Chemotherapy: Open Access
Open Access
ISSN: 2167-7700
ISSN: 2167-7700
Research Article - (2023)Volume 11, Issue 2
Background: Melanoma is one of the deadliest types of skin cancer that affects the deep layers of the skin and has a great potential to spread to the tissues. In recent years, the antitumor effect of parasitic derivatives from some protozoa and worms has been reported. Worm parasites, including Echinococcus granulosus, contain compounds that exhibit antitumor activity.
Aim: The objective of this study was to evaluate the effects of AgB (AgB) extracted from hydatid cyst fluid, Echinococcus granulosus larval stage, on melanoma B16F10 cell line.
Methods: Effect of different concentrations of AgB on B16F10 and HEK293 cells proliferation was investigated using the MTT assay. Cell cycle analysis to measure cellular DNA content in the G0/G1, S and G2/M was done using Flow cytometry. Annexin-V/PI staining method was used to determine cells apoptotic rate. Furthermore, the mRNA expression of pro-apoptotic gene BAX and anti-apoptotic gene BCL2 was assessed by RT-PCR after exposure to AgB. The effect of AgB on HEK293 and B16F10 cells showed that HEK293 cells as a normal cell line is less sensitive than cancer cell line B16F10 to AgB, and IC50 values in HEK293 and B16F10 cells were 35 ± 4.3 and 15 ± 3.1 µM, respectively. In both cell lines, the AgB induced anti-proliferative effect on the cells with increasing cell population at G0/G1, and decreasing the numbers of cells at the S and G2/M phases. Results show that AgB can induce cell apoptosis by increasing mRNA expression of BAX and decreasing mRNA expression of BCL2.
Conclusion: This study confirmed that AgB inhibits proliferation and promotes apoptosis of HEK293 and B16F10 cells and can raise hopes in the treatment of melanoma cancer after in vivo testing.
Melanoma; Cancer; Cell line; Anticancer; Antigen B; Hydatid cyst
HCF: Hydatid Cyst Fluid; FBS: Fetal Bovine Serum; PI: Propidium Iodide; DMEM: Dulbecco's Modified Eagle Medium
Skin cancer is a type of cancer that involves abnormal changes in the outer layer of the skin and is divided into three main types: melanoma, basal cell carcinoma, and squamous cell carcinoma [1]. This cancer is much more common in people with fair skin. Among the major types of skin cancer, melanoma is the deadliest because it affects the deeper layers of the skin and has the greatest potential to spread to other tissues [2]. Melanoma is a malignant tumor that originates in melanocyte cells [3]. According to 2021 statistics in the United States, melanoma is estimated to be the fourth most common new cases after breast, prostate and lung cancer with 106,110 cases per year. The death rate from this cancer is high and among the new cases, 7180 are likely to die [4]. So, in the coming years, skin cancers could be a major public health problem.
There are several treatments for melanoma, including surgery, radiotherapy, and medication (herbal and chemical). Over the last 50 years, several drugs for the treatment of melanoma have been approved by FDA, some of which are dacarbazine, interferon α-2b, vemurafenib, ipilimumab, dabrafenib, trametinib, laherparepvec and talimogene [4,5]. Despite the many therapeutic advances that have been mentioned, a definitive drug for the treatment of melanoma has not yet been introduced, on the other hand, due to the many side effects of chemical drugs that have different tissues in the body, researchers have used natural compounds of biological origin in the treatment of various cancers [6,7]. Natural compounds have been the source of medicinal products for many years, and during the last century, many useful drugs have been made from natural sources, especially plants [8].
In recent years, a different approach has been observed regarding the antitumor effect of parasitic derivatives of protozoa and worms [9,10]. These compounds affect several characteristics of cancer, but the mechanisms involved are not yet fully understood. However, some studies have suggested beneficial effects such as inducing apoptosis, activating the immune response, preventing metastasis and angiogenesis, inhibiting proliferative signals, and regulating inflammatory responses [11,12]. Hydatid cyst, which is the larval stage of Echinococcus granulosus (E. granulosus) worm, is the cause of hydatidosis in humans and animals [13]. It has been shown that the prevalence of hydatid cyst in patients with cancer was lower than the normal population [14]. In general, in cancer cells, hydrocarbon compounds such as mucins play an important role in the adhesion of cancer cells to the surrounding tissue, preventing cell death and preventing cell lysis by Natural killer cell and cytotoxic lymphocytes. These mucin-containing antigens are also supplied by hydatid cysts, so they can be used to stimulate the immune system against mucin-secreting cancer cells [15]. So, in this study, the efficacy of hydatid cyst AgB on B16F10 cell line was evaluated by cellular methods including apoptosis and cell cycle, as well as by molecular mechanisms and expression of genes involved in apoptosis.
Hydatid Cyst Fluid (HCF) was collected from livers of infected sheep at slaughterhouse in Hamadan, Iran. RPMI 1640 medium, Penicillin-Streptomycin, Fetal Bovine Serum (FBS), and trypsin were bought from GIBCO (Darmstadt, Germany). Propidium Iodide (PI) was obtained sigma. Annexin V-fluorescein Isothiocyanate (V-FITC) was bought from Invitrogen by Thermo Fisher Scientific (Waltham, Massachusetts,USA). Total RNA extraction and RNAse were obtained by RNX-Plus kit from CinnaGen (Tehran, Iran). cDNA synthesis Kit was purchased from fermentas (Waltham, Massachusetts, USA), master mix green was purchased from Amplicon (Copenhagen, Denmark) and the primers of candidate genes were purchased from the Bioneer (Daejeon, Republic of Korea). All other chemicals were of analytical grade.
Preparation of HCF AgB
Liver infected with hydatid cyst was collected from Hamadan slaughterhouse and transferred to parasitology laboratory. The surface of the cysts was disinfected using 70% Ethanol and heat, HCF of cyst was aspirated by syringe and were examined for the presence of protoscolex. Cysts containing protoscolex were used for this study. The sample stored in 50 ml sterile test tube and after three washes with normal saline was kept at -20. After 2 h, the supernatant was slowly removed and centrifuged at 1500 g for 30 min. The supernatant was dialyzed twice with acetate buffer (0.005 M, pH=5) for 24 h at 4°C. The dialyzed sample was centrifuged at 30,000 g for 30 min at 4°C; in this process insoluble proteins such as Ag B and Ag 5 were precipitated. The pellet was dissolved in 10 ml of phosphate buffer, PBS (0.2 M, pH=8) to eliminate globulins. Then, 2.31 g of ammonium sulfate (40%) was added and the mixture was centrifuged at 3000 g for 30 min. The supernatant was incubated in a water bath for 15 min; in this step Ag 5 became denatured and insoluble due to its heat sensitivity. Finally, the preparation was centrifuged at 30,000 g for 1 h and the supernatant containing Ag B was collected. After filtration (using a 0.2 µm sterile filter) sodium azide, was added as a preservative [16,17]. The mixture was stored at -70°C until use and part of it was subjected to sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) and Bradford method to determine of purity and concentration of the isolated antigen [18-20].
Bradford protein assay
To draw the standard curve, six dilutions of a bovine serum albumin as standard protein prepared; then, 100 ml of buffer was added. Moreover, 5 ml of Bradford reagent was added and completely mixed. For preparing the blank tube, 100 ml of phosphate-buffered saline and 5 ml of Bradford regent were mixed, then at room temperature for 20 min incubated. With an ELISA reader (BioRad) at 590 nm absorbance was measured for plotting the standard curve with standard samples and determining the concentration of isolated proteins [16].
SDS-PADE analysis
The purity of AgB preparation was measured by SDS-PAGE 2.5% gel. Afterwards, 10 µl of AgB was dissolved in the same sample buffer that including: 2% SDS, 0.06% bromophenol blue, 0.0625 M Tris HCl, pH=6.8, 10% glycerol and 5% ß-mercaptoethanol and boiled for 10 min. finally the product was loaded on the electrophoresis gel. Electrophoresis was performed at a constant current of 110 V for 1 h. After that, the separated bands were stained by Coomassie brilliant blue staining solution (0.1 cc Coomassie blue in 70 ml methanol, 15 ml water, and 15 ml acetic acid) and decolorized in decolorizer buffer (70 ml of methanol, 15 ml of water, and 15 ml of acetic acid) [16].
Cell culture
Melanoma cancer cell line, B16F10 and human embryonic kidney cell line, HEK293 as control group were purchased from the National Cell Bank of Iran (Pasteur Institute, Tehran, Iran). Cells were cultured in Dulbecco's Modified Eagle Medium (DMEM) with 10% fetal bovine serum, 100 ng/mL of streptomycin and 100 units/mL of penicillin and kept in a 5% CO2 incubator at 37°C [21].
MTT assay
Cytotoxicity of Ag B was assessed by MTT assay. B16F10 and HEK293 cells were seeded at 5000 cells/well in 96-well plates at 37°C in complete medium (150 μl total volume/well) for 24 h. Ag B were serially diluted in medium and added to cell cultures with final concentrations of 10, 20, 30, 40, 50, 60, 70, 80, 90 and 100 μM for 48 hours in a humidified incubator. Subsequently, MTT reagent (5 mg/ml) was added to each well. The incubation was performed for 4 hours in the incubator; next, the medium was removed and 100 µl DMSO was added to each well and shake for 20 min on gentle shaker to dissolve the purple crystal. Finally, the absorbance was recorded by ELISA Plate Reader at 630 nm and cytotoxicity was measured with below formula:
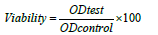
Cell cycle analysis
Cell cycle was examined in three main cellular phases (G1, S, G2/M) by flow cytometry technique. Briefly, HEK293 or B16F10 cells (5 × 105 per well) were seeded in 6-well plates and treated with AgB (at IC50 concentration). After 48 h the media containing the cells were centrifuged at 2500 rpm for 15 minutes. The supernatant was removed and 5 ml of 10% formalin was added to the cells and incubated for 10 minutes at room temperature and in the dark. After centrifuge, the supernatant was drained; subsequently triton (0.1%) was added to the final precipitate and incubated for 40 minutes at room temperature in the dark. Next, centrifuged and finally 3 ml of PBS, 2 μl of RNAse and 2 μl of PI were added to the precipitate for 30 minutes and kept at room temperature in the dark and then read by flow cytometry.
Apoptosis assay
The rate of cellular apoptosis in normal (HEK293) and cancer (B16F10) cells which were treated using AgB (at IC50 concentration) for 48 h and were measured by flow cytometry device. Apoptosis kit of annexin V-fluorescein isothiocyanate (V-FITC) was used for this purpose. After washing in PBS, 1 × 106 cells were rinsed and re-suspended in the HEPES buffer. The cells were incubated at room temperature for 5-15 min in the dark, subsequently, annexin V-FITC (5 μl) was added. Before the flow cytometry analyzing, propidium iodide staining solution (5 μl) was added to the cell suspension to detect necrotic cells [22].
RNA extraction, cDNA synthesis and qRT-PCR
For RNA extraction, about 3 × 106 cells were treated with AgB at IC50 concentrations for 72 h in the humidified incubator. After incubation, RNA was extracted using RNX™-plus reagent in RNase-free tube, according to the manufacturer’s instructions. Gel electrophoresis (1%) and NanoDrop (absorbance at 260/280 nm ratios) were used to quality and quantity of the extracted RNA, respectively. Complementary DNA (cDNA) was synthesized from the extracted RNA using random primers (N6) and hexamer primers with the purchased RT kit (K1622; Fermentas, Thermo Fischer Scientific, MA, USA). qRT-PCR reactions were performed in a Roche light cycler 96 Real Time PCR instrument using SinaSYBR Blue HS-qPCR Mix (Sinaclone, Tehran, Iran) and gene specific primers according to manufacturer instructions. GAPDH was considered as a house keeping gene. The primer characteristics of the nominated genes have been listed in Table 1. Expression fold change was calculated using F=2-∆∆CT formula [23]. Each qRT-PCR reaction was carried out in duplicate.
| Genes | Primers | Product size |
|---|---|---|
| BAX | F: 5'TTTGCTTCAGGGTTTCATCCA3' | 151 bp |
| R: 5'CTCCATGTTACTGTCCAGTTCGT3' | ||
| BCL2 | F: 5'CATGTGTGTGGAGAGCGTCAAC3' | 241 bp |
| R: 5'CAGATAGGCACCCAGGGTGAT3' | ||
| GAPDH | F: 5'GGCCAAGATCATCCATGACAACT3' | 500 bp |
| R: 5'ACCAGGACATGAGCTTGACAAAGT3' |
Table 1: Primers used for RT-PCR of BAX, BCL2 and GAPDH.
Statistical analysis
Data were analyzed using ANOVA and Student's t-tests and the differences were statistically significant at P<0.05. Data were reported as mean ± standard deviation from three independent experiments [24].
The concentration and purity of isolated AgB using Bradford protein assay and SDS-PAGE
Out of 100 ml of hydatid cyst fluid collected from sheep hepatic cysts, the concentration of B antigen protein was 0.9 mg/ml which the standard curve is shown in Figure 1. Moreover, specific bands were seen, as presented in Figure 2. SDS-PAGE results showed band on 20 KDa subunit of AgB.
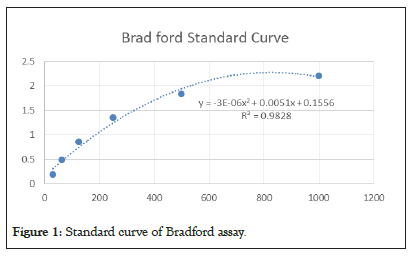
Figure 1: Standard curve of Bradford assay.

Figure 2: SDS-PAGE of Ag.B, M: Marker, 2: Purified Ag.B, 3: Ag.B.
In vitro cytotoxicity
Cytotoxicity of AgB was determined in HEK293 and B16F10 cells by the MTT assay. In both cell lines, AgB led to the toxicity than control group. AgB is more toxic to cancerous cell line than regular cell line, and IC50 values for AgB in HEK293 and B16F10 cells were 35 ± 4.3 and 15 ± 3.1 µM, respectively (Figure 3). Thus, HEK293 cells as a normal cell line that has rapid growth is less sensitive than cancer cell line B16F10 to AgB.
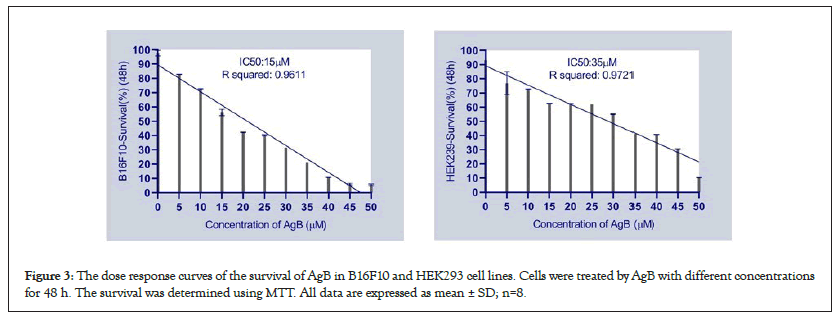
Figure 3: The dose response curves of the survival of AgB in B16F10 and HEK293 cell lines. Cells were treated by AgB with different concentrations for 48 h. The survival was determined using MTT. All data are expressed as mean ± SD; n=8.
Cell cycle analysis
Further assessment for the potential influence of AgB on the cell cycle distribution of HEK293 and B16F10 cells were conducted using flow cytometry. As shown Figure 4, AgB induced effect on DNA synthesis of the HEK293 cell cycle; at G0/G1 phase, a significantly increasing in the cell population was showed from 21.3 ± 3 to 29.5 ± 3.5% (P<005). Furthermore, AgB showed an anti-proliferative effect on the HEK293 cell with decreasing cell population in the S-phase (from 44.6 ± 5 to 40 ± 4%) and G2/M phase (from 34.1 ± 4.2 to 30.5 ± 3.2%). In B16F10 cells, the AgB induced anti-proliferative effect on the cell cycle at G0/G1 increasing its population from 9.8 ± 2 to 38.2 ± 6.6% (P<0.01) with reciprocal effect on the S-phase significantly decreasing the cell population from 60.3 ± 7.6 to 41.7 ± 6.2%, and also decreasing the B16F10 cells population at the G2/M phase from 29.9 ± 6.5 to 20.1 ± 4.3% (P<0.05). Therefore, the results showed that although AgB can be effective in anti-proliferative and cellular stages in both cell lines, its effects on cancer cell line are greater than normal cell line (Figure 4).
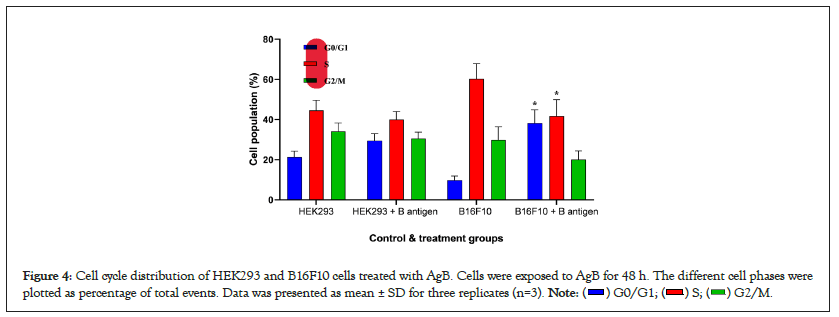
Figure 4: Cell cycle distribution of HEK293 and B16F10 cells treated with AgB. Cells were exposed to AgB for 48 h. The different cell phases were
plotted as percentage of total events. Data was presented as mean ± SD for three replicates (n=3). 
Apoptosis assessment
To evaluate the mechanisms of cell death, apoptosis induced by AgB against HEK293 and B16F10 using annexin-V/FITC staining and processed via flow cytometry. The results presented in the Figure 5 showed that both cell lines were affected by AgB. HEK293 cells in response to AgB showed a significant increase in the percentage of the total apoptotic cells compared to its control; 2.1% versus 0.9% (P<0.05). Moreover, AgB significantly increased the total apoptotic percentage of B16F10 cells (2.9 vs. 4.9%) compared to the control (P<0.05).
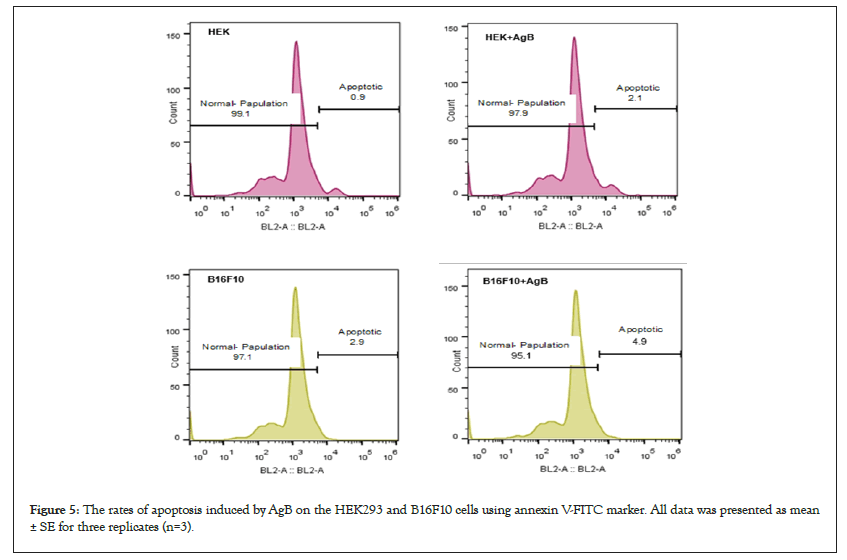
Figure 5: The rates of apoptosis induced by AgB on the HEK293 and B16F10 cells using annexin V-FITC marker. All data was presented as mean ± SE for three replicates (n=3).
Gene expression of BAX and BCL2
For further investigation in relation with apoptosis, two genes of BAX and BCL2 were selected as the most important genes involved in the internal pathway of apoptosis. Thus, their expression changes were studied in the two cell lines of HEK293 and B16F10 using qRT-PCR. qRT-PCR analysis showed that BAX mRNA expression level in the both cell lines increased after treatment with AgB, while BCL2 mRNA expression level was decreased in those groups. The highest changes in the BAX and BCL2 mRNA expression levels were showed when AgB was on the cancer cells of B16F10. The rate of fold change for all treatment groups have been shown in Figure 6.
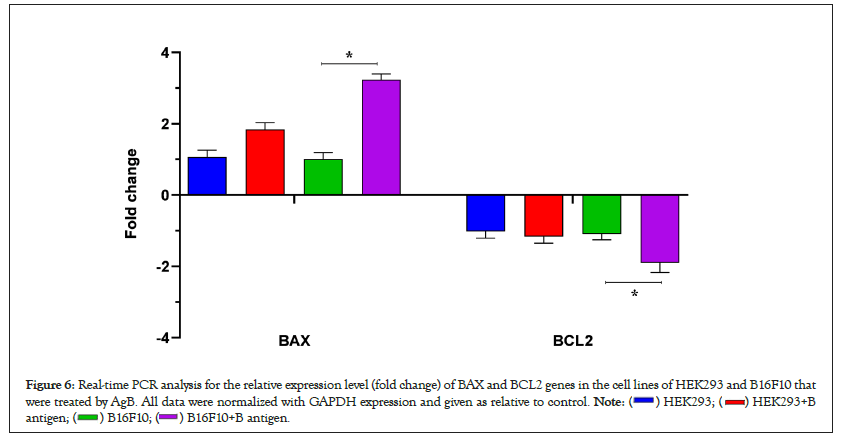
Figure 6: Real-time PCR analysis for the relative expression level (fold change) of BAX and BCL2 genes in the cell lines of HEK293 and B16F10 that
were treated by AgB. All data were normalized with GAPDH expression and given as relative to control. 
It was observed that AgB from hydatid cyst fluid can inhibits melanoma cancer cells in vitro study. Some studies highlighted the importance of a mutual cross-reaction existing between the antigens of hydatid cyst and excretory secretory products of cancer cells [25]. Chookami, et al., reported that hydatid cyst fluids have a complex mixture of antigens which stimulate the immune system [26]. So, one of the features of hydatid cyst is stimulation of cellular and humoral defense immune system [27]. In this study, the cell lines of HEK293 and B16F10 were selected to investigation. HEK293 as a normal cell line, showed the least sensitivity to AgB, while the cells of B16F10 as a cancer cell line showed the highest sensitivity. Furthermore the results showed that AgB has a special role in inhibiting the cell cycle and can reduce the cellular population in S and G2/M phases. In this regard, Darani, et al., showed that alive protoscoleces of hydatid cyst inhibited the growth of fibrosarcoma cells in culture medium [28]. Exposure to hydatid fluid increased or decreased the entry of D10 cells into the S phase. The increase in phase S population was not accompanied by an increase in the number of live, indicating that mitotic cycles induced by hydatid products were incomplete. In contrast, the main effect on B9 and A20 B leukocytic cell lines was to inhibit S-phase entry [29]. In another study by Aref, et al., effect of hydatid cyst antigen on inhibition of Hela and Vero cells growth have also been shown in vitro [30]. In this regard to role of hydatid cyst antigen on the induction of apoptosis, our results showed that the numbers of apoptotic cells were increased after exposure to AgB. In addition to, the AgB decreased the expression of anti-apoptotic of BCL2 in the mRNA level, and increased the expression of pro-apoptotic factor BAX, thereby induction cell apoptosis. When the apoptotic signal is issued, one hypothesis is that pro-apoptotic proteins accumulate on the surface of the mitochondria, causing channels in the mitochondrial membrane or changes the conformation of voltage-dependent ion channels (ADAS) in the mitochondria, thereby leaking mitochondrial contents into the cytoplasm [31]. This phenomenon neutralizes anti-apoptotic molecules [32,33]. In the study of Amirmajdi, et al., human lymphocytes were treated with hydatid fluid. Also, caspase-3 activity, the central enzyme of apoptosis cascade, was measured in the HF-treated lymphocytes and control cells. In addition, the expression of BAX and BCL2 mRNA was assessed by RT-PCR. Their results showed that ratio of BAX/BCL2 mRNA expression and caspase-3 activity was higher in the HF-treated lymphocytes relative to the control group [34]. Apoptosis is one of the main mechanisms of host defense against parasites. E. granulosus is capable of modulating immune responses of the host by several mechanisms. Some of these mechanisms are the interference with modulation of dendritic cells maturation, and induction of a non-protective Th2 cell response by AgB [35]. In the study of Mohammadi, et al., human melanoma A375 cancer cells were treated by fertile and infertile HCF. Cell viability was evaluated by MTT assay and the IC50 value on A375 cells was 85 μg/ml [36-38].
Moreover, the expression of BAX, BCL2, Caspase-9 and miR-365 (as an important tumor suppressor miRNA in cancers) mRNAs was determined by qRT-PCR. Their results showed that the fold change of BAX/BCL2 ratio, Caspase-9, miR-365 and Caspase-3 activity was higher in the fertile HCF-treated melanoma cells compared to infertile fluid treated A375 cells and human normal epithelial cell (as control cell). Other studies showed that both apoptotic pathways, including external pathways (death receptor mediators) and internal pathways (mitochondria) are active in HCF-treated host lymphocytes and PSCs-treated cestocidal drug such as benzimidazoles. However, stronger in vivo studies are needed to validate this biology of AgB before we can think of utilizing this compound further.
This study was approved by the ethics committee of Hamadan University of Medical Sciences, ethics committee code (IR.UMSHA.REC.1400.149).
The authors would like to acknowledge the Research Center of Deputy of Research, Hamadan University of Medical Sciences.
The authors declare that they have no competing interests.
All authors had full access to the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. Conceptualization, S.M., N.B. and H.T.; Methodology, N.B., H.T, S.Z. and S.S.; Formal Analysis, S.K.; Writing-Original Draft, N.B., S.M. and H.T.
This work is a part of research project and was financially supported by Deputy of Research, Hamadan University of Medical Sciences, grant number: 140002211204.
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
Citation: Barati N, Tanzadehpanah H, Zafari S, Asl SS, Khazaei S, Motavallihaghi S (2023) Anticancer Activity of Antigen B from Hydatid Cyst Fluid on Melanoma Cancer Cell Line. Chemo Open Access. 11:181.
Received: 13-Mar-2023, Manuscript No. CMT-23-22134; Editor assigned: 16-Mar-2023, Pre QC No. CMT-23-22134 (PQ); Reviewed: 30-Mar-2023, QC No. CMT-23-22134; Revised: 06-Apr-2023, Manuscript No. CMT-23-22134 (R); Published: 13-Apr-2023 , DOI: 10.35248/2167-7700.23.11.181
Copyright: © 2023 Barati N, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.