Journal of Pharmaceutical Care & Health Systems
Open Access
ISSN: 2376-0419
ISSN: 2376-0419
Research Article - (2014) Volume 1, Issue 1
Background: Much has been written about the impact of the Part D coverage gap on prescription filling behavior of Medicare beneficiaries with cardiovascular disease. However, we do not know if beneficiaries anticipating gap entry also cut spending in order to delay or avoid being in the gap. Methods and Results: We tracked 16,272 pairs of Part D enrollees with heart failure from 2006 through 2008 (half with full cost-sharing and half low-income subsidy recipients) matched on characteristics predictive of future drug spending. We estimated differences between the groups in drug spending, probability of reaching the gap and catastrophic thresholds, and December/January differences in spending. The highest drug spenders (>$600 per month) were least affected by phase transitions. Among lower spenders, 2.8% to 3.8% (p<0.05) avoided the gap through anticipatory cutbacks in 2007, rising to 6.1% to 7.7% (p<0.05) in 2008. Total reductions in drug spending attributable to Part D design features were 4.4% to 8.7% in 2007 and 11.8 to 17.1% in 2008 (p<0.05). Beneficiaries deflected part of the gap impact by shifting prescription fills from December 2007 to January 2008. The Part D design had little effect on heart failure medication spending. Conclusions: Filling the Part D coverage gap under provisions of the Affordable Care Act will provide economic benefits to most heart failure patients with mid- to high-level drug spending, but the biggest effect on drug utilization is likely among beneficiaries who anticipated entry into the coverage gap under the original benefit design.
Keywords: Medicare; Part D; Heart failure; Anticipatory behavior
Much has been written about the peculiar structure of the Medicare Part D prescription drug benefit design with its relatively generous coverage during an initial coverage phase followed by a coverage gap (aka doughnut hole) where enrollees are exposed to total drug costs up to a catastrophic threshold at which point out-of-pocket cost sharing drops to 5 percent. Considerable attention has been paid to the question of how drug use and spending change when enrollees transition into the gap [1-15]. Several of these studies have focused on Medicare beneficiaries with cardiovascular disease [6,10,12-15]. They report that the gap reduces drug adherence, increases discontinuance with essential medications, [6,10,12-15] and induces beneficiaries to switch to cheaper generic products [13]. These findings provide important insights into how beneficiaries with cardiovascular disease are likely to alter their drug regimens when the coverage gap is finally eliminated under provisions of the Affordable Care Act (ACA).
However, the potential distortions in treatment created by the original Part D benefit design have yet to be fully explored. Perhaps the most significant missing piece of the puzzle is knowledge of how beneficiary behavior is influenced by anticipation of future out-ofpocket price changes associated with the Part D benefit phases. Because cardiovascular disease treatments are dominated by chronic medications taken on a recurrent basis, most beneficiaries can reasonably anticipate their future medication expenses and some may cut back on drug use prior to reaching the coverage gap in order to postpone its impact or avoid the gap altogether. Prior studies have only investigated behavior of beneficiaries who actually reach the gap threshold, so the extent of anticipatory cutbacks is unknown. Another possible anticipatory behavior is that beneficiaries who face the coverage gap toward the end of the year may partially deflect its impact by postponing refills until the following January when benefits reset to the initial coverage phase. Alternatively, beneficiaries who face the gap in one year may permanently change their prescription filling behavior in future years in order to avoid a repeat of that experience. Neither scenario has been formally investigated to date. Finally, beneficiaries with very expensive regimens who anticipate reaching reach the catastrophic coverage phase have no incentive to cutback either prior to entry into the gap or once in it, but whether they do or not is unknown.
The research design for this article was developed to help fill these gaps in our knowledge of Part D effects, focusing specifically on heart failure. Medicare beneficiaries with this disease tend to be heavy medication users [13,16,17] and are thus more likely to be affected by the coverage gap than the average Part D enrollee. On the other hand, the mainstays of heart failure treatment-angiotensin-converting enzyme (ACE) inhibitors, beta-blockers, and diuretics are widely available as inexpensive generics, which present an opportunity to study potential heterogeneous responses to the Part D benefit design.
Analytic strategy
Our analytic strategy was driven by the following considerations. First, to measure anticipatory demand responses to Part D benefit phase transitions, we stratified the study sample into four cohorts based on total drug spending in the first quarter of the year, the lowest spending cohort members being unlikely to reach the doughnut hole threshold by year’s end (based on simple linear extrapolation) and the highest spending cohort members likely to reach the catastrophic limit. We then tracked mean monthly drug spending for members of each cohort to see if there were discontinuities before and after they reached predicted benefit phase thresholds. Second, we needed counterfactual samples of individuals with similar characteristics who were not affected by the Part D benefit phases. We used propensity score matched cohorts of Part D low-income subsidy (LIS) recipients for this purpose. LIS recipients pay the same nominal copays in both the initial coverage phase and coverage gap and thus are insulated from the effects of entry into the gap. For dual eligible beneficiaries who comprised more than 85% of all LIS recipients during our study period, copays were just $1 for generics and $2.50 for brands both before and while in the gap. Third, we wished to assess whether monthly spending patterns in one year affected beneficiary behavior in a subsequent year (particularly among those reaching the coverage gap). That aim dictated that we track all study cohorts for two years. Finally, we focused separately on heart failure medications to see whether benefit phase transitions have less impact on drug regimens with a high prevalence of generics.
Data source and study sample
Data for the study were drawn from a 5% random sample of the Medicare population as of January 2006 who survived through December 31, 2008. The data were obtained from the Chronic Condition Data Warehouse (CCW) [18]. We used CCW data from 2006 to establish baseline characteristics of study subjects with Part D drug spending patterns tracked over 2007 and 2008. The files included Medicare enrollment records for Parts A, B, C, D and Part D LIS status. We also used Part A and B claims records and Part D prescription drug event (PDE) files. The PDE files contain NDC codes for each filled prescription, days-supply, payment data, and a Part D benefit phase flag (for LIS recipients, the benefit phase flag indicates the phase the enrollee would have been in had she enrolled in a non-LIS defined standard benefit plan). Finally, we used the CCW beneficiary summary file to identify the first date of a heart failure diagnosis in Medicare claims.
The study sample comprised beneficiaries with a first heart failure diagnosis occurring prior to 2007 based on at least one hospital inpatient, hospital outpatient or carrier claim with the following ICD-9 codes: 398.91, 402.01, 402.11, 402.91, 404.01, 404.03, 404.11, 404.13, 404.91, 404.93, 428.xx. We included only beneficiaries with continuous enrollment under Part A, B and D throughout the observation period. Because Medicare Advantage plans did not report Medicare claims data to CMS during this time period, we excluded individuals with any enrollment in a Part C plan. This restriction also meant our analysis was limited to enrollees in stand-alone, fee-for-service prescription drug plans (PDPs).
Measures
Our primary variables of interest were 2007 and 2008 spending (plan payments plus enrollee cost sharing) for all Part D drugs and for medications recommended in the treatment of heart failure. The latter include ACE inhibitors, angiotensin receptor blockers (ARBs), aldosterone antagonists, digoxin, diuretics, and beta-blockers with label indications for heart failure Table 1. All drug spending variables were aggregated monthly to permit tracking across the Part D benefit phases each year.
| Drug Class and Name | ||
| Beta-Blockers | Captopril/Hydrochlorothiazide | Irbesartan/Hydrochlorothiazid |
| AcebutololHcl | Enalapril Maleate | Losartan Potassium |
| Atenolol | Enalapril Maleate/Felodipine | Losartan/Hydrochlorothiazide |
| Atenolol/Chlorthalidone | Enalapril/Hydrochlorothiazide | OlmesartanMedoxomil |
| BisoprololFumarate | EnalaprilatDihydrate | Olmesartan/Hydrochlorothiazid |
| BisoprololFumarate/Hctz | Fosinopril Sodium | Telmisartan |
| BetaxololHcl | Fosinopril/Hydrochlorothiazid | Telmisartan/Hydrochlorothiazi |
| Carvedilol | Lisinopril | Valsartan |
| Carvedilol Phosphate | Lisinopril/Hydrochlorothiazid | Valsartan/Hydrochlorothiazide |
| Labetalol Hcl | MoexiprilHcl | Cardiac Glycoside |
| Metoprolol Tartrate | Moexipril/Hydrochlorothiazide | Digoxin |
| Metoprolol/Hydrochlorothiazid | Perindopril Erbumine | Diuretics |
| Metoprolol Succinate | Quinapril Hcl | AmilorideHcl |
| NebivololHcl | Quinapril/Hydrochlorothiazide | Amiloride/Hydrochlorothiazide |
| Nadolol | Ramipril | Chlorthalidone |
| Nadolol/Bendroflumethiazide | Trandolapril | Chlorothiazide |
| Penbutolol Sulfate | Trandolapril/Verapamil Hcl | Hydrochlorothiazide |
| Pindolol | Aldosterone Antagonists | Indapamide |
| Propranolol Hcl | Eplerenone | Methyclothiazide |
| Propranolol/Hydrochlorothiazi | Spironolact/Hydrochlorothiazi | Metolazone |
| SotalolHcl | Spironolactone | Triamterene |
| Timolol Maleate | Angiotensin Receptor Blocker | Triamterene/Hydrochlorothiazi |
| Angiotensin-Converting-Enzyme Inhibitors | Candesartan Cilexetil | Loop Diuretics |
| Benazepril Hcl | Candesartan/Hydrochlorothiazi | Bumetanide |
| Benazepril/Hydrochlorothiazid | EprosartanMesylate | Ethacrynic Acid |
| Captopril | Eprosartan/Hydrochlorothiazid | Furosemide |
| Captopril/Hydrochlorothiazide | Irbesartan | Torsemide |
Table 1: Evidence-Based Drugs Used to Treat Heart Failure.
Other variables included factors hypothesized to influence drug use and spending in 2007 and 2008. These included baseline (2006) spending on heart failure and other drugs, annual drug fills, highest Part D benefit phase, and spending on Part A and B services; demographic characteristics (age, gender, race, region); selected comorbidities (other cardiovascular diseases, diabetes, chronic kidney disease, dementia, depression, and COPD); counts of medication-intensive chronic conditions based on the CMS RxHCC risk adjustment model; [19] proxy measures for severity of heart failure (incident heart failure case in 2006 and hospitalization for heart failure in 2006); Part D plan type in 2006 (defined standard benefit, actuarially equivalent, basic alternative, and enhanced alternative); and indicators for beneficiaries switching plan types in subsequent years.
Statistical analysis
We divided the full sample into four mutually exclusive cohorts to reflect mean monthly Part D spending during the first quarter of 2007 of between 0 and $200, $201 to $400, $401 to $600, and over $600 per month. For the propensity score matching algorithm we used logistic regression models to predict the probability that observations within each spending cohort belonged to a non-LIS (1) or an LIS recipient (0) as a function of all factors hypothesized to influence future drug spending listed above including actual first-quarter spending. This step allowed us to identify beneficiaries with closely matched determinants for future drug demand. Moreover, because we included drug spending in 2006 and first quarter 2007 as conditioning variables, we indirectly controlled for unobserved differences between LIS and non-LIS beneficiaries that may also be associated with drug spending trends. We then took the predicted probabilities from the logistic regression models and matched LIS and non-LIS beneficiaries using a greedy one-to-one matching algorithm starting with the closest match on the predicted probability and continuing until the remaining observations could not be matched at three decimal points or better.
Because most beneficiaries were in the initial coverage phase of the Part D benefit during the entire first quarter of 2007, matching on spending controlled for differences in drug use associated with differential copays faced by LIS and non-LIS enrollees during these months. Any divergence in drug spending patterns later in 2007 and through 2008 could thus logically be attributed to beneficiary response to price differentials faced in the coverage gap and catastrophic phases.
Another reason for this specification is that it permitted us to assess alternative theories of how beneficiaries respond to impending changes in out-of-pocket drug prices. Because few non-LIS beneficiaries in cohort 1 (first quarter monthly spending of $200 or less) would anticipate entry into the doughnut hole that year (threshold=$2,400), we hypothesized that their spending trajectory in 2007 would closely follow that of their matched LIS recipients. Similarly, we hypothesized that non-LIS beneficiaries in cohort 4 (first quarter spending averaging over $600 per month) would also closely match the spending trajectory of LIS recipients because most of them could anticipate spending through the gap and ending up in the catastrophic phase before year’s end (threshold=$5,451 in 2007).
Our primary interest revolved around behavior of beneficiaries in cohorts 2 and 3 because most of these individuals could anticipate reaching the coverage gap without spending through it. If non-LIS beneficiaries in cohorts 2 and 3 were not cognizant of impending entry into the gap, we would expect to see similar drug spending trajectories for the matched pairs up to the point that the non-LIS recipients hit the gap threshold followed by a sharp discontinuity in spending thereafter. On the other hand, if beneficiaries attempted to delay entry or avoid exposure to the coverage gap we would observe more days in the initial coverage phase and a gradual divergence in spending trajectories beginning before the gap threshold was reached.
After matching, we produced charts showing monthly spending trajectories for all drugs combined and for heart failure medications by LIS status and spending cohort from January 2007 through December 2008. We then calculated differences in the percent of LIS and non-LIS enrollees who reached the coverage gap and catastrophic thresholds within each spending cohort. Finally, we used difference-in-difference (DID) estimators to determine whether the experience by non-LIS beneficiaries with the Part D benefit phases in one year affected their drug spending patterns in the following year. These equations took the following form:
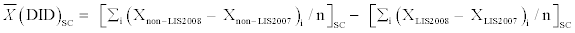
Where, i indexes the individual within each spending cohort (SC), X is the value of the variable of interest by LIS status and year, and n references the number of individuals within the spending cohort. The DID estimates of interest included differences in mean annual drug spending, percentages reaching the coverage gap and catastrophic phase thresholds, and changes in drug spending between December 2007 and January 2008.
We also tested the sensitivity of our findings to alternative assumptions. First, we repeated the analysis described above but retained individuals who died in 2008 as a test for possible survivor bias. Next, we re-estimated the propensity score models removing all variables relating to 2006 drug use and expenditure as a test for potential over-fitting that might occur when lagged values of the dependent variables are included as control variables.
The full survivor sample before matching included 101,463 beneficiaries with heart failure, of whom more than two-thirds were LIS recipients Table 2. The two groups differed significantly on virtually all measured characteristics. The propensity score-matched samples included 32,544 individuals arrayed in four spending cohorts ranging in size from 7,542 matched pairs in cohort 1 (0 to $200 per month) to 1,098 in cohort 4 (>$600 per month) as shown in Table 3. The matching algorithm produced cohorts with balanced characteristics on almost all factors hypothesized to influence future drug spending. Even in the few instances in which there were statistically significant differences in individual characteristics after matching, the magnitudes of the differences were inconsequential.
| Beneficiary Characteristics | Non-LIS (N=30,949) | LIS (N=70,514) |
|---|---|---|
| Monthly drug spending in first quarter of 2007 ($) (sd) | 262 (265) | 421* (461) |
| Annual drug spending in 2006 ($) (sd) | ||
| All drugs | 2872 (2740) | 4730*(4777) |
| Heart failure drugs | 408 (467) | 369* (461) |
| All other drugs | 2464 (2663) | 4360*(4713) |
| Annual drug fills in 2006 (sd) | ||
| All drugs | 52 (31) | 75* (48) |
| Heart failure drugs | 14 (11) | 15* (12) |
| All other drugs | 38 (26) | 60* (42) |
| Highest Part D benefit phase in 2006 (%) | ||
| Initial coverage phase | 53.2 | 30.5* |
| Doughnut hole | 38.5 | 37.1* |
| Catastrophic phase | 8.3 | 32.4* |
| Part A and B Spending in 2006 ($) (sd) | 1001 (1564) | 1429* (2221) |
| Age (%) | ||
| <65 (SSDI) | 2.4 | 22.6* |
| 65–74 | 22.9 | 22.4 |
| 75–84 | 44.4 | 31.6* |
| 85+ | 30.3 | 23.4* |
| Sex (%) | ||
| Males | 35.5 | 29.1* |
| Females | 64.5 | 70.9* |
| Race/ethnicity (%) | ||
| White | 95.3 | 65.8* |
| Black | 3.1 | 21.1* |
| Other | 1.7 | 13.1* |
| Region (%) | ||
| Northeast | 18.1 | 19.3* |
| North Central | 30.8 | 20.7* |
| South | 38.7 | 41.7* |
| West | 12.4 | 18.3* |
| First CHF diagnosis | ||
| Diagnosed before 2006 (prevalent cases) | 85.4 | 87.6* |
| Diagnosed in 2006 (incident cases) | 14.6 | 12.4* |
| Hospitalization for CHF in 2006 | ||
| Primary diagnosis | 1.5 | 1.3* |
| Diagnosis in any position | 6.1 | 5.7* |
| Comorbidities in 2006 (%) | ||
| Cardiovascular diseases | ||
| Atrial fibrillation | 21.0 | 11.3* |
| Acute myocardial infarction | 1.7 | 1.5 |
| Ischemic heart disease | 62.4 | 58.3* |
| Stroke | 5.7 | 8.8* |
| Hyperlipidemia | 72.4 | 60.4* |
| Hypertension | 86.6 | 85.9* |
| Other diseases | ||
| Diabetes | 35.4 | 47.9* |
| Chronic kidney disease | 17.0 | 21.8* |
| Dementia | 10.2 | 22.8* |
| Depression | 11.4 | 24.0* |
| COPD | 16.8 | 23.3* |
| RxHCC Count in 2006 (%) | ||
| ≤ 3 | 8.8 | 9.1 |
| 4-6 | 31.8 | 26.4* |
| 7-9 | 34.4 | 32.0* |
| 10+ | 25.0 | 32.5* |
| Initial Part D plan type in 2006 (%) | ||
| Defined standard benefit | 13.2 | 24.7* |
| Actuarially equivalent | 11.6 | 27.7* |
| Basic alternative | 46.3 | 45.9 |
| Enhanced alternative | 28.8 | 1.7* |
| Switched plan types (%) | ||
| Switched plan type in 2006 | 4.1 | 9.9* |
| Switched plan type in 2007 | 1.0 | 3.6* |
| Switched plan type in 2008 | 0.8 | 4.6* |
Table 2: Baseline (2006) Characteristics of Unadjusted Sample of Part D Enrollees with Heart Failure by Low-Income Subsidy Status.
| Beneficiary Characteristics | Spending Cohorts | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 (0-$200) | 2 ($201-$400) | 3 ($401-$600) | 4 (Over $600) | ||||||
| Non-LIS (N=7,542) | LIS (N=7,542) | Non-LIS (N=5,536) | LIS (N=5,536) | Non-LIS (N=2,096) | LIS (N=2,096) | Non-LIS (N=1,098) | LIS (N=1,098) | ||
| Monthly drug spending in first quarter of 2007 ($) (sd) | 100 (58) | 100 (61) | 288 (57) | 288 (56) | 482 (56) | 482 (56) | 918 (581) | 932 (690) | |
| Annual drug spending in 2006 ($) (sd) | |||||||||
| All drugs | 1545 (1131) | 1530* (1240) | 3151 (1327) | 3130 (1282) | 4830 (2060) | 4772 (1921) | 8668 (5937) | 8688* (8058) | |
| Heart failure drugs | 259 (310) | 260* (332) | 446 (453) | 450 (487) | 532 (534) | 540 (561) | 573 (587) | 580 (666) | |
| All other drugs | 1286 (1073) | 1271* (1177) | 2705 (1294) | 2679 (1261) | 4297 (2038) | 4232 (1903) | 8095 (5964) | 8107* (8056) | |
| Annual drug fills in 2006 (sd) | |||||||||
| All drugs | 39 (24) | 39 (25) | 61 (27) | 60 (27) | 77 (32) | 76 (31) | 98 (44) | 97 (42) | |
| Heart failure drugs | 12 (10) | 12 (11) | 16 (11) | 16 (12) | 17 (12) | 16 (12) | 17 (12) | 17 (13) | |
| All other drugs | 27 (18) | 27 (19) | 45 (22) | 45 (22) | 60 (26) | 59 (26) | 81 (38) | 80 (37) | |
| Highest Part D benefit phase in 2006 (%) | |||||||||
| Initial coverage phase | 79.2 | 79.3 | 24.1 | 24.8 | 8.5 | 8.6 | 4.8 | 4.3 | |
| Doughnut hole | 19.9 | 20.0 | 70.0 | 69.5 | 56.0 | 56.9 | 21.3 | 22.8 | |
| Catastrophic phase | 0.8 | 0.7 | 5.9 | 5.7 | 35.5 | 34.5 | 73.9 | 73.0 | |
| Part A and B Spending ($) (sd) | 824 (1451) | 840* (1540) | 1101 (1603) | 1080 (1667) | 1401 (2028) | 1351 (1844) | 1729 (2096) | 1667 (2087) | |
| Age (%) | |||||||||
| <65 (SSDI) | 3.6 | 3.5 | 3.5 | 3.6 | 3.8 | 3.7 | 7.7 | 7.7 | |
| 65–74 | 23.1 | 23.4 | 23.1 | 22.8 | 25.3 | 24.1 | 27.2 | 26.0 | |
| 75–84 | 40.9 | 41.1 | 41.7 | 42.0 | 41.7 | 43.0 | 39.8 | 41.8 | |
| 85+ | 32.5 | 32.1 | 31.6 | 31.6 | 29.2 | 29.2 | 25.3 | 24.4 | |
| Sex (%) | |||||||||
| Males | 31.2 | 31.9 | 28.4 | 28.4 | 29.2 | 29.6 | 28.6 | 29.1 | |
| Females | 68.8 | 68.1 | 71.6 | 71.6 | 70.8 | 70.4 | 71.4 | 70.9 | |
| Race/ethnicity (%) | |||||||||
| White | 90.3 | 90.7 | 92.6 | 92.8 | 94.0 | 93.6 | 94.4 | 94.4 | |
| Black | 6.4 | 6.2 | 4.9 | 4.6 | 3.9 | 4.3 | 3.2 | 2.8 | |
| Other | 3.3 | 3.1 | 2.5 | 2.5 | 2.1 | 2.1 | 2.4 | 2.7 | |
| Region (%) | |||||||||
| Northeast | 19.9 | 20.0 | 20.2 | 19.7 | 22.0 | 22.8 | 25.4 | 23.7 | |
| North Central | 25.4 | 24.9 | 25.5 | 25.1 | 23.4 | 24.1 | 21.7 | 22.6 | |
| South | 38.6 | 39.2 | 40.9 | 40.9 | 40.7 | 39.5 | 40.8 | 42.7 | |
| West | 16.1 | 16.0 | 13.4 | 14.2 | 13.8 | 13.6 | 12.1 | 11.0 | |
| First CHF diagnosis | |||||||||
| Diagnosed before 2006 (prevalent cases) | 86.6 | 86.3 | 86.5 | 86.1 | 85.0 | 85.7 | 85.0 | 85.1 | |
| Diagnosed in 2006 (incident cases) | 13.4 | 13.7 | 13.5 | 13.9 | 15.0 | 14.3 | 15.0 | 14.9 | |
| Hospitalization for CHF in 2006 | |||||||||
| Primary diagnosis | 1.3 | 1.3 | 1.6 | 1.7 | 1.6 | 1.7 | 1.5 | 1.7 | |
| Diagnosis in any position | 5.2 | 5.3 | 6.8 | 6.3 | 7.3 | 7.0 | 8.3 | 8.8 | |
| Comorbidities in 2006 (%) | |||||||||
| Cardiovascular diseases | |||||||||
| Atrial fibrillation | 16.5 | 16.9 | 19.2 | 18.7 | 18.0 | 18.5 | 16.0 | 15.8 | |
| Acute myocardial infarction | 1.1 | 0.9 | 2.2 | 2.3 | 2.7 | 2.0 | 2.7 | 2.2 | |
| Ischemic heart disease | 53.5 | 53.7 | 64.8 | 64.7 | 69.9 | 68.6 | 69.4 | 68.4 | |
| Stroke | 4.4 | 4.7 | 6.9 | 7.0 | 9.2 | 8.6 | 9.3 | 7.9 | |
| Hyperlipidemia | 60.6 | 61.4 | 72.3 | 72.8 | 75.6 | 74.4 | 74.5 | 74.4 | |
| Hypertension | 82.5 | 82.9 | 89.2 | 89.4 | 90.7 | 90.7 | 89.9 | 90.3 | |
| Other diseases | |||||||||
| Diabetes | 29.7 | 29.9 | 41.4 | 40.8 | 49.2 | 49.3 | 53.8 | 55.3 | |
| Chronic kidney disease | 12.6 | 13.0 | 18.1 | 18.3 | 23.2 | 22.8 | 27.8 | 26.4 | |
| Dementia | 9.8 | 9.4 | 13.5 | 13.2 | 20.1 | 20.5 | 27.3 | 27.8 | |
| Depression | 9.5 | 9.4 | 14.3 | 13.6 | 19.8 | 19.5 | 27.4 | 27.5 | |
| COPD | 15.3 | 15.5 | 20.0 | 19.8 | 23.8 | 23.1 | 29.6 | 29.6 | |
| RxHCC Count in 2006 (%) | |||||||||
| ≤ 3 | 15.8 | 15.5 | 6.2 | 6.5 | 2.6 | 3.1 | 1.9 | 1.5 | |
| 4-6 | 37.4 | 37.4 | 29.3 | 29.6 | 21.7 | 21.2 | 14.3 | 14.1 | |
| 7-9 | 30.4 | 30.8 | 36.8 | 36.5 | 36.8 | 37.4 | 31.8 | 33.7 | |
| 10+ | 16.4 | 16.3 | 27.8 | 27.3 | 38.9 | 38.3 | 52.0 | 50.7 | |
| Initial Part D plan type in 2006 (%) | |||||||||
| Defined standard benefit | 22.3 | 22.2* | 18.6 | 18.7 | 15.3 | 15.8 | 14.2 | 14.2 | |
| Actuarially equivalent | 19.9 | 19.3* | 19.7 | 19.8 | 18.5 | 18.3 | 22.2 | 20.9 | |
| Basic alternative | 52.6 | 54.2* | 54.8 | 55.3 | 56.5 | 57.3 | 53.0 | 55.9 | |
| Enhanced alternative | 5.2 | 4.2* | 6.9 | 6.2 | 9.7 | 8.6 | 10.6 | 9.0 | |
| Switched plan types (%) | |||||||||
| Switched plan type in 2006 | 4.9 | 4.7 | 5.6 | 5.5 | 5.8 | 5.6 | 6.8 | 7.3 | |
| Switched plan type in 2007 | 1.4 | 1.4 | 1.5 | 1.7 | 1.5 | 1.4 | 1.7 | 1.1 | |
| Switched plan type in 2008 | 1.1 | 1.2 | 1.4 | 1.4 | 1.3 | 1.3 | 1.7 | 0.9 | |
Table 3: Baseline (2006) Characteristics of Propensity Score Matched Cohorts of Part D Enrollees with Heart Failure by Monthly Part D Spending in the First Quarter of 2007.
Figure 1 shows mean monthly total Part D spending trends during 2007 and 2008 for each cohort together with projected phase transitions based on linear projections of first quarter spending in 2007. Several features of these timelines stand out. As hypothesized, we see much closer parallels in monthly spending for LIS and non-LIS beneficiaries in cohorts 1 and 4 compared to the middle cohorts. In both cohorts 2 and 3, LIS beneficiaries experienced relatively flat monthly drug spending throughout 2007 and 2008. For non-LIS beneficiaries in the cohort 2, we begin to see divergence from the LIS group in July 2007, two months before these individuals would have entered the doughnut hole based on simple linear projections. For beneficiaries in cohort 3, the divergence began in May, about a month before projected entry into the doughnut hole. In both cases, however, pre-gap differences were small compared to post-gap differences. Except for the highest spenders in cohort 4, we observe a distinct reset in monthly spending in January 2008 resulting in a roller coaster spending pattern over time. After January 2008 we observed widening differentials in spending between LIS and non-LIS beneficiaries in all cohorts.
Figure 2 presents monthly timelines for spending on heart failure medications in 2007 and 2008. As in 2006 (see Table 1) spending on these drugs represented a small share of total Part D spending for LIS and non-LIS beneficiaries in every spending cohort. Moreover, except in cohort 1, expenditures on heart failure medications declined both in dollar volume and as a share of total drug expenditures over the two years. As with total drug spending we see a marked increase in heart failure medication spending between December 2007 and January 2008 for all beneficiaries except those in cohort 4.
Statistics on mean annual spending for all drugs and heart failure medications by study cohort and LIS status are presented in Table 4. In every cohort, non-LIS beneficiaries had lower total drug spending compared to their LIS controls. In both years the largest percentage differences between non-LIS and LIS beneficiaries were in cohorts 2 and 3 the two groups we hypothesized would express the greatest anticipatory response to the coverage gap. LIS/non-LIS spending differences for all medications rose substantially between 2007 and 2008, reflecting declining spending among all non-LIS beneficiaries except those in cohort 1. Results from the DID analysis indicated that relative to LIS beneficiaries, non-LIS beneficiaries systematically cut back their drug spending between 2007 and 2008. The differences ranged between $152 (p<0.05) for those in cohort 1 to $388 (p<0.05) in cohort 3 (the difference in cohort 4 was not statistically significant). We found no significant differences in annual spending for heart failure medications by LIS status in 2007, but spending by non-LIS beneficiaries in 2008 was significantly lower in cohorts 2 and 3.
| Year and LIS Status | Spending Cohorts | |||
|---|---|---|---|---|
| 1 (0-$200) |
2 ($201-$400) |
3 ($401–$600) |
4 (Over $600) |
|
| Total Drug Spending | ||||
| 2007 | ||||
| Non-LIS ($) | 1,520 | 3,313 | 5,247 | 10,117 |
| LIS ($) | 1,587 | 3,555 | 5,702 | 10,548 |
| Difference ($) | -67 | -240* | -455* | -431* |
| Difference (%) | -4.4 | -7.2* | -8.7* | -4.3* |
| 2008 | ||||
| Non-LIS ($) | 1,863 | 3,276 | 4,978 | 9,380 |
| LIS ($) | 2,082 | 3,835 | 5,821 | 10,143 |
| Difference ($) | -219* | -559* | -843* | -763* |
| Difference (%) | -11.8 | -17.1 | -16.9 | -8.1 |
| Difference-in-difference ($) | -152* | -317* | -388* | -332 |
| Heart Failure Drug Spending | ||||
| 2007 | ||||
| Non-LIS ($) | 267 | 473 | 571 | 636 |
| LIS ($) | 275 | 490 | 601 | 649 |
| Difference ($) | -8 | -17 | -30 | -13 |
| Difference (%) | -2.9 | -3.5 | -5.0 | -2.0 |
| 2008 | ||||
| Non-LIS ($) | 265 | 397 | 447 | 510 |
| LIS ($) | 274 | 415 | 480 | 491 |
| Difference ($) | -9 | -18* | -33* | 19 |
| Difference (%) | -3.3 | -4.3* | -6.9* | 3.9 |
| Difference-in-difference ($) | -0.4 | -0.8 | -1.9 | 1.9 |
Table 4: Annual Drug Spending in 2007 and 2008 for LIS and Non-LIS Beneficiaries with Heart Failure by Level of Drug Spending in the First Quarter of 2007.
Underpinning these patterns were differential percentages of beneficiaries reaching the gap and catastrophic thresholds each year Table 5. In 2007, 17.5% of LIS beneficiaries in cohort 1 reached the gap threshold compared to just 14.2% of non-LIS beneficiaries. Between 82% and 100% of beneficiaries in cohorts 2 to 4 reached this threshold in 2007 with small differences by LIS status. In 2008 we see what appears to be regression to the mean in spending trajectories for beneficiaries in cohorts 1 to 3, with a much higher proportion of those in cohort 1 (both LIS and non-LIS) reaching the gap that year compared to 2007 and fewer reaching it among those in cohorts 2 and 3. Downward regression to the mean was least noticeable among the highest spenders as most of them were also exposed to the catastrophic phase each year. The DID results indicate that non-LIS beneficiaries’ experience with the Part D benefit phases in 2007 reduced the likelihood of reaching the gap threshold the following year in 2008 for cohort 1 (-2.8%; p<0.05) and cohort 2 (-4.9%; p<0.05). There was no significant impact on beneficiaries in cohorts 3 and 4.
| Year and LIS Status | Spending Cohorts | |||
|---|---|---|---|---|
| 1 (0-$200) |
2 ($201-$400) |
3 ($401–$600) |
4 (Over $600) |
|
| Percent Beneficiaries Reaching Coverage Gap | ||||
| 2007 | ||||
| Non-LIS (%) | 14.2 | 82.0 | 95.4 | 98.1 |
| LIS (%) | 17.5 | 84.8 | 99.2 | 100 |
| Difference | -3.3* | -2.8* | -3.8* | -1.9* |
| 2008 | ||||
| Non-LIS (%) | 22.4 | 66.4 | 89.5 | 96.1 |
| LIS (%) | 28.5 | 74.1 | 92.8 | 97.7 |
| Difference | -6.1* | -7.7* | -3.3* | -1.6* |
| Difference-in-difference | -2.8* | -4.9* | 0.03 | 0.03 |
| Percent Beneficiaries Reaching Catastrophic Threshold | ||||
| 2007 | ||||
| Non-LIS (%) | 0.3 | 3.0 | 32.4 | 80.8 |
| LIS (%) | 0.8 | 5.7 | 51.4 | 92.9 |
| Difference | -0.5 | -2.7* | -19.0* | -7.9* |
| 2008 | ||||
| Non-LIS (%) | 1.7 | 6.2 | 26.3 | 65.6 |
| LIS (%) | 4.0 | 12.9 | 44.2 | 78.1 |
| Difference | -2.3* | -6.7* | -17.9 | -12.5 |
| Difference-in-difference | -2.8* | -3.9* | 1.1 | -0.5 |
Table 5: Percent of LIS and Non-LIS Beneficiaries with Heart Failure Who Reached the Coverage Gap and Catastrophic Threshold by Level of Drug Spending in the First Quarter of 2007.
Few beneficiaries in cohorts 1 and 2 were exposed to the catastrophic phase either year. However, the rates were consistently higher among LIS recipients (from 0.5% to 6.7% higher depending on cohort and year). The DID results on non-LIS beneficiaries’ probability of reaching the catastrophic threshold were similar to their probability of reaching the coverage gap: -2.8% (p<0.05) in cohort 1 and -3.9% (p<0.05) in cohort 2.
Finally, consistent with the visual patterns evident in Figures 1 and 2, we found strong evidence that all but the highest spending non- LIS beneficiaries delayed filling prescriptions from December 2007 to January 2008, which was the start of a new coverage period Table 6. The biggest impact was seen in cohorts 2 and 3 where non-LIS spending was $102 and $71 higher, respectively in January 2008 compared to December 2007 (p<0.05). The differences were significantly smaller for LIS beneficiaries, resulting in a net differences of $86 and $44 higher January 2008 drug spending relative to LIS recipients (p<0.05).
| LIS Status and Month | Spending Cohorts | |||
|---|---|---|---|---|
| 1 (0-$200) |
2 ($201-$400) |
3 ($401–$600) |
4 (Over $600) |
|
| Non-LIS | ||||
| January 2008 ($) | 163 | 330 | 475 | 798 |
| December 2007 ($) | 138 | 228 | 403 | 852 |
| Difference ($) | 25* | 102* | 71* | -54 |
| LIS | ||||
| January 2008 ($) | 159 | 318 | 497 | 839 |
| December 2007 ($) | 154 | 302 | 470 | 890 |
| Difference ($) | 6 | 16* | 27* | -51 |
| Difference-in-difference ($) | 20* | 86* | 44* | -3 |
Table 6: Changes in Drug Spending Between December 2007 and January 2008 for LIS and Non-LIS Beneficiaries by Level of Drug Spending in First Quarter of 2007.
Results from the sensitivity tests confirmed the main study findings (not shown). The monthly timelines for spending on all Part D drugs and heart failure medications for cohorts including decedents in 2008 were virtually identical to those presented in Figures 1 and 2, indicating that the findings are not subject to survivor bias. Our test for model over-fitting (excluding 2006 Part D variables in the propensity score matching algorithms) resulted in slight changes in the time paths but did not substantively affect the relationships between LIS and non-LIS beneficiaries in any spending cohort.
These results present a more nuanced view of how beneficiaries cope with the Part D benefit phases than reported in previous studies of cardiovascular disease treatments [6,10,12-15]. First, we found that, except for high spenders, Part D enrollees facing the prospect of reaching the coverage gap cut back their drug spending prior to gap entry. We estimate that between 2.8% and 3.8% avoided the gap entirely through such anticipatory cutbacks in 2007 and that the number avoiding the gap in 2008 more than doubled to between 6.1% and 7.7%. For these beneficiaries the total reduction in drug spending attributable to both gap avoidance and reduced spending within the gap ranged from 4.4% to 8.7% in 2007 and 11.8% to 17.1% in 2008. Although we cannot isolate the actual factors behind the larger response in 2008, it is reasonable to presume that beneficiary experiences with Part D benefit phases in 2007 played a major role. This also suggests that by 2008, Part D enrollees had a much better appreciation and knowledge of the coverage gap compared to earlier assessments shortly after Part D was implemented in 2006 [20].
Secondly, as hypothesized, we found that high spending beneficiaries who could reasonably expect to reach the Part D catastrophic threshold during the calendar year were less affected by the coverage gap. We estimate that only 1.6% to 1.9% of such beneficiaries avoided the gap and that the overall spending reduction attributable to the Part D benefit design-related cutbacks ranged between 4.3% (2007) and 8.1% (2008).
Third, we found evidence suggesting that Part D enrollees (again excluding high spenders) postponed some drug fills at the end of the calendar year, refilling them in January instead. While the mean spending differences between December and January were modest ($25 to $102), they provide further evidence of purposive responses to the financial incentives embedded in the Part D design.
Finally, we found that spending on heart failure medications was much less sensitive to Part D benefit phase transitions than for all drugs considered together, a finding also reported by previous researchers [14,15]. We did observe cutbacks in heart failure drug purchased late in each calendar year among non-LIS beneficiaries in cohorts 2 and 3 that could plausibly be attributed to a coverage gap effect, but the magnitude was small and was compensated by higher spending in the following January. Low price sensitivity for heart failure drugs could be explained by a combination of generally low generic prices and perceived effectiveness of drugs considered critical in the treatment of the disease. This is important to both clinicians and Medicare policy makers given that the medication regimens for other common diseases like diabetes, hypertension, and hyperlipidemia are also dominated by generic products.
These findings have other important implications for Medicare policy makers. It is generally believed that the adverse effects of gap entry will disappear as the coverage gap is over the next few years under ACA provisions [14,21]. This seems like a reasonable assumption with respect to beneficiaries who postpone prescriptions fills to the new calendar year. It is also reasonable to expect that short-term cutbacks in anticipation of gap entry will disappear. However, it is an open question whether longer-term behavioral trends based on prior exposure to the coverage gap will revert back to pre-ACA levels.
Our analysis is subject to a number of important caveats. Foremost, our results hang on the critical assumption that the propensity scorematched cohorts of non-LIS and LIS beneficiaries are essentially identical on factors predictive of future drug spending, and thus any deviation in spending trajectories can be attributed to Part D phase transitions. This assumption might appear tenuous given known dissimilarities between beneficiary populations that do and do not receive LIS subsidies including differences in income, assets, education, health status, and other factors [22]. Our approach was based on population restrictions designed to achieve equality by: (1) matching on an extensive list of observable variables likely to influence future drug spending, (2) matching on first quarter 2007 drug spending which accounts for unobserved factors related to spending in that quarter, (3) including 2006 drug spending as an additional matching criterion which, by establishing two historical spending points, should control for potential differences in future spending trajectories, and finally, (4) by estimating difference-in-difference equations in which each beneficiary acts as his or her own control.
A second limitation is that while our sample was randomly selected among all Medicare beneficiaries, we were forced to exclude beneficiaries enrolled in Medicare Advantage plans because they lacked Medicare claims data. For this reason, our results can only be generalized to enrollees in stand-alone fee-for-service plans. Because we restricted the sample to Medicare beneficiaries with heart failure, we cannot generalize the findings to the Medicare population at large. However, we would expect to find similar results for beneficiaries with other chronic diseases.
Third, we focused on Part D spending rather than drug utilization and did not investigate whether spending reductions associated with the Part D benefit design reflected decreased adherence or outright discontinuation with certain medications. This represents a fruitful avenue for future research, but was beyond the scope of the present paper.
Lastly, our results should not be taken to suggest that the filling the doughnut hole will have no major impact on significant segments of Part D enrollment. For one thing, it will significantly reduce out-ofpocket obligations for all Part D enrollees with spending in the (former) coverage gap. It will also eliminate uncertainty regarding cost sharing schedules and should smooth out the roller coaster pattern in drug utilization and spending observed among beneficiaries with repeated exposure to the coverage gap.
The analysis for this paper was supported by a contract from the Novartis Pharmaceutical Corporation awarded to Dr. Stuart. The sponsor had no role in obtaining data for the study or with the design and conduct of the analysis. The authors are solely responsible for the preparation of this manuscript.
Dr. Stuart is a consultant to Merck and has received funding from PhRMA and NACDS. Dr. Magder and Mr. Shaffer report no disclosures. Drs. Park and Zacker are employees of Novartis. All opinions expressed in this article are those of the authors and do not necessarily represent those of the authors’ organizations.