Advanced Techniques in Biology & Medicine
Open Access
ISSN: 2379-1764
ISSN: 2379-1764
Research Article - (2019)Volume 7, Issue 2
The chemical composition, functional properties, antioxidant activity (reducing power, DPPH•, DNA nicking test, ferrous chelating and ß-carotene bleaching assays) and anti-inflammatory (anti-5-lipoxygenase (5-LOX)) activities comparison between undigested Liza aurata head protein (ULAHP) and its protein hydrolysates (LAHPHs), prepared by Neutrase, testing three degrees of hydrolysis (DH), was evaluated. This study was designed to examine how the increase of degree of hydrolysis was effective for destroying protein-lipid complexes LAHPHs. Results showed that fatty acids and lipid content were distributed unequally between the fractions and the distribution was affected by the DH. Oleic acid is the most abundant in the hydrolysate fraction. The percentage of oleic acid in the LAHPHs range between 34.6% and 36.6%. The protein content of LAHPHs ranged from 61.72% to 63.44%, and the highest value was obtained in DH1. In terms of antioxidant activities, it was found that DH3 exhibited better capacities (DPPH: 72.03%, ferrous chelating: 97.14%, reducing power: 1.621 and β-carotene bleaching inhibition: 58.33%) and the activity increased as the DH increased. However, the interfacial activities and the DH are inversely proportional. Moreover, LAHPHs showed powerful inhibitory activity towards the enzyme 5-LOX.
Overall, these results indicated that proteins hydrolysates from L. aurata by-product are valuable source of bioactive peptides and show promise as functional foods ingredients and biotechnological application.
By-product; Degree of hydrolysis; Free fatty acid; Functional properties; Anti-inflammatory; Antioxidant activity
Studies have been developed aiming at producing protein hydrolysates of high nutritional value from non-conventional sources of animal origin, such as the liver, head, lungs, heart, kidneys, brain and guts [1]. This is justified by the fact that these byproducts are excellent sources of functional peptides, essential amino acids, vitamins and minerals [2].
Enzymatic hydrolysis has been shown to increase solubility, modify foaming and emulsifying properties as well as producing bioactive peptides from certain proteins [3]. It is possible to produce the desirable physicochemical properties of hydrolysate protein by controlling the hydrolysis parameters such as pH, time, DH, enzyme concentration and temperature [4]. The choice of substrate, protease employed and degree of hydrolysis generally affects the physicochemical properties of the resulting hydrolysates [5]. Peptides carried out under mild conditions, giving rise to final products of high functionality, good organoleptic properties and excellent nutritional value without formation of toxic compounds [6]. The hydrolysis of protein, which is measured in terms of DH, is a potential factor in the investigation of the functional characteristics of hydrolysates preparations [7]. DH affects the size and hence the amino acid profile of the peptides, which can affect the taste of protein hydrolysate by producing bitter peptides at high DH [7].
Furthermore, the size of peptides is very important for interfacial/ surface activity of protein hydrolysate. Extensive hydrolysis can have enormously negative effects on the functional properties [7]. Several reports have suggested that there is an optimum molecular size or chain length for peptides to provide good functional properties and limited hydrolysis generally leads to improved functional properties [8]. Besides their functional properties, some authors have reported bioactivities on several proteins from fish muscle including anti-diacetic, anti-inflammatory; antioxidative, antihypertensive, and antimicrobial [9-12].
As reported by bioactive peptides from natural sources have gained interest in recent years due to consumers’ preferences and health concerns associated to the use of synthetic food additives such as butylated hydroxyanisole, butylated hydroxytoluen, tertbutylhydroquinone and propyl gallate [13]. Recently, a great deal of interest has been expressed regarding marine-derived bioactive peptides because of their numerous health benefits.
Golden grey mullet (L. aurata), one of the mullet species, is very widespread on the sides of the Mediterranean Sea and Madeira northward to the British Isles, Atlantic coasts from the Azores and Black Sea, and the southern coasts of Norway and Sweden [14]. L. aurata is among the most important species in the fishing of Tunisia, and is utilized for human alimentation [15].
This is the first research that deals with the preparation of value-added head protein hydrolysates from L. aurata by using neutrase. There are no data explaining the effect of DH on the anti-inflammatory and antioxidant properties in byproducts L. auarata hydrolysates. For these reasons, the present study aimed at investigating the effects of DH in the functional and biological properties of the obtained product.
Materials
Golden grey mullet (L. aurata) was provided by the fish market. The samples were preserved in thermostatic bag, moved to the laboratory within 30 minutes. Fresh fish heads were then separated, cleaned with cold water, and then stored at -20°C until further use.
Chemicals and enzyme
Neutrase® 0.5 L, were purchased from Sigma Chemical Co (St. Louis, MO, USA) and Bio-Rad Laboratories (Hercules, CA, USA). All reagents and chemicals used in the experiments were of high purity and analytical grade.
Preparation of L. aurata heads protein hydrolysates (LAHPHs)
750 g of L. aurata heads was minced with 1.5 L distilled water, and then cooked for twenty minutes at 90 ± 2°C to inhibit the endogenous enzymes. The sample was adjusted to optimal conditions of Neutrase (pH 7.0, temperature 50°C, E/S 3(U/mg) enzyme/protein ratio). Before starting hydrolysis, the protein sample was allowed to equilibrate for ½ hour. During the incubation, the pH of the mixture was kept constant. After the required hydrolysis time, the enzyme was denatured by heating the mixture at 80°C during twenty min. Then, the mixture was cooled and centrifuged at 8500 g for thirty min to collect the soluble fractions. Finally, the supernatant was freeze-dried and stored at -20°C.
Hydrolysates obtained with neutrase after 1, 2 and 3 hours of hydrolysis are referred to DH1, DH2 and DH3, respectively. ULAHP, prepared without the addition of enzymes, were used as control
Chemical analysis and determination of color
Moisture, fat and ash contents of LAHPHs were measured according to AOAC methods 930.15, 922.06 and 942.05 respectively. Total nitrogen (N) content was estimated by Kjeldahl nitrogen method according to AOAC method [16]. Total crude protein content was determined by multiplying total nitrogen by 6.25.
Analyses of mineral content (magnesium (Mg), calcium (Ca), potassium (K), and sodium (Na)) in three hydrolysates were determined by using the analytical technique (ICP-OES: Model 4300 DV, Perkin Elmer, Shelton, CT, USA). 1 g of sample was homogenized with 1 ml nitric acid (70%). The mixture was heated until reaction was completed. The volume of treated mixture was adjusted, with deionized water, to ten ml. The concentration of minerals was expressed as mg / kg sample.
The color of three hydrolysates was evaluated using a Color Flex EZ spectrocolorimeter and reported as L*, a* and b* values, in which a* represents the color ladder from green to red, L* is a measure of lightness, and b* represents the color scale from blue to yellow [17]. The sample was filled in a 64 mm glass sample cup with three readings in the same place. The white tile and black glass were used to standardize the equipment.
Functional properties of LAHPHs
Solubility of LAHPHs: The solubility of LAHPHs was tested over a range of pH values from pH 2.0 to 9.0. The nitrogen content in the supernatant of sample was determined by the gornal method. Solubility was calculated using the following formula:

Emulsifying capacity: The emulsifying capacity and stability index (EAI, ESI) were measured according to the method described by Pearce and Kinsella [18]. To calculate the EAI and the ESI, the absorbance of the samples, at T0 (A0) and after 10 min (A10), were used:
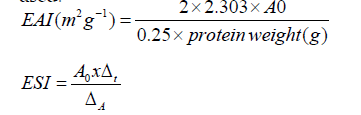
Foaming ability and stability: The foaming properties (capacity (FC) and stability (FS)) of hydrolysates samples were performed following the method of Shahidi et al. [19]. The foaming volume at 0 min corresponds to FC, while the FS was expressed as the foam volume after 30 min.
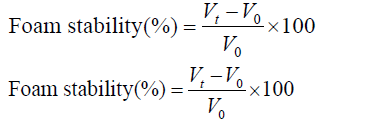
where VT, V0 are the total volume after, before whipping ( ml), respectively; and Vt is the total volume after leaving for 30 min.
Fat and water absorption capacity: The ability of LAHPHs to absorb fat was determined using a modified method of Shahidi et al. [19]. The mixture (500 mg of LAHPHs and 10 ml of oil) was kept at 37°C for 30 min. The sample was vigorously mixed, and then centrifuged at 2000 g for 25 min. Free oil was then decanted and the fat absorption (FA) was measured from the weight difference.
FA was reported in terms of ml oil absorbed /g LAHPHs.
WH capacity was evaluated following the method of Okezie and Bello [20] 500 mg of LAHPHs was dispersed in distilled water (50 ml) and homogenized (2 min). The homogeneous solutions were kept at 37°C for 30 min and then centrifuged at 5000 g for ½ hour. The supernatant was decanted and the volume recovered was measured. The WHC was examined as ml water absorbed per g of LAHPHs.
Preparation and analysis of fatty acid methyl esters (FAME)
The total lipids from the three LAHPH were extracted as per method of Bligh and Dyer [21] FAME was prepared by acid-catalyzed trans-esterification [22]. FAME were analyzed in a Varian CP 3800 (Walnut Creek, CA, USA) gas chromatograph, equipped with an auto-sampler and fitted with an injector and a flame ionization detector (FID), both at 250°C. The separation was achieved using a capillary column DB-Wax (30 m length, 0.25 mm internal diameter and 0.25 μm film thickness) from Agilent (Albertville, MN, USA). After holding the oven temperature at 180°C for 5 min, it was raised to 220°C at 4°C min-1, and maintained at 220°C for 25 min. FAME were identified by comparison of their retention times (Rt) with those from chromatographic standards (Sigma). The quantification was done using the internal standard 21:0 area.
Free fatty acid determination
Free fatty acids (FFA) content was evaluated by a colorimetric method [23]. Approximately, 50mg of oil was placed into 15 ml Pyrex tubes and 3 ml cyclohexane and 1 ml of cupric acetate– pyridine reagent (5% w/v) was added. Tubes were vortexed and centrifuged for 10min. The upper layer was read at 710 nm. FFA quantification was based on a calibration curve from oleic acid.
RP-HPLC analysis of LAHPHs
RP-HPLC analysis of LAHPHs (20 mg/ ml) was performed using a 1100 Agilent liquid chromatography system (Agilent Technologies, Palo Alto, CA, USA) equipped with a diode array detector and a C18 column (250 mm × 4.6 mm, Waters, Milford, MA, USA) at 30°C. Solvent A (0.1% trifluoroacetic acid (TFA) in bidistilled water) and solvent B (TFA (0.085% v/v) in ACN:bidistilled water (60:40v/v), were filtered through a 0.45 μm nylon membrane filter and degassed prior to any analytical run. The elution started with 100% solvent A for 2min, followed by a linear gradient from 0 to 25% of solvent B during 30 min and reached 100% after 15 min. The flow rate used was fixed at 1 ml/min and the separation was monitored at 214 nm RP-HPLC.
Antioxidant potential of LAHHs
DPPH assay: DPPH free radical-scavenging activity of LAHPHs was performed as developed by Bersuder et al. [24]. The reduction of DPPH radical by each sample at various concentrations (1-6mg/ ml) was recorded at 517nm. The control (BHA) was used as reference. The percentage inhibition of DPPH was calculated as follows:
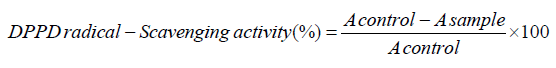
where A control is the absorbance of the control reaction, and A sample is the absorbance of LAHPHs or ULAHP (with the DPPH solution).
Determination of reducing power: The ability of LAHPHs to reduce Fe3+ was carried out according to the method of Xie et al. [25]. A sample solution (1 ml) of LAHPHs at various concentrations (1– 6 mg/ml) was mixed with 1.25 ml of phosphate buffer (pH 6.6; 0.2M) and 1.25 ml of potassium ferricyanide solution. After incubation at 50°C for 30min, 1.25 ml of TCA (10%) was added to each sample and the reactions mixtures were centrifuged (10 min at 3.000 g). 2.5 ml of the supernatant of each sample, was added to 2.5 ml of distilled water and 0.5 ml of ferric chloride solution (0.1%). The absorbance was recorded at 700 nm.
ß-Carotene bleaching assay: The capacity of LAHPHs to inhibit bleaching of β-carotene was performed as described by Koleva et al [26] 500 μl of LAHPH sample at various concentrations was added to 2.5 ml of the β-carotene/linoleic acid emulsion. The obtained samples were incubated for 2 h at 50°C and absorbance was recorded at 470 nm. The antioxidant activity of the LAHPHs was calculated using the following formula:
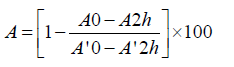
A0, A10, A2h and A12h: absorbance of the sample and the blank, respectively, measured at T0, and after incubation for 2h.
Ferrous chelating activity: According to Dinis et al. [27] method, chelating potential of the LAHPHs and ULAHP towards Fe2+ was determined. A volume of 1.6 ml of distilled water and 0.05 ml of FeCl2 (2mM) were added, to 0.5 ml of sample at tested concentrations. The reaction started after 15 min by the addition of ferrozine (0.1 ml; 5mM). The absorbance of the Fe2+-ferrozine complex was measured, after 10min, at 562nm. The chelating antioxidant activity was calculated according to the following formula:
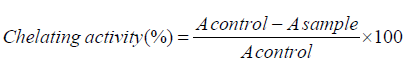
Where A control and A sample are the absorbance of the control and LAHPHs, respectively. EDTA was used as reference.
DNA protection test: The assay procedure followed the method of Lee et al [28] using pCRII™TOPO plasmid (Invitrogen). 10μl of LAHPHs (2 mg/ ml) and plasmid DNA (0.5 μg/well) was incubated for 10min at 37°C followed by adding Fenton's reagent (10 μl). The mixture was then incubated for additional 10min at 37°C. The DNA plasmid was analysed on agarose gel (1% (w/v)) and visualised under UV-light after staining with ethidium bromide.
Anti-inflammatory activity
The anti-inflammatory activity was termed using the spectrophotometric measurement of a conjugated diene the result of linoleic acid oxidation by the enzyme 5-LOX [29]. Briefly, 500 μL of buffer (pH=7.4) (Na2HPO4, 2H2O; KH2PO4; NaCl) were mixed with 200 μL of protein hydrolysate, 200 μL of linoleic acid and 100 μL of 5-LOX enzyme solution. After homogenization the mixture was incubated for 10 min at 25°C. The absorbance at 234 nm against a blank was determined and Naprosyn (NAP) was used as positive control.
Statistical analysis
Statistical analyses were performed with SPSS ver. 2.0, professional edition using ANOVA analysis. Differences were considered significant at p<0.05. All tests were carried out in triplicate.
Preparation of LAHPHs
Enzymatically hydrolyzed fish proteins exhibited different physicochemical and biological activities correlated to the enzyme specificity, substrate, and to the DH. In this study Neutrase, as a commercial enzyme, was used for the preparation of protein hydrolysates. To examine how the extent of enzymatic hydrolysis can influence the evolution of bioactivities as well as the functional properties (emulsifying capacity, foaming ability and oil and water holding capacity), hydrolysates with DH values of 12.4%, 14.5% and 15.4% were prepared by hydrolysis of heads from golden grey mullet.
The hydrolysis curve of golden grey mullet head proteins after 210 min of incubation is shown in Figure 1. The DH of LAHPHs gradually increased with increasing the hydrolysis time. Rapid hydrolysis was observed within the first 1 h. Subsequently, the rate of hydrolysis decreased and the enzymatic reaction reached a saturated state phase. The final degree of hydrolysis (DH), recorded after 180 min of processing, was estimated to be 15.4%. The shape of the hydrolysis curve is similar with result published for hydrolysates from octopus [30] and goby [11].
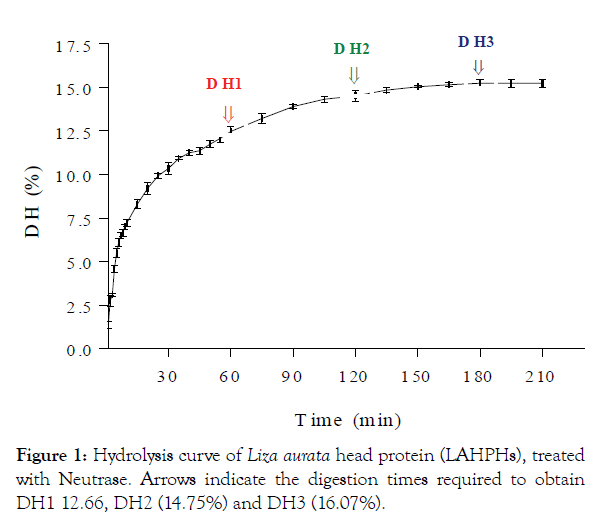
Figure 1: Hydrolysis curve of Liza aurata head protein (LAHPHs), treated with Neutrase. Arrows indicate the digestion times required to obtain DH1 12.66, DH2 (14.75%) and DH3 (16.07%).
Chemical composition
The chemical composition of LAHPHs was determined and compared with that of ULAHP. The protein contents of DH1, DH2 and DH3 were found to be 63.4% ± 0.47, 62.7% ± 0.56 and 61.7% ± 0.83 (Table 1). The protein content decreased slightly with the increase of degree of hydrolysis. This result was similar to a previous study reported by Jamdar et al. [31]. The relatively high protein content was a result of the solubilisation of the proteins during hydrolysis, the removal of insoluble undigested non-protein substances and the partial removal of lipids after hydrolysis. Lower protein content in the ULAHP (57.3% ± 0.43) was due to higher content of crude fat (11.1% ± 0.48). All protein hydrolysates had relatively low levels of fat (7.4%–4.4%) than did ULAHP (11.1%). This is in accordance with the results obtained by Ktari et al. [32]. Based on the dry weight, fat content in LAHPHs samples are considered lower than the fat content of those reported by Noman et al. [6] who found that the fat content in sturgeon samples was 23.62% based on the dry weight.
The ash content was 12.5% ± 0.65, 14.9% ± 0.63 and 17.3% ± 0.62% in DH1, DH2 and DH3, respectively, which were higher compared to that of ULAHP (10.7% ± 0.26). The high ash content of heads protein hydrolysates probably due to increasing alkali volume required to control the pH during enzymatic hydrolysis of head proteins. Our findings are in line with the study reported by Kristinsson and Rasco [7]. Chemical composition of three LAHPHs was approximately the same. The freeze-dried LAHPHs consisted of different minerals at different levels as shown in Table 1. Na+, K+ and Ca2+were found at high concentrations, while Mg2+ was found at a low level. The sodium levels in the LAHPHs samples (DH1; 3.488 g/100 g, DH2; 4.357 g/100 g and DH3; 5.297 g/100 g) were higher than in ULAHP (1.086 g/100 g) with a significant difference (p< 0.05). Similar results were found by Ktari et al. [32]. The increase in the sodium ion may be due to the NaOH added during proteolysis to keep the pH constant. Fish protein hydrolysates usually contain a moderate NaCl content due to salting for conservation or pH adjustments during the pH shift process. The apparent metal ions could act as pro-oxidants in the hydrolysate. Sathivel et al. [33] reported that Na, K+, Ca2+and Mg2+ were abundant in herring and herring byproduct hydrolysates and varied with the substrate used.
Determination of colour
Colour influences the overall acceptability of food products. The colour of LAHPHs was determined by colorimeter (Table 1). When the DH was increased, L, a and b values of LAHPHs significantly (p<0.05) decreased. During hydrolysis, LAHPHs turned brownish. Indeed, ULAHP was the lightest (L*=46.11±0.43) and more yellow (b*=+30.08 ± 1.5). Whereas, DH1, DH2 and DH3 were darker with values (L*=44.36 ± 0.8; 28.1 ± 1.5 and 25.57 ± 0.16) and less yellow (b*=+27.66 ±2; +10.80 ± 1.05 and +9.10 ± 0.67), respectively (p<0.05). These results appear to indicate that colour of protein hydrolysates is greatly influenced by DH. As the proteins were hydrolyzed extensively, the product became dark. The dark colour of fish protein hydrolysate was believed to be due to the oxidation of myoglobin and melanin in the meat [34]. Moreover, the formation of brown pigments might result from an aldol condensation of carbonyls produced from lipid oxidation and the non-enzymatic Maillard browning reaction [35]. The pigment oxidation and Maillard reactions in the meat were accelerated when the hydrolysis time was prolonged and the DH was enhanced. Enzymatic browning reactions are assumed to have contributed to the reduction in the luminosity, giving a darker colour in LAHPHs. Dark fleshed fish, such as sardine and mackerel, contained a high amount of myoglobin, which is susceptible to oxidation [36].
| Composition (%) | DH1 | DH2 | DH3 | ULAHP |
|---|---|---|---|---|
| Protein | 63.4 ± 0.47a | 62.9 ± 0.56b | 61.7 ± 0.83c | 57.3 ± 0.43d |
| Fat | 7.4 ± 0.82b | 5.6 ± 0.59c | 4.4 ± 1.2d | 11.1 ± 0.48a |
| Moisture | 16.5 ± 1.63b | 16.4 ± 0.87b,c | 16.4 ± 0.75b,c | 20.7 ± 0.92a |
| Ash | 12.5 ± 0.65c | 14.9 ± 0.63b | 17.3 ± 0.62a | 10.7 ± 0.26c,d |
| Mineral contents (g/100 g) | ||||
| Ca2+ | 0.676 | 0.407 | 0.346 | 0.219 |
| Na+ | 3.488 | 4.357 | 5.297 | 1.086 |
| K+ | 2.0575 | 1.644 | 1.029 | 2.150 |
| Mg2+ | 0.254 | 0.115 | 0.092 | 0.266 |
| Colour | ||||
| L* | 44.3 ± 0.8b | 28.1 ± 1.5c | 25.6 ± 0.16d | 46.1 ± 0.43a |
| a* | +11.2 ± 0.75b | +7.7 ± 0.3c | +4.5 ± 0.19d | +13.2 ± 1.2a |
| b* | +27.6 ± 2b | +10.8 ± 1.05c | +9.1 ± 0.67c,d | +30.0 ± 1.5a |
Physico-chemical composition was calculated based on the dry matter. Different letters in the same line indicate significant differences (a>b>c>d; p<0.05). L. aurata head protein hydrolysates were obtained by treatment with Neutrase (DH1), (DH2), (DH3) and undigested L. aurata head protein (ULAHP).
Table 1: Chemical constituents of L. aurata head protein hydrolysates (LAHPHs) and undigested L. aurata head protein (ULAHP).
Influence of DH on the oil fraction and fatty acid content
The effect of DH on the oil percentages in the oil fraction (OF) and hydrolysate fraction (HF) was evaluated and shown in Figure 2. The maximum percentage of free oil released from the LAHPHs on the hydrolysate fractions was obtained with DH1, which attained about 37.17% of the total amount of oil in the ULAHP. The composition of fatty acids in LAHPHS and ULAHP is shown in Table 2. The most abundant fatty acids found in the HF, were oleic acid ranged between 34.6% and 36.6%, followed by palmitoleic acid (21.9% and 23.7%), and palmitic acid (14.1%; 14.3%). The contents of saturated fatty acids (SFAs) and monounsaturated (MUFAs) fatty acids in the HF of LAHPHs were 36.9%, 60.3% for DH3, 39.6%, 58.5% for DH2 and 42.1%, 57.5% for DH1, respectively. As expected, in comparison with ULAHP, a significant decrease in the levels of SFAs as well as a significant increase of MUFAs was observed in HF of LAHPHs. The content of MUFAs in the present study was higher than that reported in the study of Noman et al. [37], who found that the contents of MUFAs in Chinese sturgeon (Acipenser sinensis) were 35.40% ± 0.53%.
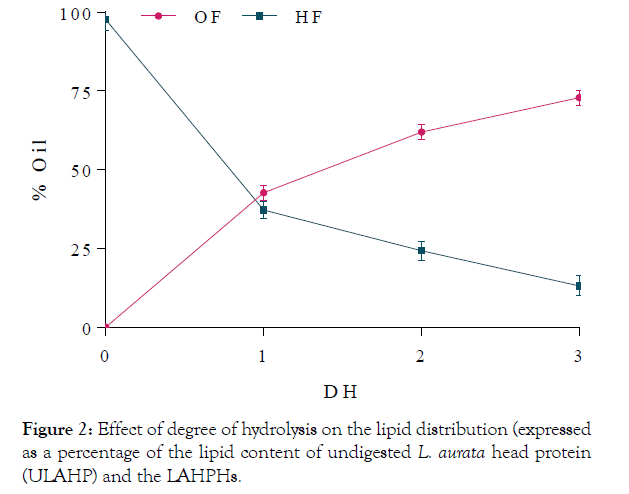
Figure 2: Effect of degree of hydrolysis on the lipid distribution (expressed as a percentage of the lipid content of undigested L. aurata head protein (ULAHP) and the LAHPHs.
| Fatty | ULAHP | DH1 | DH2 | DH3 | |||
|---|---|---|---|---|---|---|---|
| Acids | OF | HF | OF | HF | OF | HF | |
| Myristic acid C14:0 | 12.9 ± 0.25c | 13.3 ± 0.13c | 8.7 ± 0.3d | 14.2 ± 0.11b | 6.7 ± 0.4e | 15.5 ± 0.27a | 8.5 ± 0.1d |
| Palmitic acid C16:0 | 20.1 ± 0.34a | 16.8 ± 0.20d | 14.2 ± 0.12e | 19.1 ± 0.20b | 14.3 ± 0.23f | 18.8 ± 0.50c | 14.1 ± 0.3b |
| Stearic acid C18:0 | 2.4 ± 0.09b | 3.4 ± 0.05a | 0 ± 0d | 2.1 ± 0.02c | 0 ± 0d | 2.4 ± 0.07b | 0 ± 0d |
| Others | 19.9 ± 0.33a | 17.9 ± 0.37bc | 19.2 ± 0.61c,d | 18.8 ± 0.57ab | 18.6 ± 0.41ab | 18.7 ± 0.19ab | 14.3 ± 0.0e |
| ƩSFA | 55.3 ± 0.29ab | 50.4 ± 0.36c | 42.1 ± 0.20 | 54.3 ± 0.24b | 39.6 ± 0.50e | 55.4 ± 0.26a | 36.9 ± 0.26e |
| Palmitoleic acid C16:1 | 26.4 ± 0.0c,d | 29.5 ± 0.08a | 21.9 ± 0.20e | 28.1 ± 0.30b | 22.6 ± 0.20c | 27.9 ± 0.42b,c | 23.7 ± 0.70f |
| Oleic acid C18:1 | 18.1 ± 0.14d | 18.9 ± 0.74d | 34.6 ± 0.49c | 17.5 ± 0.80e | 35.9 ± 0.49a | 16.6 ± 0.9f | 36.6 ± 0.68a,b |
| ƩMUFA | 44.6 ± 0.07d | 48.5 ± 0.41c | 57.5 ± 0.69b | 45.6 ± 0.55d | 58.5 ± 0.35a | 44.5 ± 0.66d | 60.3 ± 0.35a |
Table 2 : Fatty acid profile of LAHPHs lipids from the fractions obtained by hydrolysis with different degree of hydrolysis and ULAHP: oil fraction (OF) and Hydrolysate fraction (HF).
Palomer et al. [38] reported that high levels of SFAs, in particular palmitic acid, induced cardiovascular disease. In the other hand, the monounsaturated fatty acids such as oleic acid elicits beneficial effects on insulin sensitivity, and prevents palmitic acid induced inflammation and insulin resistance in adipose tissue, liver, skeletal muscle, and pancreas. According to the results in Table 2, it is quite obvious that the content of the oleic acid and palmitic acid in LAHPHs was higher than the content of those obtained by Njinkoue et al. [39].
In the same context, our findings revealed that, bioactive peptides generated by hydrolysis of L aurata byproduct, can be used for the treatment of various diseases.
Functional properties
Protein solubility: Solubility is one of the most important physicochemical properties of proteins and protein hydrolysates. High solubility of protein hydrolysates is necessary for their use in many manufactured foods to improve other functional properties such as emulsification and foaming.
Protein solubility expressed in terms of percent nitrogen solubility (% NS) of LAHPHs and ULAHP, carried out over the pH range of 2.0–9.0 is shown in Figure 3. All LAHPHs showed same solubility profiles and they had minimum solubility at pH 5.0. Solubility increased gradually below and above pH 5.0, which is the isoelectric point of L. aurata head proteins. The solubility increased as a function of degree of hydrolysis. At low DH (12.4%), the solubility increased up to 75% at pH 9.0. Similarly, at DH of 15.4%, more than 70% nitrogen solubility was observed at above pH 6.0 and was about 90.5% at pH 9.0. However, ULAHP was less soluble than the hydrolysates, having solubility below 35% at pH 2–9.0, indicating that enzymatic hydrolysis considerably improved (p< 0.05) the solubility of L. aurata head proteins at all pH values tested. The enhancement in solubility with the increase of DH, may be attributed to the decrease in the molecular size and the formation of smaller peptides 12 and also the formation of carboxylic and amine groups from amino acids, which increased the hydrophilicity of the LAHPHs. The obtained results demonstrated that proteins solubility depends in several factors, including pH and molecular size.
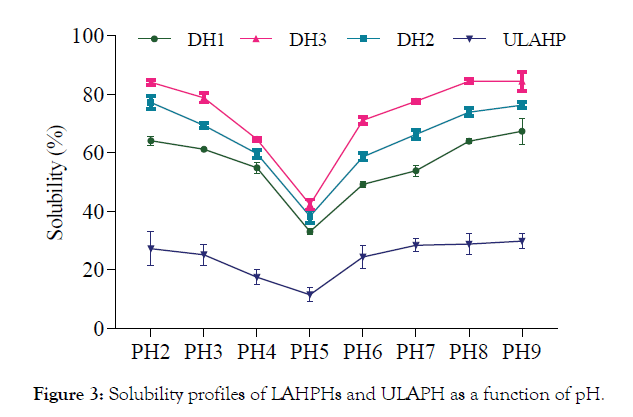
Figure 3:Solubility profiles of LAHPHs and ULAPH as a function of pH.
Emulsifying properties: The EAI and ESI of LAHPHs and ULAHP at different concentrations are shown in Table 3. As can be seen, LAHPHs had higher EAI and ESI than undigested proteins, indicating that hydrolysis of process has significantly increased the emulsifying properties. DH1 hydrolysate, with the lowest DH, showed the highest emulsifying activity and stability over all concentrations used than did DH2 and DH3. These results were in agreement with those of Hmidet et al. [40] where they found that EAI of protein hydrolysates decreased as the DH increases. Results illustrated in Table 3 also show that both EAI and ESI increased with the increase of protein hydrolysate concentrations.
| Concentration (%) | DH (%) | EAI (m2/g) | ESI (min) | FE (%) | FS (%) | ||
|---|---|---|---|---|---|---|---|
| 0.1 | ULAHP | 2.5 ± 0.71dA | 1.1 ± 0.22dB | 3.1 ± 0.43dA | 2.1 ± 0.29dB | ||
| DH1 | 25.3 ± 1.05aD | 21.8 ± 1.25aD | 40.3 ± 1.12aC | 24.6 ± 0.45aC | |||
| DH2 | 21.4 ± 1.39bD | 19.9 ± 0.76bC | 24.1 ± 0.87bC | 16.1 ± 0.59bC | |||
| DH3 | 19.3 ± 0.65cD | 9.9 ± 1.19cC | 12.3 ± 2.29cC | 8.5 ± 2.37cC | |||
| 0.25 | ULAHP | 3.1 ± 0.53dA | 1.44 ± 0.39daB | 3.3 ± 0.27dA | 4.2 ± 0.33dA | ||
| DH1 | 36.5 ± 0.88aC | 27.14 ± 1.67aC | 46.2 ± 0.56aB | 30.7 ± 1.62aB | |||
| DH2 | 30.0 ± 1.35bC | 24.19 ± 2.09bC | 32.4 ± 1.42bB | 19.5 ± 0.41bbC | |||
| DH3 | 24.8 ± 2.06cC | 12.55 ± 0.66cC | 15.6 ± 0.65cbC | 10.7 ± 0.79cbC | |||
| 0.5 | ULAHP | 3.7 ± 0.21dA | 1.92 ± 0.13daB | 3.9 ± 0.37dA | 2.6 ± 0.20dB | ||
| DH1 | 44.7 ± 0.92aB | 39.04 ± 1.42aB | 50.1 ± 1.47aB | 34.0 ± 0.44aB | |||
| DH2 | 38.2 ± 1.77bB | 31.58 ± 1.87bB | 36.2 ± 0.72baB | 23.4 ± 2.35baB | |||
| DH3 | 30.4 ± 0.35cB | 23.18 ± 0.93cB | 19.7 ± 0.84caB | 15.1 ± 1.58caB | |||
| 1.0 | ULAHP | 4.1 ± 0.43dA | 2.28 ± 0.11dA | 4.5 ± 0.62dA | 3.9 ± 0.37dA | ||
| DH1 | 59.0 ± 1.47aA | 45.31 ± 2.55aA | 58.7 ± 0.95aA | 40.3 ± 0.78aA | |||
| DH2 | 46.2 ± 2.28bA | 43.72 ± 1.34bA | 38.3 ± 2.14bA | 27.2 ± 0.8bA | |||
| DH3 | 38.8 ± 0.94cA | 35.65 ± 1.07cA | 23.0 ± 0.76cA | 19.1 ± 0.91cA | |||
| Sample | Fat absorption (ml oil g−1 LAHPH) | Water-holding capacity (ml water g−1 LAHPH) | |||||
| ULAHP | 0.6 ± 0.15c | 1.2 ± 0.35d | |||||
| DH1 | 1.6 ± 0.85a | 2.5 ± 0.5b,c | |||||
| DH2 | 1.4 ± 0.62a | 3 ± 1aB | |||||
| DH3 | 1.2 ± 0.25aB | 4 ± 0.5a | |||||
Values are given as mean ± SD from triplicate determinations (n=3) L. aurata head protein hydrolysates were obtained by treatment with Neutrase (DH1), (DH2), (DH3) and undigested L. aurata head protein (ULAHP).
a-d Different letters in the same column within the same concentration and different sample indicate significant differences (p< 0.05).A-D Different capital letters in the same column within the same hydrolysate sample and different concentration indicate significant differences (p< 0.05).
FE: foam expansion; FS: foam stability; EAI: Emulsion activity index; ESI: emulsion stability index
Table 3: Emulsifying and foaming properties, fat absorption and water-holding capacity of LAHPHs and ULAHP at various concentrations.
The decrease in the emulsifying activity of protein in solution during enzymatic hydrolysis is probably due to the reduction of hydrophobicity.
A peptide is required to have a minimum length of about 20 residues to possess good emulsifying and interfacial properties [41]. Thus, limited protein hydrolysis is a good procedure to improve functional properties of LAHPHs. But the extent of hydrolysis is critical for maximizing the functionality of the product. So, a compromise must be reached between DH and functionality.
Foaming capacity and stability: Some food proteins are capable of forming good foams, and their capacity to form and stabilize foams depends on the type of protein, degree of denaturation, pH, temperature and whipping methods. Foam expansion (FE) and foam stability (FS) of LAHPHs with different DH at various concentrations (0.10%, 0.25%, 0.50 % and 1%) and ULAHP, are shown in Table 3. At the same concentrations of hydrolysates used, FE and FS decreased with the increase of DH (p< 0.05). This is possibly due to a lesser alignment of small peptides at the air–water interface [42].
Further, both FE and FS of the three hydrolysates increased with increasing concentration. This is in line with the work of Nalinanon et al. [42] who reported that increasing concentration leas to increase levels of FE and FS.
The results show a significant increase in the foaming capacity and stability of the three hydrolysates compared with ULAHP. The hydrolysate with high DH more likely had the smaller peptides, which could migrate to the air–water interface faster. With higher amount of peptides in LAHPHs, a lower FS was attained. The higher concentration of LAHPHs might result in the self-aggregation and lowered the migration of peptides to the air–water interface.
DH1 exhibited high FS could be attributed to its high content of high molecular weight peptides, which could form flexible films around the air bubbles.
Fat absorption: The ability of fish protein hydrolysate to absord and hold oil is another important functional property. It influences not only the taste of the product but is also an important functional characteristic, especially for the meat industry [7]. Fat binding capacity of proteins hydrolysates also correlates with surface hydrophobicity [7], degree of hydrolysis [43], enzyme substrate specificity [44], such as bulk density of the protein [45]. Fat absorption of LAHPHs was determined and compared with that of ULAHP. All LAHPHs exhibited excellent fat absorption, greater than that of ULAHP (Table 3). DH1 had the highest fat absorption (1.6 ml oil g−1 LAHPH), followed by DH2 (1.4 ml oil g−1 LAHPH) and DH3 (1.2 ml oil g−1 LAHPH) (Table 3). The decrease in fat absorption as the DH increased might be due to hydrolytic degradation of the protein structure. DH1 contained larger peptides and more medium-hydrophobic peptides than the other hydrolysates. Other studies indicated that hydrophobic interactions are primarily responsible for the decrease in fat absorption [44].
The results showed that LAHPHs exhibited good fat absorption and could be very useful in the meat and confectionary industries.
Water-holding capacity: Several studies have shown that fish protein hydrolysates have excellent water-holding capacity (WHC) and can increase the cooking yield when added to minced meat. As shown in Table 3, WHC increased with the extent of hydrolysis. DH3 had significantly higher WHC than the control (ULAHP). These findings support the hypothesis that functional properties of peptides are highly influenced by their respective molecular weight [44]. The observed increase in WHC with increasing DH (and solubility) could be due to the increased presence of polar groups such as –COOH and –NH2 during enzymatic hydrolysis.
Characterisation of hydrolysates by RP-HPLC: RP-HPLC profile was used to analyse the hydrophilic/hydrophobic peptide ratio of the protein hydrolysates. Several researchers have used this technique in studying protein hydrolysates from octopus [30] and sardinelle muscle hydrolysates [46]. Several peaks are detectable by RP-HPLC, illustrating the heterogeneous composition of LAHPHs. The retention time of peptides depends generally on their size and their polarity. The order of elution of peptides from a reverse phase column is dependent on their size. This is probably due to the more hydrophobic character of large peptides relative to short peptides. The order in which hydrophilic and hydrophobic residues appear in a peptide has also been shown to influence the elution profile of small peptides. The RP-HPLC profiles (Figure 4) showed that DH3 contain a higher concentration of hydrophilic peptides than DH1 and DH2. The differences in RP-HPLC profiles of LAHPHs are essentially due to the difference in the time of hydrolysis.
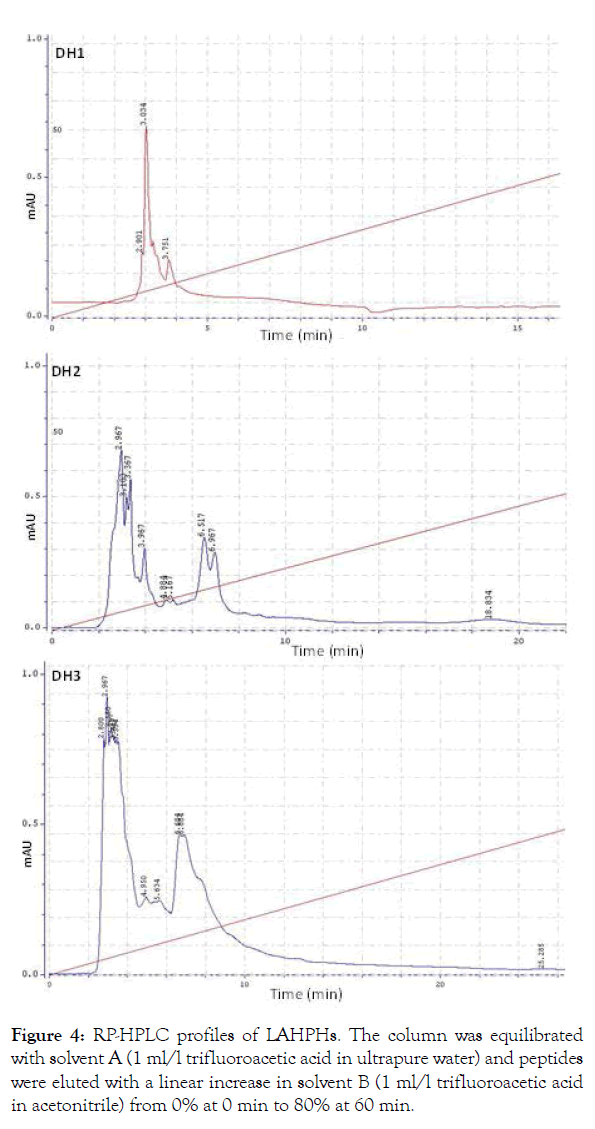
Figure 4:RP-HPLC profiles of LAHPHs. The column was equilibrated with solvent A (1 ml/l trifluoroacetic acid in ultrapure water) and peptides were eluted with a linear increase in solvent B (1 ml/l trifluoroacetic acid in acetonitrile) from 0% at 0 min to 80% at 60 min.
DPPH free radical-scavenging activity: It is well known that antioxidants can interact with free radicals and form stable species, which prevent the oxidation. The DPPH radical-scavenging assay has been widely used to investigate the scavenging activities of antioxidant compounds. DPPH radical scavenging activities of LAHPHs with three DH at various concentrations are depicted in Figure 5a. As can be seen, all LAHPHs showed DPPH scavenging activities in a dose-dependent manner.
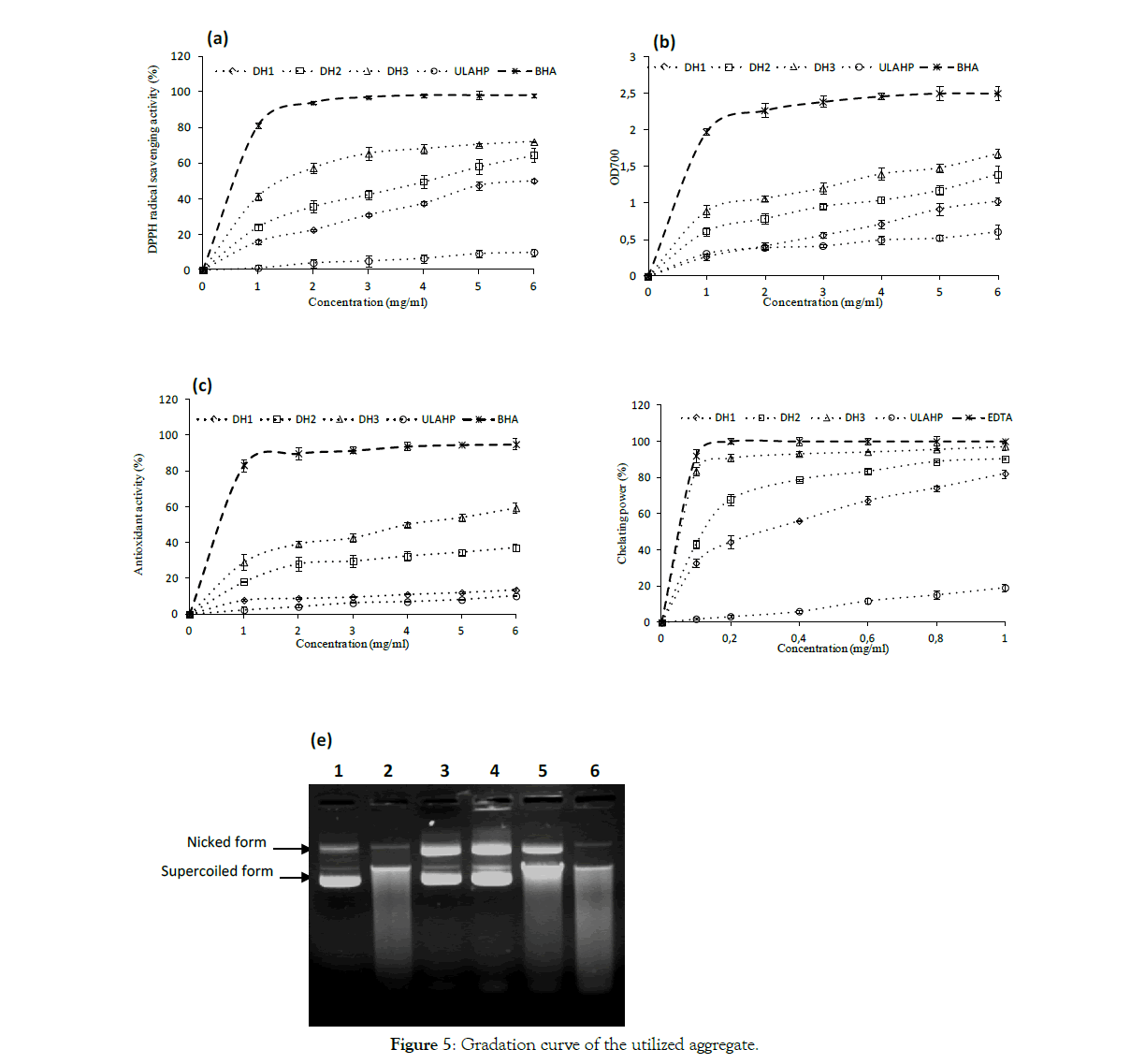
Figure 5:Gradation curve of the utilized aggregate.
Further, the DPPH radical scavenging activity increased with increasing DH (p<0.05). When DH increased from 12.4% to 15.4%, the DPPH radical-scavenging activity increased from 50.1% to 72% at a final concentration assay of 6 mg/ ml.
The obtained results indicate that the antioxidant activity of LAHPHs was affected by proteolysis. The higher radical-scavenging activity of DH3 with the highest DH could be due to higher content of low molecular weight peptides as well as free amino acids released during the enzymatic hydrolysis [46]. Indeed, several works have reported that hydrolysates with low molecular weight peptides generally possessed higher DPPH radical-scavenging activity than high molecular weight hydrolysates [40,47]. Our findings are in good accordance with previous research reported by Nasri et al. [11] and Sabeena Farvin et al. [48] who reported that protein hydrolysates exhibited significant DPPH scavenging activity in a concentration-dependent manner.
The above results indicated that the antioxidative activity of peptides depends on their molecular weights. DH3 hydrolysate possibly has more peptides which acted as a proton donor and could react with free radicals to convert them to more stable products and prevent the radical chain reaction.
Reducing power assay: In this assay, the presence of bioactive peptide in the samples resulted in reducing the Fe3+/ferricyanide complex to ferrous form and thus can be used to evaluate its potential antioxidant activity [49]. As presented in Figure 5b, the reducing power of all LAHPHs exhibited a dose-dependent effect, since the activity increased with the increase of concentration. Further, the reducing power increase as the DH increase. The highest activity (1.621% ±0.021) was observed for DH3, while lowest activity (0.995 ± 0.089) was exhibited by DH1.
Several works also reported the increase of antioxidant activity with the increase of DH [50–52]. The reducing power results revealed that LAHPHs had peptides which may exhibit donating capacity by neutralizing and/or converting free radicals to form stable products, and thereby, may terminate the chain reactions initiated by free radicals. Our results showed that DH have a greatly influence in the peptide chain length and therefore the bioactivity.
β-Carotene bleaching assay: The bioactivities of peptides using the discoloration of β-carotene is widely used to prove the antioxidant activity, as β-carotene is very sensitive to free radical mediated oxidation of linoleic acid [53]. In the absence of antioxidant β-carotene is rapidly discolored in the solution, which results in the increase in absorbance of the sample. However, the addition of antioxidant suppresses the extent of bleaching by neutralizing the linoleic hydroperoxyl free radical formed. Antioxidant potential of LAHPHs analyzed using β-carotene bleaching assay is presented in Figure 5c. All LAHPHs prevent the discoloration of β-carotene at different levels by donating hydrogen atoms to peroxyl radicals of linoleic acid. Hydrolysate with high DH showed the highest β-carotene bleaching ability compared to those of the other hydrolysates. However, the inhibitions of β-carotene bleaching by all LAHPHs were lower than that observed by BHA. Several works reported that the antioxidant activity of peptides in hydrolysates varies depending on their amino acids composition and lengths of peptides [54]. Since hydrolysates showed antioxidant activity in the emulsion model, this would imply the potential application of LAHPHs as antioxidants in food emulsion.
Ferrous ion chelating potential: The chelation of Fe2+ was used to demonstrate the ability of three hydrolysates in metal chelating activity. As reported in Figure 5d, LAHPHs, with different DH, showed very strong metal (Fe2+) chelating activity. Further, chelating activity of LAHPHs against Fe2+is inversely proportional to the DH value, and hydrolysate with a DH of 15.4% exhibited the highest ferrous- chelating activity value (97.14% at 1 mg/ ml) followed by DH2 (90.28% at 1 mg/ ml), while the lowest ferrous-chelating ability was obtained with DH1 (82.15% at 1mg/ ml) (p< 0.05) [55].
The above results indicate that the metal ion-binding capacity of LAHPHs was enhanced by enzymatic hydrolysis. A similar finding was reported by Klompong et al. [56] for Selaroides leptolepis hydrolysates, and by Thiansilakul et al. [12] for hydrolysates from Decapterus maruadsi muscle. However, LAHPHs showed lower metal chelating potential than obtained for positive control (EDTA), at all concentrations tested. Chelating potential of LAHPHs was higher than that of hydrolysates from zebra blenny obtained by crude enzyme treatment [32].
The obtained results correlated with antioxidant assays tested, and showed that antioxidant activity increases with DH.
DNA protection test: Antioxidative potential of LAHPHs using DNA protection assay was also studied. Figure 5e shows the agarose gel electrophoresis pattern of plasmid DNA treated with Fenton’s reagent with or without LAHPHs. Line 1 represents the native DNA with its nicked and supercoiled forms. Incubation of the plasmid DNA with Fenton's reagent in the absence of LAHPHs caused the degradation of the two DNA bands (lane 2).
Interestingly, addition of DH1 and DH2 hydrolysates prior to incubation with oxidizing agent resulted in a prevention of DNA breakage against hydroxyl radical. An increase in the intensity band of nicked form was observed, which may result from the conversion of the supercoiled form to the open circular form. The addition of the undigested L. aurata protein hydrolysate (ULAHP) caused the degradation of the two DNA bands (lane 6).
However, a little protection was obtained with hydrolysate having the highest DH. Most of the supercoild DNA was converted to open circular form, linear form and degraded DNA.
In terms of antioxidant assays tested, except the DNA nicking assay, DH3 hydrolysate possessed the highest activity. The differences observed between three LAHPHs in terms of antioxidant activities might be due to the difference in chain length as well as amino acid composition.
Anti-inflammatory activity: The 5-LOX inhibition by L. aurata hydrolysate byproduct was reported for the first time in the literature in this study. Table 4 depicts the anti-inflammatory activity of LAHPHs, ULAHP and Naprosyn (NAP). As expected, the DH3 exhibited the highest anti-inflammatory activity (57.4% ± 2.11) in comparison with DH2 (46.3% ± 1.09) and DH1 (35.0% ± 1.21), at the equal concentration. This result might be attributed to its richness in condensed anti-inflammatory peptides and oleic acid who is knows with his anti-inflammatory potential. The presented data correspond well with other results obtained for protein hydrolysates where noted high LOX inhibitory potential IC50 values of 0.79 ± 0.02 mg and 3.14 ± 0.06 for the peptide fractions from Tenebrio molitor and Gryllodes sigillatu [56]. ULAHP did not show any inhibition of the 5-LOX enzyme activity at the same concentration.
| Sample | Concentration (µg/ml) | Inhibition |
|---|---|---|
| NAP | 2.5 | 12.6 ± 0.93 |
| 5 | 35.9 ± 0.52 | |
| 25 | 97.1 ± 2.64 | |
| DH1 | 50 | 19.8c ± 0.37 |
| 100 | 28.2c ± 0.66 | |
| 200 | 35.0c ± 1.21 | |
| DH2 | 50 | 22.5b ± 0.28 |
| 100 | 34.9b ± 0.57 | |
| 200 | 46.3b ± 1.09 | |
| DH3 | 50 | 31.6a ± 1.43 |
| 100 | 43.8a ± 0.75 | |
| 200 | 57.4a ± 2.11 | |
| ULAHP | 50 | 0d |
| 100 | 0d | |
| 200 | 3.6d ± 0.78 |
Values are given as mean ± SD from triplicate determinations (n=3) L. aurata head protein hydrolysates were obtained by treatment with Neutrase (DH1), (DH2), (DH3) and undigested L. aurata head protein (ULAHP) and NAP as a positive control.
a-d Different letters within the same concentration and different sample indicate significant differences (p< 0.05).
Table 4: Anti-inflammatory activity of LAHPHs and ULAHP at various concentrations.
We should notice that the most prominent anti-inflammatory and antioxydant activities were found in the same most active protein hydrolysate: DH3. This result may be explained by the possible presence of a correlation between these activities such as a radical mechanism link.
Our work highlighted the significant influence of degree of hydrolysis, using L. aurata byproduct as substrate and neutrase as enzyme, in chemical composition, functional properties and on the antioxidant and anti-inflammatory activities. The protein content was determined to be highest in DH1, however the fatty acid content has demonstrated that oleic acid is much more abundant in DH3. The results indicated that the DH1 had high solubility, and good properties of emulsification. However, DH3 exerted more interesting inhibitory activity towards 5-LOX enzyme. Both DH2 and DH3 are endowed with very potent antioxidant activity, especially DH3, proved by four different spectrophotometric tests. These findings lead us to conclude that L. aurata head protein hydrolysate with highest degree of hydrolysis, could be considered as potential alternatives for synthetic antioxidants used in the food industry.
This work was funded by the Ministry of Higher Education and Scientific Research, Tunisia.
Citation: Bkhairia I, Kolsi RBA, Ghorbel S, Azzabou S, Ktari N, Nasri M (2019) Anti-inflammatory, antioxidant activities and fatty acid profile of three hydrolysates from Liza aurata by-product influenced by hydrolysis degree. Adv Tech Biol Med 7: 268.
Received: 22-Mar-2019 Accepted: 10-May-2019 Published: 17-May-2019
Copyright: © 2019 Bkhairia I, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.