Journal of Developing Drugs
Open Access
ISSN: 2329-6631
ISSN: 2329-6631
Research Article - (2016) Volume 5, Issue 2
Inflammation causes discomfort, suffering and lower productivity of the victims. Synthetic anti-inflammatory drugs are not readily available and have adverse side effects. Alternative herbal medicines possess bioactive compounds that are safer and efficient in the management of various diseases and disorders. The present study evaluated for the anti-inflammatory activity of methanolic extracts of Kigelia africana and Acacia hockii in mice to scientifically validate their traditional use among the Embu and Mbeere communities in Kenya. The plant samples were collected with the help of local herbalists in Embu County, Kenya and transported to Kenyatta University biochemistry and biotechnology laboratories for cleaning, air drying, milling, and extraction. Swiss albino mice of either sex were randomly divided into six groups of 5 animals each; normal control, negative control, positive control and three experimental groups. The anti-inflammatory activity was tested using carrageenan-induced hind paw edema method. The anti-inflammatory activity of the extracts was compared to reference drug diclofenac. The leaf extract of K. africana reduced inflamed hind paw diameter of mice by between 0.21%- 4.98% while the stem bark extract of A. hockii reduced inflamed hind paw diameter by between 0.6%-5.38%. The diclofenac reduced inflamed hind paw diameter by between 1.11%-4.9%. The qualitative phytochemical screening indicated the presence of saponins, flavonoid, alkaloids, terpenoids, phenolics, and cardiac glycosides. The present study demonstrated potent antiinflammatory activities of methanolic extracts of K. africana and A. hockii in a dose-dependent manner, which supports their traditional use. The present study, therefore, recommends the ethnomedicinal use of K. africana and A. hockii in the management of inflammation.
<Keywords: Kigelia africana, Acacia hockii, Anti-inflammatory, PGE2, Paw edema
Inflammation refers to body’s normal protective response to tissue injury caused by physical trauma, toxic chemicals or microbiological agents [1]. The classical signs of inflammation are skin redness, swelling, pain, heat, and loss of function [2]. The process of inflammation involves changes in blood flow, destruction of tissues, increased vascular permeability and the synthesis of pro-inflammatory mediators [3]. The injured cells, lymphocytes, phagocytes, mast cells and blood proteins are the sources of inflammatory mediators. The most important inflammatory mediators include bradykinins, serotonins, histamine, tumor necrosis factor-α, interleukin-6, interleukin-1β, leukotrienes, phospholipase A2, nitric oxide (NO), lipoxygenases and cyclooxygenase 2 (COX-2) [3,4].
Inflammatory process has two phases: acute and chronic. The acute inflammation occurs a few minutes after tissue damage. It is characterized by an increase in permeability of blood vessels, extravasation of fluid and proteins and accumulation of white blood cells for a short period [5]. The primary mediators of acute inflammation include histamine, serotonin, and C0X-2 [6]. The failure of the management of acute inflammation and an autoimmune response to a self-antigen lead to chronic inflammation and disease [7]. Chronic inflammation is mediated by inflammatory mediators such as PGE2, nitric oxide and lipoxygenases. Chronic inflammation may results in ailments such as chronic peptic ulcers, rheumatoid arthritis, systemic lupus, asthma, chronic periodontitis and cancer [8].
During the inflammatory response, the PGE2 are at low levels in tissues with no inflammation and increase immediately in acute inflammation. As immune cells infiltrate the tissues, further increases in PGE2 levels is observed [9]. The non-steroidal anti-inflammatory drugs (NSAIDs) such as naproxen, indomethacin, ibuprofen, diclofenac, and ketoprofen are the most commonly used conventional medicinal products in the treatment of inflammation [10]. The NSAIDs inhibit the expression of cyclooxygenase 2 (COX-2) enzyme responsible for the production of PGE2 which induces pyrexia [11]. However, the prolonged use of NSAIDs is linked with severe effects on the gastrointestinal tract, kidney, and cardiovascular system [12].
The demand for herbal medicine is increasing due to the growing recognition of natural products having fewer side effects, easily available, better cultural acceptability and being comparatively affordable [13]. Kigelia Africana (Lam) Benth and Acacia Hockii De Wild are used traditionally to manage inflammation among Embu and Mbeere communities in Embu County Kenya but lack validated scientific data to confirm their use [14]. The present study was, therefore, designed to evaluate for the anti-inflammatory potential of the two extracts to act as a preliminary step towards the development of more efficient plant-derived anti-inflammatory agents.
Collection and preparation of plant materials
The fresh leaves of K. africana and stem bark of A. hockii were collected in Mbeere North sub-county, Embu County, Kenya with the help of local herbalists in August, 2015. The plant samples were availed to an acknowledged taxonomist for botanical authentication and a sample voucher deposited at Kenyatta University herbarium. The plant samples were transported in polyethene bags to biochemistry and biotechnology laboratories at Kenyatta University, where they were sorted out, cleaned with tap water and rinsed with distilled water. The plant materials were separately chopped into small pieces, and air dried at room temperature until dry. The dried sample materials were ground into fine homogenous powder using an electric mill.
Extraction
For each sample, 400 grams of powder was soaked in 2 litres of methanol, stirred for four hours and left for 48 hours. The extracts were then filtered using Whatman’s No.1 filter paper. The filtrate was concentrated to dryness under reduced pressure using a rotary evaporator at a maximum temperature of 64°C. The concentrate was put in an airtight container and stored at 40°C until use in the bioassay.
Experimental design
Laboratory animals: Swiss albino aged between 2-3 months and weighing between 19-25 grams were used in this study. The animals breeding colonies were acquired and bred in the animal breeding and experimentation facility in the department of biochemistry and biotechnology, Kenyatta University. The animals were allowed to acclimatize for two days prior to experimentation. The experimental animals were kept in the standard cages in the animal house maintained under standard laboratory condition of an ambient temperature of 25°C with 12 hours daylight and 12 hours darkness cycles. The experimental animals were fed on standard rodent pellets and provided with water ad libitum [15].
Evaluation of anti-inflammatory activity: Thirty Swiss albino mice of either sex were divided randomly into six groups of five mice each and treated as follows; Group I (normal control) was not induced with inflammation but received 4% DSMO. Group II (negative control) was induced with inflammation and received 4% DMSO. Group III (positive control) was induced with inflammation and received diclofenac (reference drug) at a dose of 15 mg/kg body weight. Groups IV, V and VI (experimental groups) were induced with inflammation and received the extracts at the dose levels of 50 mg/kg, 100 mg/kg and 150 mg/kg body weight. This design is summarized in Table 1.
| Group | Status | Treatment |
|---|---|---|
| I | Control | DMSO |
| II | Negative control | Carrageenan+DMSO |
| III | Positive control | Carrageenan+15 mg/kg/bwdiclofenac |
| IV | Experimentalgroup A | Carrageenan+DMSO+50 mg/kg bw extract |
| V | Experimentalgroup B | Carrageenan+DMSO+100 mg/kg bw extract |
| VI | Experimentalgroup C | Carrageenan+DMSO+150 mg/kg bw extract |
Table 1: Treatment protocol for evaluation of anti-inflammatory activities of methanolic extracts of Kigelia Africana (Lam) Benth and Acacia Hockii De Wild in Swiss albino mice; Carrageenan=1%, DMSO=4%.
The anti-inflammatory activity of the extracts was assessed using carrageenan-induced right paw edema in mice as described by Winter. Acute inflammation was induced by sub-plantar injection of 0.05 ml 1% carrageenan (sigma-type I) in normal saline 30 minutes after treatment. The change in paw diameter was measured using a digital vernier caliper 30 minutes before injection of carrageenan and at 1, 2, 3 and 4 hours after induction of inflammation [17]. The percentages inhibition in inflammation was calculated using the formula described by Uma mageswari A [18], as follows;
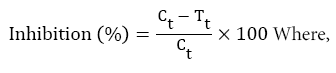
Ct=Paw diameter at 1 hour after carrageenan administration
Tt=Paw diameter after Treatment
Qualitative phytochemical screening
The plant extracts were subjected to qualitative phytochemical screening to identify the absence or the presence of various phytochemicals using methods of analysis described by Ref. [19,20]. Phytochemicals tested include alkaloids, terpenoids, saponins, flavonoids, phenolics, cardiac glycosides and steroids. These phytochemicals are reported to possess anti-inflammatory activity [21].
Data management and statistical analysis
Paw diameter measured was recorded and tabulated on spreadsheet. The data was exported to Minitab statistical software version 17.0 (State College, Pennsylvania) for analysis. The data was subjected to descriptive statistics and expressed as mean ± standard error of mean (SEM). One-way analysis of variance (ANOVA) was used to determine whether there was any significant difference between the means of different groups. This was followed by Turkey’s tests to separate means and obtain the specific significant differences among the various treatment groups. Unpaired student t-test was done to compare between the mean activities of the two extracts. The values of p ≤0.05 were considered significant. The data was presented in tables and graphs.
Anti-inflammatory activity of methanolic leaf extract of Kigelia Africana (Lam.) Benth on carrageenan-induced inflammation in Swiss albino mice
The methanolic leaf extract of K. africana showed significant antiinflammatory activity on carrageenan-induced paw edema, which was demonstrated by the reduction in inflamed hind paw diameter after extract administration (Figure 1; Table 2). In the first hour after treatment, the leaf extract of K. africana at the dose level of 150 mg/kg and the diclofenac (reference drug) at the dosage of 15 mg/kg body weight showed anti-inflammatory effect by reducing hind paw diameter by 0.21% and 1.10% respectively (Figure 1; Table 2). However, the extract at the dose levels of 50 mg/kg and 100 mg/kg body weight never showed anti-inflammatory activity at this hour (Figure 1; Table 2). The anti-inflammatory activity of the extract at the dose levels of 50 mg/kg, 100 mg/kg and 150 mg/kg body weight showed no significant difference and were comparable to normal control (p>0.05; Table 2). In addition, the anti-inflammatory activity of extract at the dose level of 150 mg/kg body weight was comparable to aspirin (reference drug) (p>0.05; Table 2).
| Group | Treatment | % change in paw diameter (mm) after treatment | ||||
|---|---|---|---|---|---|---|
| Normal Control | 10% DMSO | 0 hr | 1 hr | 2 hr | 3 hr | 4 hr |
| 100 ± 0.00 (0.00) | 99.84 ± 0.10b(0.16) | 99.84 ± 0.10b (0.16) |
99.84±0.10b (0.16) |
99.93 ± 0.07b (0.07) |
||
| Negative Control | Carrageenan+DMSO | 100 ± 0.00 (0.00) | 102.13 ± 0.20a(2.13) | 103.61 ± 0.08a (3.61) |
104.60 ± 0.30a (4.6) |
104.95 ± 0.48a (4.95) |
| Positive Control | Carrageenan +diclofenac+DMSO | 100 ± 0.00 (0.00) | 98.89 ± 0.22c(1.11) | 97.20 ± 0.53e (2.8) |
95.98 ± 0.44bc (4.02) |
95.57 ± 0.47de(4.43) |
| Methanolic Extracts |
Carrageenan+50 mg/kg bw+DMSO | 100 ± 0.00 (0.00) | 101.74 ± 0.15b (1.74) |
100.37 ± 0.26bc (0.37) |
99.14 ± 0.18b (0.86) |
98.05 ± 0.21c (1.95) |
| Carrageenan+100 mg/kg bw+DMSO | 100 ± 0.00 (0.00) | 101.1 0 ± 0.25b (1.1) |
99.58 ± 0.77cd (0.42) |
97.75 ± 0.60b (2.25) |
97.02 ± 0.45cd (2.98) |
|
| Carrageenan+150 mg/kg bw+DMSO | 100 ± 0.00 (0.00) | 99.79 ± 0.14bc (0.21) |
98.59 ± 0.25de (1.42) |
96.59 ± 0.26b (3.41) |
95.02 ± 0.20e (4.98) | |
Table 2: Effects of intraperitoneal administration of methanolic leaf extract of Kigelia Africana (Lam) Benth on carrageenan-induced inflammation in Swiss albino mice. Values expressed as Mean ± SEM for five animals per group. Values with the same superscript letter are not significantly different by one-way ANOVA followed by Turkey’s test (p>0.05). Percentage reduction in rectal temperature is given within parenthesis. Carrageenan=1%; 15 mg/kg bw diclofenac and 4% DMSO.
In the second hour, the leaf extract of A. hockii at the dosage of 100 mg/kg and 150 mg/kg body weight as well as the diclofenac (reference drug) reduced inflamed paw diameter by 0.42%, 1.42% and 2.8% respectively (Figure 1; Table 2). However, the extract at the dosage of 50 mg/kg body weight never showed anti-inflammatory activity at this hour (Figure 1; Table 2). The anti-inflammatory activity of the extract at the dose level of 50 mg/kg and 100 mg/kg showed no significant difference (p>0.05; Table 2). Besides, the anti-inflammatory activity of the extract at the dose level of 150 mg/kg body weight was comparable to diclofenac (reference drug) (p>0.05; Table 2).
In the third hour after treatment, the extract at the dose levels of 50 mg/kg, 100 mg/kg, and 150 mg/kg body weight, as well as the diclofenac (reference drug) reduced the inflamed hind paw diameter by 0.86%, 2.25%, 3.41% and 4.02% respectively (Figure 1; Table 2). The anti-inflammatory activity of the extract at the dosage of 50 mg/kg and 100 mg/kg and 150 mg/kg body weight showed no significant difference and were comparable to diclofenac (reference drug) (p>0.05; Table 2).
In the fourth hour after treatment, the leaf extract of K. africana at the dose levels of 50 mg/kg, 100 mg/kg, and 150 mg/kg body weight reduced inflamed hind paw diameter by 1.95%, 2.98% and 4.98% respectively (Figure 1; Table 2). Similarly, the diclofenac (reference drug) reduced the inflamed paw diameter by 4.43% at this hour (Figure 1; Table 2). The anti-inflammatory activity of the extract at the dosages of 50 mg/kg and 100 mg/kg body weight showed no significant difference (p>0.05; Table 2). In addition, the antipyretic activity of the extract at the dose level of 150 mg/kg body weight was comparable to diclofenac (reference drug) (p>0.05; Table 2).
Anti-inflammatory activity of methanolic stem bark extract of Acacia Hockii De Wild on carrageenan-induced inflammation in Swiss albino mice
The methanolic stem bark extract of A. hockii demonstrated antiinflammatory activity on carrageenan-induced paw edema in mice, which was indicated by the reduction in paw edema after extract administration (Figure 2 and Table 3). In the first hour after treatment, the stem bark extract of A. hockii at the dose levels of 100 mg/kg and 150 mg/kg body weight as well as the diclofenac (reference drug) at the dosage of 15 mg/kg body weight reduced inflamed hind paw diameter by 0.6%, 0.77% and 1.48% respectively. However, the extract at the dose level of 50 mg/kg body weight never showed anti-inflammatory activity at this hour (Figure 2; Table 3).
| Group | Treatment | % change in paw diameter (mm) after treatment | ||||
|---|---|---|---|---|---|---|
| 0 hr | 1 hr | 2 hr | 3 hr | 4 hr | ||
| Normal Control | DMSO | 100 ± 0.00(0.00) | 99.99 ± 0.12bc(0.01) | 99.99 ± 0.12bc(0.01) | 99.99 ± 0.12b(0.01) | 100.08 ± 0.08b(0.08) |
| Negative Control | Carrageenan+DMSO | 100 ± 0.00(0.00) | 101.49 ± 0.17a(1.49) | 102.66 ± 0.26a(2.66) | 103.30 ± 0.26a(3.30) | 103.37 ± 0.45a(3.37) |
| Positive Control | Carrageenan +Diclofenac +DMSO | 100 ± 0.00(0.00) | 98.52 ± 0.28d(1.48) | 96.63 ± 0.44d(3.37) | 95.50 ± 0.36c(4.5) | 95.10 ± 0.47c(4.9) |
| Methanolic Extracts | Carrageenan +50 mg/kg bw+DMSO | 100 ± 0.00(0.00) | 101.24 ± 0.24ab(1.24) | 100.35 ± 0.25b(0.35) | 99.04 ± 0.17b(0.96) | 98.22 ± 0.29b(1.78) |
| Carrageenan +100 mg/kg bw+DMSO | 100 ± 0.00(0.00) | 99.40 ± 0.56cd(0.6) | 98.57 ± 0.58c(1.43) | 96.89 ± 0.74c(3.11) | 95.93 ± 0.63c(4.07) | |
| Carrageenan +150 mg/kg bw+DMSO | 100 ± 0.00(0.00) | 99.23 ± 0.41cd(0.77) | 96.97 ± 0.28d(3.03) | 95.72 ± 0.47c(4.28) | 94.62 ± 0.41c(5.38) | |
Table 3: Effects of intraperitoneal administration of methanolic stem bark extracts of Acacia Hockii De Wild on carrageenan-induced inflammation in Swiss albino mice. Values expressed as Mean ± SEM for five animals per group. Values with the same superscript letter are not significantly different by one-way ANOVA followed by Turkey’s test (p>0.05). Percentage reduction in rectal temperature is given within parenthesis. Carrageenan=1%; 15 mg/kg bw diclofenac and 4% DMSO.
The anti-inflammation activity of the extract at the dosage of 100 mg/kg and 150 mg/kg body weight showed no significant difference and was comparable to diclofenac (reference drug) (P>0.05; Table 3). Besides, the anti-inflammatory activity of the extract at the dose level of 50 mg/kg body weight was significantly different (p<0.05; Table 4) from 100 mg/kg and 150 mg/kg body weight and comparable to negative control (p<0.05; Table 3).
| Phytochemical | Leaf Kigelia Africana | Stem bark Acacia Hockii |
|---|---|---|
| Alkaloids | - | + |
| Flavonoids | + | + |
| Steroids | + | + |
| Saponins | - | + |
| Terpenoids | + | + |
| Cardiac glycosides | + | + |
| Phenolics | + | + |
Table 4: Qualitative phytochemical composition of methanolic leaf extract of Kigelia Africana and stem bark extract of Acacia Hockii . Present phytochemical is denoted by (+) sign; absent phytochemical is indicated by (-) sign.
In the second hour after treatment, the extract at the dose levels of 100 mg/kg and 150 mg/kg body weight, as well as the diclofenac (reference drug) reduced inflamed paw diameter of mice by 1.43% and 3.03% and 3.37% respectively (Figure 2; Table 3). However, the extract at the dose level of 50 mg/kg body weight never showed the antiinflammatory effect at this hour (Figure 2; Table 3). The antiinflammatory activity of the extract of at the dose levels of 50 mg/kg, 100mg/kg and 150 mg/kg body weight were significantly different (p<0.05; Table 3). However, the anti-inflammatory activity of the extract of at the dosage of 150 mg/kg body weight was comparable to the diclofenac (reference drug) (p>0.05; Table 3).
In the third hour after treatment, the extract at dose levels of 50 mg/kg, 100 mg/kg and 150 mg/kg body weight, as well as the diclofenac (reference drug) reduced the inflamed hind paw diameter by 0.96%, 3.11%, 4.28% and 4.5% respectively (Figure 2; Table 3). The anti-inflammatory activity of the extract at the dosage of 100 mg/kg and 150 mg/kg body weight demonstrated no significant difference and were comparable to diclofenac (reference drug) (p>0.05; Table 3). However, the anti-inflammatory activity of the extract at the dose level of 50 mg/kg body weight was significantly different from 100 mg/kg and 150 mg/kg body weight (p<0.05; Table 3) and comparable to normal control (p>0.05; Table 3).
In the fourth hour, the extract at the dose levels of 50 mg/kg, 100 mg/kg and 150 mg/kg body weight, as well as the diclofenac reduced inflamed hind paw diameter by 1.78%, 4.05%, 5.38% and 4.9% respectively (Figure 2; Table 3). The anti-inflammatory activity of the extract at the dosage of 100 mg/kg and 150 mg/kg showed no significant difference and were comparable to aspirin (reference drug) (p>0.05; Table 3). However, the anti-inflammatory activity of the extract at the dose level of 50 mg/kg body weight was significantly different from 100 mg/kg and 150 mg/kg body weight (p<0.05; Table 3) and comparable to normal control (p>0.05; Table 3).
Comparison between the anti-inflammatory activities of Kigelia Africana and Acacia Hockii
In comparison, the anti-inflammatory activity of the two extracts at the dose level of 50 mg/kg body weight in the 1, 2, 3 and 4 hours of the test period was not significantly different with p values of 0.12, 0.96, 0.70 and 0.64 respectively (p>0.05). The anti-inflammatory activity of the two extracts at the dose level of 100 mg/kg body weight was significantly different in the first hour after treatment with p values of 0.04 (p<0.05). However, the anti-inflammatory activity of the two extracts at the dosage of 100 mg/kg body weight in the 2, 3 and 4 hours was not significantly different with p values of 0.32, 0.40 and 0.20 respectively (p>0.05). The anti-inflammatory activity of the two extracts at the dose level of 150 mg/kg body weight in the 1, 3 and 4 hours of the test period showed no significant difference with p values of 0.27, 0.16 and 0.42 respectively (p>0.05). However, the antiinflammatory activity of the two extracts the dose level of 150 mg/kg body weight in the 2 hour of the treatment period showed significant difference with p values of 0.004 (p<0.05). Both extracts were more efficient in the fourth hour at the dose level of 150 mg/kg body weight (Figure 3).
Qualitative phytochemical screening
The qualitative phytochemical screening of the methanolic leaf extract of K. africana showed the presence of flavonoids, phenolics, cardiac glycosides, steroids, and terpenoids (Table 4). However, the methanolic stem bark extract of A. acacia demonstrated the presence of alkaloids, cardiac glycosides, flavonoids, saponins, phenolics, steroids, and terpenoids (Table 4).
The present study evaluated for the anti-inflammatory (antiphlogistic) activity of methanolic leaf extract of Kigelia Africana (Lam.) Benth and methanolic stem bark extract of Acacia Hockii De Wild on carrageenan-induced paw edema in mice. Carrageenan, dextran, arachidonic acid, dextran, histamine, serotonin and formalininduced paw edema; cotton pellet induced granuloma; Freund’s adjuvants are the standard agents for causing acute, sub-acute and chronic inflammation respectively in animal models [22-25].
Carrageenan is a natural carbohydrate obtained from edible red seaweeds [26]. It is widely used to induce acute inflammation in experimental animals [27] and hence the choice in the present study. A freshly prepared solution of 1% carrageenan in normal saline as an intraplantar injection at a dose of 50-150 μl is commonly used to induce inflammation [28].
The carrageenan-induced inflammation is described as a biphasic event in which various mediators operates to produce an inflammatory response [29]. The first mediators detectable in the early phase (1 hour) include histamine, serotonin, and cyclooxygenase. On the other hand, the late phase (over 1 hour) is sustained by the production of PGE2 and it is mediated by bradykinin and leukotrienes [6, 30]. Inducible nitric oxide synthase (iNOS) and COX-2 enzyme are responsible for the production of an enormous amount of inflammatory mediators [26, 31]. Carrageenan-induced inflammation is also associated with enhanced levels of the endogenous pyrogenic cytokines such as tumor necrosis factor and interleukins (IL-1 and IL-6) which act as pro-inflammatory mediators [32].
The evaluation of anti-inflammatory activity of methanolic leaf extracts of K. africana and stem bark extract of A. hockii demonstrated a significant anti-inflammatory activity on carrageenan-induced paw edema in Swiss albino mice (Figure 1 and 2; Tables 2 and 3). These findings were consistent with other studies carried out on herbal medicines using animal models. A similar study carried out by [33], demonstrated a significant anti-inflammatory activity of dichloromethane: methanolic leaf extracts of Caesalpina volkensii and Maytemus obscura on carrageenan-induced paw edema in mice. Similarly, methanolic stem bark extract of Tinospora cordifolia Wild, fruits of Emblica officinalis and rhizomes of Cyperus rotundus Linn demonstrated anti-inflammatory effect in rodents [34].
The NSAIDs such as diclofenac, ibuprofen, indomethacin, naproxen and acetaminophen are commonly prescribed in the medication of inflammation [35]. The NSAIDs block the enzyme cyclooxygenase 2 (COX-2) which stimulate biosynthesis PGE2. There are two types COX enzymes; COX-1, and COX-2. The COX-2 produces prostaglandins that promote inflammation while the COX-1 produces prostaglandins that support platelets and protect the stomach [36]. The NSAIDs less inhibit the initial phase of carrageenan-induced paw edema, and this is attributed to the release of histamine, serotonin and bradykinin. However, the second phase is attributed to the induction of inducible COX-2 and can be blocked using NSAIDs [37]. It is therefore believed that the anti-inflammatory activities of leaf extract of K. africana and stem bark extract of A. hockii , inhibited the synthesis and release of prostaglandins to manage edema.
The present study used a dose range of 50 mg/kg, 100 mg/kg, and 150 mg/kg body weight to test for the anti-inflammatory activity of the extracts in mice. A similar study carried out by [38], evaluated for the anti-inflammatory activity of Cissus quadrangularis using a dose range of 50 mg/kg, 100 mg/kg, and 150 mg/kg body weight in rats. Similarly, a study carried out on the anti-inflammatory activity of leaf extract of Musanga cecropioides used a dose range of 50 mg/kg, 100 mg/kg, 150 mg/kg, and 200 mg/kg [39].
The methanolic leaf extract of K. africana and stem bark extract of A. hockii demonstrated dose-dependent response on carrageenaninduced paw edema in mice (Figure 1 and 2; Table 2 and 3). These findings were in agreement with a study carried out on the antiinflammatory properties of dichloromethane: methanolic extracts of Caesalpiina volkensii Harms and Maytemus obscura in mice [33]. Similarly, another study carried out on the evaluation of antiinflammatory activity of Strophanthus hispidus in experimental animals, showed a dose-dependent manner [40]. Furthermore, another study carried out by [41], on anti-inflammatory properties of Terbium chamaedrys showed a dose-dependent response in animal models.
The methanolic leaf extract of K. africana and stem bark extract of A. hockii showed minimal anti-inflammatory activities at lower dose levels of 50 and 100 mg/kg body weight compared to 150 mg/kg body weight (Figure 1 and 2; Table 2 and 3). The reference drug (diclofenac) achieved its maximum anti-inflammatory activity in the third hour (Figure 1 and 2; Table 2 and 3); its activity decreased subsequently probably due to metabolism and excretion of the drugs. The maximum anti-inflammatory activity of methanolic leaf extracts of K. africana and stem bark extract A. hockii was achieved at the dosage of 150 mg/kg body weight in the fourth hour (Figure 1 and 2; Table 2 and 3), indicating slow but steady passive diffusion of the bioactive constituent’s across the cell membrane in the peritoneal cavity [42].
The methanolic leaf extract of K. africana and stem bark extract of A. hockii at different dose levels did not reduce paw diameter in the first and second hours compared to the third and fourth hours (Figure 1 and 2; Table 2 and 3). However, the extract of K. africana and stem bark extract of A. hockii at the dose of 150 mg/kg body weight was more effective in the fourth hour of treatment compared to diclofenac (reference drug) in the same hour (Figure 1 and 2; Table 2 and Table 3). These findings showed that the leaf extract of K. africana and stem bark extract A. hockii were able to inhibit the synthesis of prostaglandins more than the conventional drug diclofenac (Figure 1 and 2; Table 2 and Table 3).
The antiphlogistic activity of methanolic leaf extract of K. africana and stem bark extract of A. hockii , could be due to the presence of bioactive constituents that exhibit anti-inflammatory action. This could be through inhibition of inflammatory mediators such as prostaglandins, histamine, serotonin and lysosome [43]. The qualitative phytochemical screening of methanolic leaf extract of K. africana indicated the presence of flavonoids, steroids, terpenoids, cardiac glycosides and phenolics while the stem bark extract of A. hockii indicated the presence of flavonoids, alkaloids, steroids, saponins, terpenoids, cardiac glycosides and phenolics (Table 4). The presence of some these bioactive compounds such as alkaloids, flavonoids, terpenoids, and steroids have shown to exhibit antiphlogistic activity in experimental animals [44, 45].
Flavonoids have been reported to inhibit pro-inflammatory mediators such as TNF-α and phospholipase A2 [44]. Furthermore, some flavonoids respond by blocking both the cyclooxygenase and lipoxygenase pathways of the arachidonate cascade at relatively high concentration while at the lower level only the lipoxygenase pathway is blocked [46]. Research findings have revealed that triterpenoids suppresses some function of macrophages, neutrophils and also inhibit nitric oxide (NO), NF-κB signaling and PGE2 production responsible for inflammation induction [47]. The NF-κB can detect noxious stimuli, such as infectious agents, cellular injuries and free radicals, and then directs DNA to synthesize inflammatory cytokines. Thus, their inhibition leads to management of edema [48]. Steroids also attenuate inflammation by inhibiting phospholipase A2, which hydrolyzes arachidonic acid from membrane phospholipids and subsequent formation of prostanoids and leukotrienes via the cyclooxygenase and lipoxygenase pathways.
The methanolic leaf extract of K. africana and stem bark extract of A. hockii showed potent anti-inflammatory activity on carrageenaninduced paw edema in mice. The anti-inflammatory activity of leaf extract of K. africana and stem bark extract of A. hockii demonstrated a dose-dependent response and were comparable to diclofenac (reference drug). The extracts were most active at the dose level of 150 mg/kg body weight in the fourth hour of treatment.
The extracts of K. africana and A. hockii could, therefore, be an alternative bio-resource for generating anti-inflammatory agents. However, further studies are necessary to elucidate the mechanism behind this effect and their active compounds. The present study, therefore, scientifically confirms and supports the traditional use of K. africana and A. hockii in the management of inflammation.