | S |
| 8 weeks | S | S | S | S | + | S | +++ | S |
| 10 weeks | S | S | S | S | + | S | +++ | S |
| 12 weeks | S | S | S | S | ++ | S | ++++ | S |
S: Stable; +: Liquefaction; ++: Coalescence; +++: Partial Phase Separation; ++++: Complete Phase Separation
Table 1: Pharmaceutical stability of Base (B) and Formulation (F).
Colour
The fresh prepared base and formulation were found shining light pink in colour. No change in colour of formulation up to observation period of 12 weeks at all storage condition 8 ± 0.1°C, 25 ± 0.1°C, 40 ± 0.1°C, 40 ± 0.1°C (with relative humidity (RH)) 75%. However, slight change in colour occurred in the base at 40 ± 0.1°C in 12th week of study and at 40 ± 0.1°C (with RH 75%), light brown colour occurred at 8th week and brown colour occurred at 10th week of study (Table 2). This study observed no change in colour of formulation. It may be attributed to different factors contributing to emulsion stability such as component of oil phase that is paraffin oil which is a transparent, non-fluorescent, colorless liquid having mixture of hydrocarbon [22] and Abil-EM which is a colourless, clear and non-toxic liquid emulsifier [23].
| Time | 8 ± 0.1 | 25 ± 0.1 | 40 ± 0.1 | 40 ± 0.1+75% RH | ||||
|---|---|---|---|---|---|---|---|---|
| B | F | B | F | B | F | B | F | |
| 0 hour | LP | LP | LP | LP | LP | LP | LP | LP |
| 24 hour | LP | LP | LP | LP | LP | LP | LP | LP |
| 1 week | LP | LP | LP | LP | LP | LP | LP | LP |
| 2 weeks | LP | LP | LP | LP | LP | LP | LP | LP |
| 4 weeks | LP | LP | LP | LP | LP | LP | LP | LP |
| 8 weeks | LP | LP | LP | LP | LP | LP | LB | LP |
| 10 weeks | LP | LP | LP | LP | LP | LP | B | LP |
| 12 weeks | LP | LP | LP | LP | LB | LP | B | LP |
LP: Light pink; LB: Light brown; B: Brown
Table 2: Colour of base & formulation.
pH measurements
pH is considered as one of significant parameters for evaluating effectiveness of an emulsion. Normal pH of human ’ s skin usually ranges from 4.5 to 6.0. Average pH of the human’s skin is 5.5 [24]. Therefore, the formulation containing GSE must have pH within the range.
The pH value for formulation remained in the normal range of pH of human skin at all storing conditions 8 ± 0.1°C, 25 ± 0.1°C, 40 ± 0.1°C, 40 ± 0.1°C (with RH 75%) during the study period of 3 months while value of base at 40 ± 0.1°C and 40 ± 0.1°C (with RH 75%) decreased i.e. 4.31 and 4.03 respectively at 12th week of study (Tables 3 and 4). The decrease in pH of base may be caused by oxidation of paraffin oil which produced aldehyde and organic acid (Figures 1 and 2) [23].
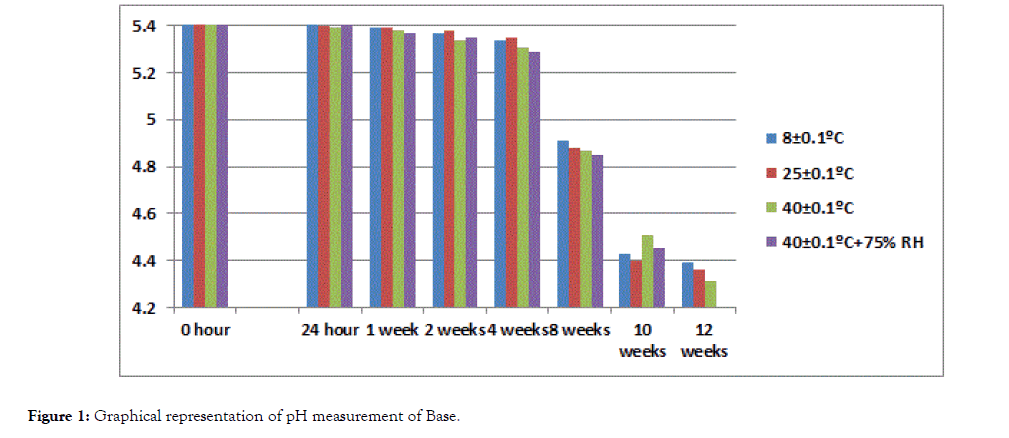
Figure 1. Graphical representation of pH measurement of Base.
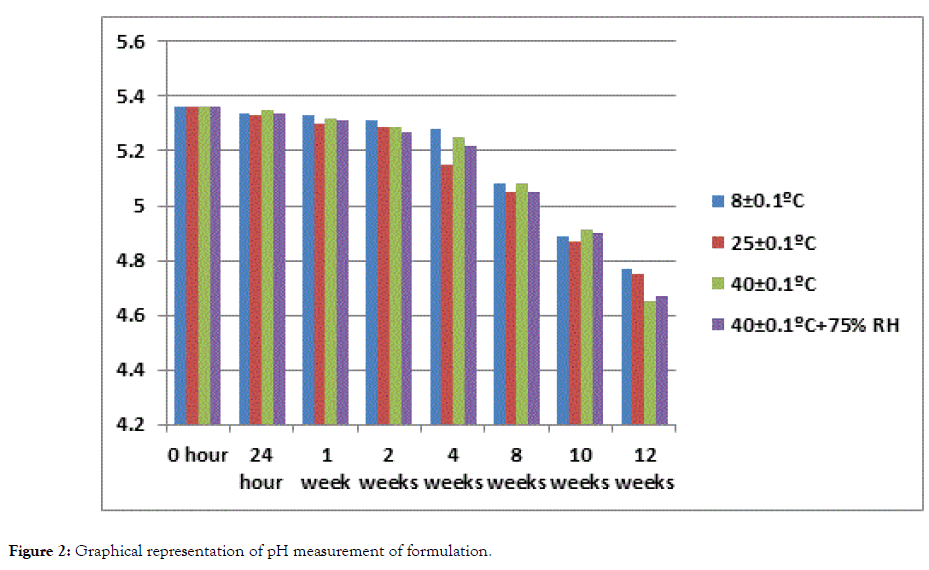
Figure 2. Graphical representation of pH measurement of formulation.
| Time | 8 ± 0.1 | 25 ± 0.1 | 40 ± 0.1 | 40 ± 0.1+75% RH | Mean ± Standard Deviation |
|---|---|---|---|---|---|
| 0 hour | 5.42 | 5.42 | 5.42 | 5.42 | 5.42 ± 0 |
| 24 hour | 5.41 | 5.4 | 5.39 | 5.41 | 5.40 ± 0.008 |
| 1 week | 5.39 | 5.39 | 5.38 | 5.37 | 5.38 ± 0.008 |
| 2 weeks | 5.37 | 5.38 | 5.34 | 5.35 | 5.36 ± 0.015 |
| 4 weeks | 5.34 | 5.35 | 5.31 | 5.29 | 5.32 ± 0.023 |
| 8 weeks | 4.91 | 4.88 | 4.87 | 4.85 | 4.87 ± 0.021 |
| 10 weeks | 4.43 | 4.4 | 4.51 | 4.45 | 4.44 ± 0.040 |
| 12 weeks | 4.39 | 4.36 | 4.31 | 4.03 | 4.27 ± 0.142 |
Table 3: pH measurements of Base.
| Time | 8 ± 0.1 | 25 ± 0.1 | 40 ± 0.1 | 40 ± 0.1+75% RH | Mean ± Standard Deviation |
|---|---|---|---|---|---|
| 0 hour | 5.36 | 5.36 | 5.36 | 5.36 | 5.36 ± 0.00 |
| 24 hour | 5.34 | 5.33 | 5.35 | 5.34 | 5.34 ± 0.01 |
| 1 week | 5.33 | 5.3 | 5.32 | 5.31 | 5.32 ± 0.01 |
| 2 weeks | 5.31 | 5.29 | 5.29 | 5.27 | 5.29 ± 0.01 |
| 4 weeks | 5.28 | 5.15 | 5.25 | 5.22 | 5.23 ± 0.05 |
| 8 weeks | 5.08 | 5.05 | 5.08 | 5.05 | 5.07 ± 0.02 |
| 10 weeks | 4.89 | 4.87 | 4.91 | 4.9 | 4.89 ± 0.01 |
| 12 weeks | 4.77 | 4.75 | 4.65 | 4.67 | 4.71 ± 0.05 |
Table 4: pH measurements of formulation.
Conductivity test
Conductivity difference arises when oil proportion increases in upper layer of an emulsion and water proportion increases in lower part of an emulsion [25]. In this study, no conductivity was observed in formulation kept at different storage condition 8 ± 0.1°C, 25 ± 0.1°C, 40 ± 0.1°C, 40 ± 0.1°C with RH 75% while slight conductivity was observed in base kept at 40 ± 0.1°C, 40 ± 0.1°C with RH 75% at period of 8th week and 2nd weeks respectively.
Study design
The study was initiated on 20 healthy female volunteers between an age group of 28-58 after they signed a written informed consent form. Exclusion criteria for volunteers are as follows:
• Pregnancy and lactation cases for women
• Persons who have started hormonal therapy before 12 weeks or less prior to the study
• Persons with extreme sensitivity in the selected region
• Persons who cannot adapt to the study
• Persons with a significant systemic story
• Use of systemic or topical medication for skin diseases within the same period
• Knowing that the person had hypersensitivity previously to formulation content
Ethical standards
This study was sanctioned by the Board of Advance Studies and Research (BASR), Department of Pharmacy, Bahauddin Zakariya University Multan, Pakistan in compliance with Helsinki Declaration.
Dermatological testing (Patch test)
0.5 gm of product was applied on the forearm of each volunteer on the 1st day of sampling to determine if any adverse effect occurred on the skin of volunteers. The volunteers were informed to avoid from contact with water and direct sunlight during 48 hour’s observation period. The patch test material was removed at the end of 48 hour’s period and it was checked if a reaction such as erythema and edema occurred in the skin of volunteers.
Preparation of test environment
If the tests are carried out in an environment receiving direct light, the skin warms up, sweating increases and as a result sebum secretion decreases. Consequently, hydrophilic film layer of the skin varied to a large extent. Therefore, it is avoided to make measurements under exposure to direct light. Other environmental factors that can affect the measurements are humidity and temperature of the environment.
Therefore, the instrumental measurements were taken after the volunteers rested in the air-conditioned room at 20 ± 1 temperature and at 40-50% relative humidity for 30 minutes.
Biophysical methods
During the 12-weeks test period, the volunteers were not permitted to use any skin care product on their cheeks where the product was used.
Measurements were taken four times before starting to the use the product (T0), in the 4th week (T4), in the 8th week (T8) and in the 12th week by non-invasive biophysical methods from the application region of the volunteers and various parameters were evaluated. The moisture content, pH value, sebum content, elasticity and average pore size of skin were determined by corneometer (working according to capacitance method), skin pH meter®, sebum meter®, cutometer® and Aramo TS skin diagnosis system respectively taking 5 different measurements from the test area for each parameter.
Subjective evaluation
A panel test was conducted to support the results of biophysical measurements after 12 weeks period of product application of volunteers. The volunteers were asked seven questions with regard to the effect of formulation and base on the skin. They were requested to respond the following queries by awarding score between 0 and 5 (Table 5).
| Question | Score |
|---|---|
| 1. Did the cream you used decrease skin moisture loss? | 4.4 |
| 2. Did the cream you used cause an increase skin oiliness? | 0.4 |
| 3. Did the cream you used give softness to your skin? | 4.6 |
| 4. Did the cream you used provide a reduction in appearance of wrinkles? | 3.8 |
| 5. Did the cream you used absorbed easily by your skin? | 4.7 |
| 6. Did the cream you used increase skin elasticity? | 3.95 |
| 7. Did the cream you used increase skin brightness? | 3.7 |
Table 5: Questionnaire scores evaluated by the panelists.
Statistical analysis
The results obtained from clinical trials were evaluated through utilization of Microsoft Excel 2010. Two-way analysis of variance (ANOVA) was applied to determine variation between different time intervals. Statistically, a significant difference was considered at a p value of less than 5% (p<0.05).
Hydration or water content of the outermost layer of (stratum corneum) is associated with intrinsic aging and menopause. It is considered that reduction in the amount of glycosaminoglycans having a hydrophilic structure make a direct reduction in water content of the skin. Moisturizers help normalize the protective functions of the skin and create a smooth, flexible and healthier looking skin [26] while the hydration value of the skin was 54.45 before the application of the product (T0), it was found to be 66.76,72.92 and 80.35 after 4th, 8th and 12th weeks of application respectively by corneometer CM 825® working with capacitance principle. The results revealed that formulation increased the moisture content of skin significantly (p<0.05) throughout the time period while base showed insignificant result (p>0.05).
Sebum has amphiphilic properties due to its free fatty acid and waxes content. This causes a little hydration of skin. It is protective against intensive dehydration in the skin. Furthermore, it has a nutrient function for the useful bacteria species in the organism, while it ensures protection of fungi static activity and functional quality of hair sebum secretion rate reaches its highest level in the teenage years and decrease gradually thereafter. Older people have a very low rate of sebum secretion. This reduction in sebum production is accompanied by a decrease in antimicrobial fatty acids lead to the formation of skin infections in the elderly people [27].
According to the measurements taken from volunteers by sebumeter® SM 815, while the sebum content (mg/cm2) was found 1.79 before application of the product (T0). It was found to be 2.05, 2.68, 3.47 after 4th (T4) 8th (T8) and 12th (T12) weeks of application respectively. The product showed significant results (p<0.05) and the base showed insignificant results (p>0.05).
Skin is a complex structure with elastic and viscous properties. Visco-elastic properties of the skin are dependent to collagen and elastin fibers in the dermis [28]. A cutometer probe was used to measure elasticity of skin which had a measurement principle based on the measurement of skin deformation as a result of the vertical vacuum that was applied to the surface. While elasticity value of the skin was 42.48 before the application of the product (T0), the elasticity value was found to be 57.45, 59.68, and 62.85 after 4th, 8th and 12th weeks respectively in the measurements taken from the volunteers. The result revealed that the application of the product significantly increased the elasticity of the skin (p<0.05) and the base showed the insignificant result throughout the period of this study (p>0.05).
Clinical signs of dry skin are roughness, redness, flaking and superficial skin loss [29]. Moisturizers hydrate the skin and also smoothens its surface. Effectiveness of the moisturizers can be increased according to target skin condition by selecting proper moisturizing agents [30]. X-60 triple lens of the Aramo TS skin analyzer was used to determine the average pore size and roughness of the application area. While the skin's average pore size and roughness value was 34.68 and 80.47 respectively before the application of the product (T0), it was found to be 19.77 and 74.63 after 4th weeks respectively and 17.55 and 70.15 respectively after 8th weeks of application and 14.98 and 65.19 after 12th week respectively (Tables 6 and 7). The results revealed that application the product for 4th, 8th and 12th weeks significantly decreased the roughness of the skin (p<0.05) while the base showed an insignificant result throughout the period of study (p>0.05) (Figures 3 and 4).
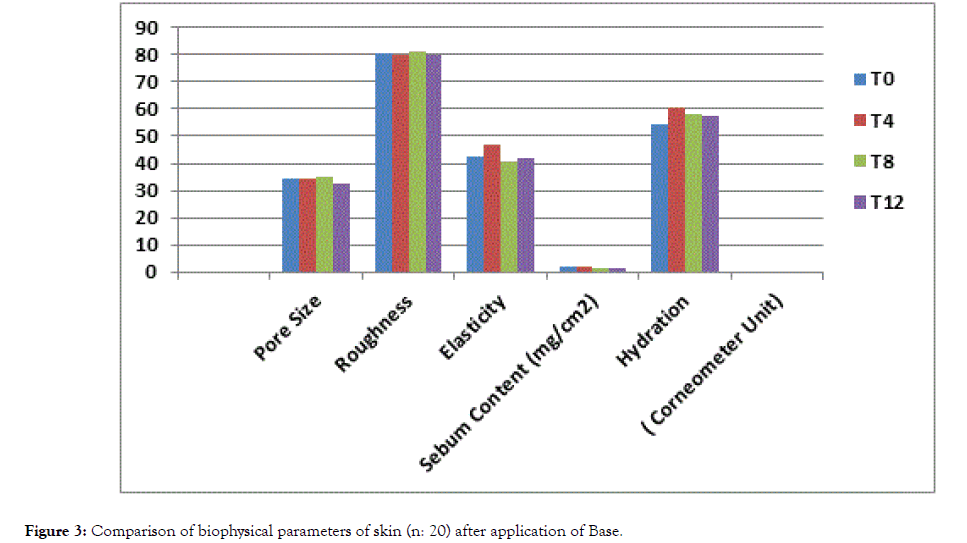
Figure 3. Comparison of biophysical parameters of skin (n: 20) after application of Base.
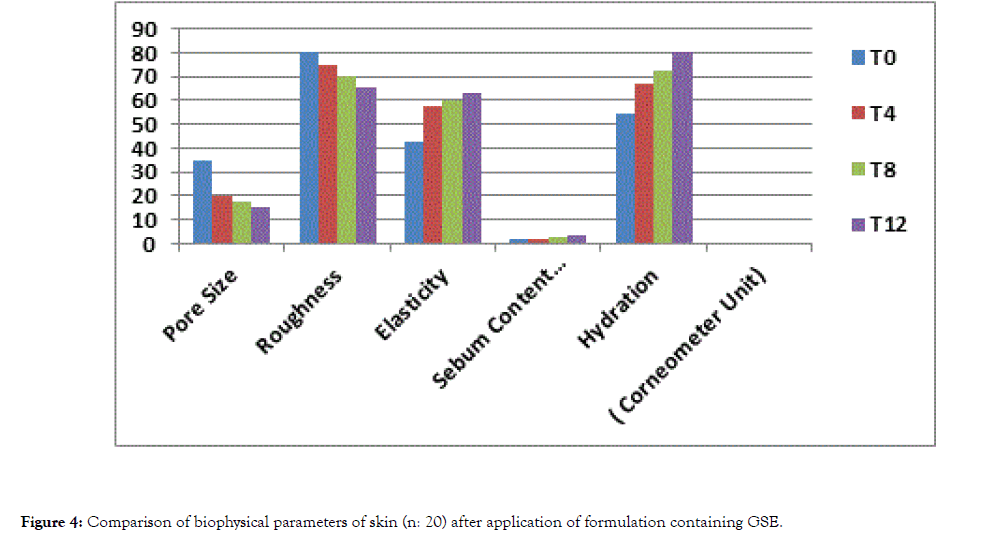
Figure 4. Comparison of biophysical parameters of skin (n: 20) after application of formulation containing GSE.
| Biophysical Parameters | T0 | T4 | T8 | T12 | Statistical Significance | |||
|---|---|---|---|---|---|---|---|---|
| Mean | Mean | % Variation | Mean | % Variation | Mean | % Variation | (p<0.05) | |
| Pore Size | 34.68 | 34.6 | -0.23 | 35.06 | 1.09 | 32.56 | -6.11 | NO |
| Roughness | 80.47 | 79.85 | -0.77 | 81.23 | 0.94 | 79.95 | 0.64 | NO |
| Elasticity | 42.48 | 46.67 | 9.86 | 40.86 | -3.81 | 41.73 | -1.76 | NO |
| Sebum Content (mg/cm2) | 1.79 | 1.83 | 2.23 | 1.66 | -7.26 | 1.47 | -17.87 | NO |
| Hydration (Corneometer Unit) | 54.45 | 60.76 | 11.58 | 58.19 | 6.86 | 57.33 | 5.28 | NO |
Table 6: Comparison of Biophysical Parameters of Skin (n: 20) after the application of Base.
| Biophysical Parameters | T0 | T4 | T8 | T12 | Statistical Significance | |||
|---|---|---|---|---|---|---|---|---|
| Mean | Mean | % Variation | Mean | % Variation | Mean | % Variation | (p<0.05) | |
| Pore Size | 34.68 | 19.77 | -42.99 | 17.55 | -49.45 | 14.98 | -56.8 | YES |
| Roughness | 80.47 | 74.63 | -7.25 | 70.15 | -12.82 | 65.19 | -18.98 | YES |
| Elasticity | 42.48 | 57.45 | 35.24 | 59.68 | 40.48 | 62.85 | 47.95 | YES |
| Sebum Content (mg/cm2) | 1.79 | 2.05 | 14.52 | 2.68 | 49.72 | 3.47 | 93.85 | YES |
| Hydration (Corneometer Unit) | 54.45 | 66.76 | 22.6 | 72.92 | 33.92 | 80.35 | 47.56 | YES |
Table 7: Comparison of Biophysical Parameters of Skin (n: 20) after application of formulation containing GSE.
This study reveals that GSE possess significant antioxidant potential and its anti-oxidant activity is also confirmed by DPPH assay. The results revealed that topical skin care cream (W/O emulsion) having grape seed extract (GSE) possesses anti-aging effects as it increase skin hydration, elasticity and sebum content and decrease pore size and roughness.
Citation: Rafique M, Shah SNH (2019) Anti-Ageing Potential of a Cream (W/O Emulsion) Containing Grape Seed Extract (GSE): Formulation and in vivo Evaluation of Effectiveness using Non-Invasive Biophysical Technique. J Clin Exp Dermatol Res 10:500. doi: 10.35248/2155-9554.19.10.500
Received: 22-May-2019 Accepted: 31-May-2019 Published: 07-Jun-2019 , DOI: 10.35248/2155-9554.19.10.500
Copyright: © 2019 Rafique M, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.