Research Article - (2016) Volume 2, Issue 2
Antimicrobial and Anti-Inflammatory Activity of Press Cake of Jatropha curcas
*Corresponding Author: Yamini Bhushan Tripathi, Department of Medicinal Chemistry, Institute of Medical Sciences, Banaras Hindu University, Varanasi - 221005, Uttar Pradesh, India, Tel: 09415694450, Fax: 0542-2317074 Email:
Abstract
Several compounds present in fruits as Polyphenol, are able to kill or inhibit the growth of microorganisms. These properties are relevant mainly in tropical areas, as Amazonian region where infectious are highly prevalent. Therefore, this study investigated the antimicrobial activity of Jatropha curcas seed cake against microorganisms. The results showed antibacterial effect of Jatropha curcas seed cake methanolic extracts on two Gram-positive bacteria and Gram- negative bacteria. Effect of Jatropha curcas seed cake on Superoxide and nitric oxide production was also estimated in macrophage cells. This result suggests its anti-inflammatory and antibacterial potential of the herb, which could be due to the bio-active principles which are anti-inflammatory and antibacterial in nature. The present study, therefore emphasizes the use of Jatropha curcas seeds as an anti-inflammatory and antibacterial drug against macrophage cells and bacteria respectively.
Keywords: Anti-inflammatory; Jatropha curcas seed cake; Superoxide; Nitric oxide; Macrophage cells
Introduction
Jatropha curcas is also known as an industrial crop which belongs to the Euphorbiaceae family and cultivated mainly in tropical America, Africa, and Asia [1]. Since, its seed kernels contain a high amount of oil [58-60% (w/w)] [2], the seeds serve as a potential source of biodiesel fuel currently being used in India, other South East Asian countries and Thailand [3]. Its seeds also contain high protein, antinutritional factors, including phytic acid, saponin, trypsin inhibitor, lectin, and phorbol esters which is a toxic compound [3]. It has been known that part of J. curcas can be used for a wide range of purposes. Extracts from various parts of J. curcas, such as seeds, seed oil, leaves, root and stem have shown antimicrobial activities [4-6]. Leaf extract was found to be very effective in preventing azolla disease which is caused by the fungal pathogen Sclerotium sp. [7]. The chemicals responsible for those effects were suggested to be phorbol esters in the extract [8,9]. It also states that some derivatives of phorbol esters are known to have antimicrobial and antitumor properties. These are diterpenes having 20 carbon atoms which are made up of four isoprene units. They are generally found in plant species of the families Euphorbiaceae and Thymelaeceae. Recently, various forms of phorbol esters have been isolated from J. curcas aerial parts and seed oil [10-12]. Generally, due to toxicity it causes tumor promotion, skin inflammation, activation of blood platelets, tissue damage, lymphocyte mitogenesis, stimulation of degranulation in neutrophils in living cells and prostaglandin production [8,13]. J. curcas seed cake is generated in considerable quantities as a by-product of J. curcas seed oil extraction. This byproduct cannot be utilized owing to the presence of antinutritional factors and toxic compounds. The compounds, especially phorbol esters, can be extracted by using methanol and dichloromethane from J. curcas seed as an extracting solvent [3,14]. These solvents, however, are both harmful and relatively expensive. For extraction from various plant parts, such as Funtumia elastica bark extract, Mallotus oppositifolius leave extract [15], Casearia sylvestris leave extract [16], and Opuntia ficus-indica stem extract [17]. Ethanol is used as extracting solvent, but this was never used for phorbol ester extraction from J. curcas parts. In addition, to our knowledge, an antifungal activity of the extract from J. curcas seed cake has not been studied. In this report, antifungal properties along with antiinflammatory and anti-bacterial activity of the J. curcas seed cake were investigated. We tested ethanoic extract of the seed cake containing phorbol esters to determine its natural antifungal properties against important fungal phytopathogens and also for its anti-inflammatory and anti-bacterial activity.
Material and Method
Plant material and extraction
The identification of the plant was done by Dr K N Diwedi, Department of Dravayguna, Faculty of Ayurveda, Institute of Medical Sciences, Banaras Hindu University, Varanasi (India) Reference number “DG /KND/11-12/603” was given to plant sample.
Jatropha press cake (machine cake) was procured from the Government of India, DST Funded Speller Machine from Surya pharmaceutical Company D-17, Industrial area Ramnagar, Chandauli, India. The cake was dried in oven; maintained at 50ºC. One part of the cake was subjected to organic solvent extraction by using reflux method and obtained as methanolic extract of mechanically prepared seed-cake (MEMJC). And then hexane washed seed cake extracted with methanol, called as hexane washed seed cake total methanol fraction (MEHJC.)
All cultures were obtained from the American Type Culture Collection (ATCC), Microbial type Culture Collection (MTCC), and preserved at Department of Microbiology, Institute of Medical Sciences, BHU, and Varanasi, India. The young bacterial broth cultures were prepared before the screening procedure.
Preparation of sample
About 1g of each extract was dissolved in 10 ml (200 mg/ml and100 mg/ml) of peptone water to obtain a stock solution. The working solution was prepared by the dilution of stock solution i.e., 1:10 & 1:5 which was equivalent to 10 mg/ml and 20 mg/ml respectively from 5μl were L dispensed on a sterile disc of Whatmans filter paper No.1 of 6 mm diameter for susceptibility testing.
Antimicrobial susceptibility test
The disc diffusion method was used to screen the antibacterial activity [18,19] and antifungal activity. Muller Hinton agar (MHA) plates were prepared by pouring 15 ml of molten media into sterile petriplates [20-22]. The fresh grown bacteria were suspended in sterile saline to achieve concentration of 107 CFU/ml. This suspension was spread on the surface of MHA agar plates. The plates were allowed to dry for 5 min. The concentrations of extracts (200 mg/ml) were put on 6 mm, sterile disc of what man filter paper No.1. The disc was then placed on the surface of the medium and the compound was allowed to diffuse for 5 min and the plates were kept in incubation at 37°C for 24 hours for bacteria and 48 hours at 25°C for fungal agents. Inhibition zones were examined around the disc appeared at the end of incubation, which if present, were measured with a transparent ruler in millimetres. This study was performed in triplicate.
Determination of minimum inhibitory concentration (MIC) and minimum bactericidal concentration (MBC) and minimum fungicidal concentration (MFC)
MIC was determined by micro-dilution method [23] using serially diluted (2 folds) plant extracts according to the National Committee for Clinical Laboratory Standards (NCCLS) (National Committee for Clinical Laboratory Standards, 2000). The MIC of the extracts was determined by dilution of the polyherbal drug of various concentrations. Equal volume of each extract and nutrient broth were mixed in wells of Microtiter plate. Specifically 0.1 ml of standardized inoculums (1-2 × 107 CFU/ml) was added in each tube. The plates were incubated aerobically at 37°C for 18-24 h for bacteria and 48h at 250°C for fungal growth. Two control wells were maintained for each test batch. These included antibiotic control (containing extracts and growth media without inoculum) and organism control (a tube containing the growth medium, saline and the inoculum). The lowest concentration (highest dilution) of the extract that produced no visible bacterial growth (no turbidity) when compared with the control were regarded as MIC. However, the MBC and MFC were determined by sub-culturing the test dilution on to a fresh drug free solid medium and incubated further. The highest dilution that yielded no bacterial or fungal colony was taken as MBC and MFC
Media used
Muller-Hinton agar and broth (Hi-media, Mumbai, India), Sabouraud dextrose agar pH 7.3 ± 0.2 (Hi-media), were used for antibacterial and antifungal activity respectively.
Toxicity of drugs on macrophages
The toxicity study was done to select a safe dose for in vitro study. Adhered macrophages were treated with different concentration of drugs and drug vectors and placed in 5% CO2 at 37°C in incubator for 24 hrs. Cell viability was measured by MTT assay and % viability was calculated with respect to control (no treated cells).
MTT assay
Mitochondria of living cells produce succinate dehydrogenase enzyme which reduces MTT into blue colored formazan. For this assay MTT was dissolved in PBS at 5 mg/ml and its 20 μl was added to each well and incubated at 37°C for 4 hrs. The plate was centrifuged at 1500 RPM for 10 min and the supernatant was discarded. The formazan crystals were dissolved in 200 μl of Dimethyl Sulfoxide (DMSO). The absorbance was recorded in ELISA plate reader at 540 nm. Viability was related directly with absorbance. Increase in absorbance shows the concentration of living cells. With increasing the death of cells, absorbance decreases.
Estimation of NO production
Adhered cells were treated with different concentration of drugs and incubated for 30 min. These cells were further treated with 20 ng/ml LPS and re-incubated for 16 hrs in same condition. Supernatant (100 μL) was mixed with the Griess reagent (1% sulfonamide in water and 0.1% N-(-napthyl) ethylamine dihydrochloride in 5% H3PO4 and mixed as 1:1. (Griess 1879) After mixing supernatant with Griess reagent, it was left for 10 min at room temperature and absorbance was recorded at 540 nm. The amount of nitrite produced was compared with standard curve of NaNO2 and reported as μM NO2 produced by a fixed number of cells.
Super oxide production
Adhered macrophages were treated with different concentration of drugs parallel with their drug vector and incubated for 30 min at 37°C in 5% CO2. The cells were treated with 50 μl 100 μM H2O2 followed by 150 μL 750 μM NBT (dissolved in SOD buffer pH 7.8) and placed in fluorescent light for 1 hour. The absorbance was recorded in the Elisa plate reader at 540nm and comparison was made with respect to control group, i.e. cells treated with H2O2 only not a drug.
Results
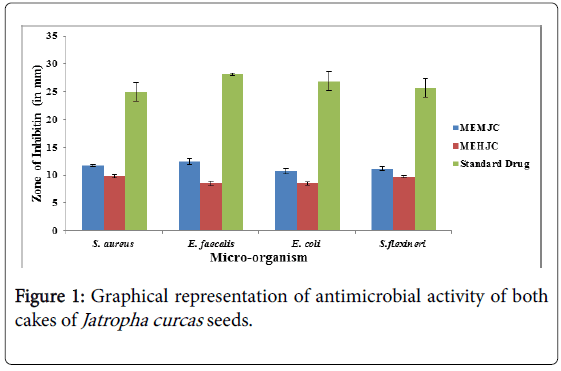
The result showed that the zone of inhibition was highest in the extract of MEMJC listed in (Figure 1) (methanolic extract of mechanical cake of Jatropha curcas) i.e.12.45 ± 0. 56, and minimum in MEHJC i.e. 8.53 ± 0. 42 against E. faecalis was used Ciprofloxacin as positive control and its zone of inhibition was 28.12 ± 2.52 and in the case of S. aureus maximum zone of inhibition was obtained in MEMJC i.e. 11.73 ± 0.20 and minimum zone of inhibition MEHJC i.e. 9.81 ± 0.22 and ampicilin were used as the positive control and its zone of inhibition was 24.93 ± 1.65.
The results of MIC and MBC for two herbal extracts of Jatropha curcas against pathogenic and spoilage micro-organisms are listed in (Table 1) which were expressed in mg/mL. The MIC values were ranging from 25 to 6.25 mg/ml. The MIC and MBC value was highest for MEMJC (i.e. 25 mg/ml and 25 mg/ml against S. flexineri respectively, and the minimum MIC and MBC was for MEMJC i.e. 6.25 mg/ml, 12.5 mg/ml against S. aureus respectively, which showed that the methanolic extract of mechanical cake of Jatropha curcas was more effective against S. aureus and the other microorganism.
| Strain | S. aureus | E. faecalis | E. coli | S. flexineri | ||||
|---|---|---|---|---|---|---|---|---|
| Extract | MIC | MBC | MIC | MBC | MIC | MBC | MIC | MBC |
| MEMJC | 6.25 | 12.5 | 12.5 | 25 | 12.5 | 12.5 | 25 | 50 |
| MEHJC | 12.5 | 25 | 25 | 50 | 25 | 25 | 25 | 50 |
Table 1: Determination of MIC, MBC (mg/ml) of both cakes of Jatropha curcas seeds.
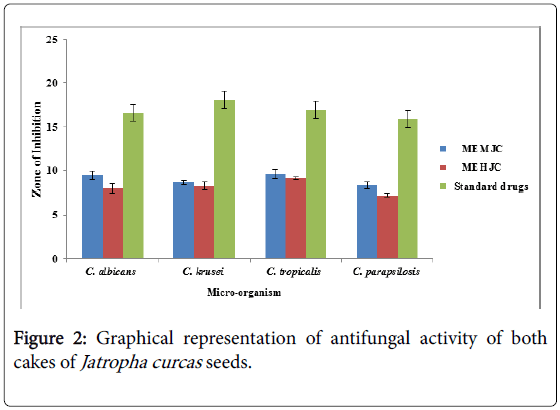
MEMJC listed in (Figure 2) exhibited the highest zone of inhibition in antifungal activity i.e. 9.60 ± 0.67, against C. tropicalis. It was the extract which showed high activity as compared to other extract of Jatropha curcas. And the minimum zone of inhibition MEHJC (methanolic extract of hexane washed seed cake of Jatropha curcas ) 8 ± 0.53, against C. albicans. The results of MIC and MFC for two herbal extracts of Jatropha curcas against pathogenic and spoilage microorganisms are listed in (Table 2) which were expressed in mg/ml. The MIC and MFC values were ranging from 12.5 to 50 mg/ml. The MIC and MFC value was highest for MEMJC 25 mg/ml, 50 mg/ml against C. albicans. The MIC and MFC lowest value was for MEMJC 12.5 mg/ml, 25 mg/ml against C. tropicalis which shows that the MEMJC were more effective against C. tropicalis.
| Strains | C. albicans ATCC 90028 | C.kruseiATCC 6258 | C. tropicalisATCC 750 | C. parapsilosisATCC 22019 | ||||
|---|---|---|---|---|---|---|---|---|
| Extract | MIC | MFC | MIC | MFC | MIC | MFC | MIC | MFC |
| MEMJC | 25 | 50 | 25 | 50 | 12.5 | 25 | 12.5 | 25 |
| MEHJC | 25 | 50 | 25 | 50 | 25 | 25 | 25 | 25 |
Table 2: Determination of the MIC, MFC (mg/ml) of Jatropha curcas seeds.
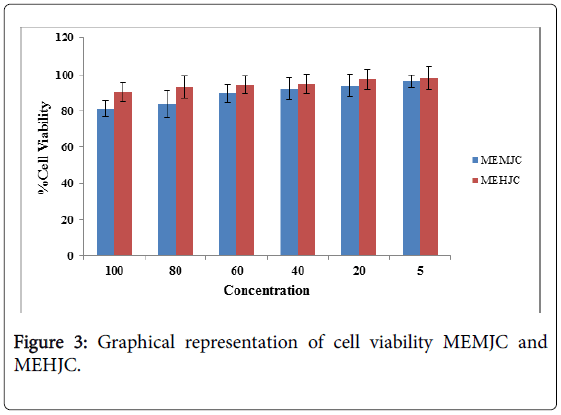
On the basis of MTT assay the cell viability with MEHJC is more than MEMJC at same concentration i.e. MEHJC is less toxic MEMJC in listed (Table 3 and Figure 3). Result was expressed in terms of % inhibition in comparison to control. No significant effect up to 100 to 20 ng conc. of MEMJC and MEHJC on superoxide production, but at concentrations of 5 ng in MEMJC and MEHJC showed significant in Superoxide production (Table 4).
| Drug Concentration ng/ml | MEMJC (%) | MEHJC (%) |
|---|---|---|
| 100 | 81.25±4.23 | 90.56 ± 5.34 |
| 80 | 83.93±7.23 | 93.18 ± 6.23 |
| 60 | 89.74±5.23 | 94.49 ± 4.67 |
| 40 | 92.19±6.23 | 95.09 ± 5.23 |
| 20 | 94.20±6.12 | 97.38 ± 5.67 |
| 5 | 96.43±3.45 | 98.17 ± 6.45 |
Table 3: Effect of cell viability MEMJC and Jatropha curcas MEHJC.
| Drug Concentration ng/ml | % Inhibition (Mean ± SD) | |
|---|---|---|
| MEMJC | MEHJC | |
| 5 | 60.12 ± 2.34 | 6.7 ± 1.23 |
| 20 | 30.8 ± 5.23 | 9.34 ± 1.56 |
| 40 | 28.1 ± 2.34 | 13.56 ± 3.12 |
| 60 | 26.6 ± 2.56 | 19.02 ± 2.12 |
| 80 | 12.2 ± 3.12 | 23.1 ± 3.78 |
| 100 | 9.5 ± 2.12 | 42.34 ± 2.23 |
Table 4: Effect of different concentration of MEMJC and MEHJC on LPS induced super oxide production in rat peritoneal macrophages in terms of % inhibition.
No significant effect up to 10 to 20 ng concentration of MEMJC and MEHJC on nitric oxide production, but at concentrations of 5 ng in MEMJC and MEHJC showed significant in nitric oxide production (Table 5).
| Drug Concentration ng/ml | % Inhibition (Mean ± SD) | |
|---|---|---|
| MEMJC | MEHJC | |
| 5 | 55.22±2.45 | 44.14±1.23 |
| 20 | 39.18±5.23 | 25.1±2.58 |
| 40 | 25.1±2.34 | 18.02±2.12 |
| 60 | 21.6±2.56 | 11.56±3.12 |
| 80 | 19.2±2.12 | 7.34±1.06 |
| 100 | 11.5±1.12 | 5.7±1.03 |
Table 5: Effect of different concentration of MEMJC and MEHJC on LPS induced Nitric oxide production in rat peritoneal macrophages in terms of % inhibition.
Discussion
Ancient Indian system of medicine (Ayurveda) was mainly based on herbal treatment. These classes (alkaloids, saponins, tannins, anthraquinones and flavonoids) of compound were known to have activity against several pathogens and therefore aid the antimicrobial activity of Jatropha curcas and suggested their traditional use for the treatment of various illnesses [24-26]. In all the four extracts, tannin was present that resulting in the inhibition of cell protein synthesis as it forms irreversible complexes with proline rich protein [27]. Tannin containing herbs have been reported for treatment of intestinal disorders such as diarrhea and dysentery, thus exhibiting antimicrobial activity [28] treatment of inflamed or ulcerated tissues, it was suggested that the presence of some compounds or groups in the extract with similar mechanism of action to that of the standard drug used in bacterial and fungal activity [29]. These observations therefore supported the use of Jatropha curcas in herbal cure remedies. Microorganism shows variable sensitivity to chemical substance related to different resistance level between strains [30]. Also reported that the crude methanol extract from the root of Jatropha curcas exhibited anti-diarrhoeal activity. Recently, reported that the presence of some secondary metabolites in the root extract of Jatropha curcas inhibited some microorganism isolated from sexually transmitted infections [31]. This may be attributed to the presence of soluble phenolic and polyphenolic compounds [32].
The crude Methanolic extracts of plants showed significant antimicrobial activity against test strains of gram-positive bacteria: B. amyloliquefaciens and S. aureus and gram-negative bacteria: E. coli and P. aeruginosa. The extracts revealed equal or higher broadspectrum antimicrobial activity as compared with standard antibiotics tested viz. ampicillin, erythromycin, and tetracycline. This indicated the great potential of these plant extracts as effective antimicrobial agents that can be used as single or in combination in medicines or can be used as natural food preservatives to retain the quality of food and prevent its spoilage. The both cakes of Jatropha curcas seeds were found rich in phytochemical constituents, which are responsible for their potent antimicrobial properties. These individual constituents of the extracts can be isolated, and further characterization as well as quantification can be done to explore the potential of these antimicrobial agents present in the extracts Free radicals of oxygen and nitrogen species (RONS) have several normal physiological roles, but their overproduction may cause many diseases such as diabetes, arthritis, ageing etc. RONS are natural and physiological modulators of cellular redox milieu and there by involving in signaling cascade and in control of a wide range of known and unknown physiological and patho-physiological processes. Despite of the multi-line antioxidant systems, the level of RON’s generation can exceed the capability of defense network, leading to oxidative stress [33]. It is generally assumed that an increase in aerobic metabolism or hypoxia easily generates increased level of RONS and causes oxidative damage to lipids, proteins and DNA. The increased level of RON’s production is not only due to the mitochondrial respiration, anaerobic exercise also could cause oxidative damage [34]. In normal respiration, molecular oxygen was released and a membrane bound protein diffuses molecular oxygen into radical form called super oxide radicals. These SO radicals further produce many other radicals such as hydroxyl radicals, nitric oxide, peroxy nitrite etc. by different mechanisms [35].
Several thousand molecules, having poly phenol structure (i.e., hydroxyl groups on aromatic rings) have been identified in higher plants as scavengers of these free radicals, and several hundred are found in edible plants. These compounds includes phenolic acids (Protocatechuric acid, Gallic acid), flavonoids, anthocyanidins Catechins, Gallocatechins, Phenyl propanoids (Eugenol) etc. [36]. These phytomolecules acts as scavengers of free radicals by rapid donation of hydrogen atom [37]. Addition to having antioxidant properties, polyphenols have several other specific biological actions and modulate the activity of a wide range of enzymes and cell receptors [38]. Here are several herbal preparations, studied by many authors for their antioxidant potentials e.g. Pueraria tuberosa [39], Vitex negundo [40], Mucuna pruriens [41], Scilla indica [42], Semicarpus anacardium [43], Rubia cardifolia [44] etc. In continuation of these herbal studies, We have made a study related to different concentration of Methanolic extract of mechanical cake of Jatropha curcas (MEMJC) and Methanolic extract of hexane washed seed cake of Jatropha curcas (MEHJC).
We induced superoxide and nitric oxide e by LPS (20 ng/ml) in macrophages as described earlier. Different concentration of MEMJC and MEHJC was checked for scavenging these free radicals (SO radicals and NO production in different extent in concentration dependent manner but we found that the % inhibition was maximum in concentration of 5ng of MEMJC and MEHJC only because of its low phenolic content. We didn’t get concentration dependent response.
Conclusion
MEMJC is showing higher degree of haemolysis and antimicrobial activity (Zone of inhibition and MIC) than MEHJC. It could be because of the presence of more curcin and phorbol ester in MEMJC. Hexane extract of Jatropha curcas seeds (MEHJC). It appears that after chemical processing, oil content of seed is completely being extracted. Because chemically isolated oil is more pure and more toxic towards haemolysis and antimicrobial activity. Different concentration of MEMJC and MEHJC was checked for scavenging these free radicals (SO radicals) and NO production in different extent in a concentration dependent manner, but we found that the % inhibition was maximum in concentration of 5 ng of MEMJC and MEHJC only because of its low phenolic content. We didn’t get concentration dependent response.
Acknowledgement
Authors are thankful to the Department of Medicinal Chemistry, Microbiology, Institute of Medical sciences, Banaras Hindu University, Varanasi India for providing the necessary laboratory facilities for the work. Authors are also thankful to the University Grant Commission (UGC-Delhi) for financial support.
Conflict of interest statement
We declare that we have no conflict of interest.
References
- Heller J (1996) Physic Nut: Jatropha Curcas L. Promoting the conservation and use of underutilized and neglected crops. Bioversity International, Italy.
- Aderibigbe AO, Cole Johnson, HPS Makkar, K Becker, N Foidl (1997) Chemical composition and effect of heat on organic matter and nitrogen-degradability and some antinutritional components of Jatropha meal. Anim Feed Sci Tech 67: 223-243.
- Martínez-Herrera J, Siddhuraju P, Francis G, Dávila-Ortíz G, Becker K (2006) Chemical composition, toxic/antimetabolic constituents, and effects of different treatments on their levels, in four provenances of Jatropha curcas L. from Mexico. Food Chem 96: 80-89.
- Sharma AK (2012) Comparative In Vitro Antimicrobial and phytochemical evaluation of methanolic extract of root, stem and leaf of Jatropha curcas Linn. Pharmacogn J 4: 34-40.
- Nwosu MO, JI Okafor (2016) Preliminary studies of the antifungal activities of some medicinal plants against Basidiobolus and some other pathogenic fungi. Mycoses 38: 191–95.
- Rug M, A Ruppel (2000) Toxic activities of the plant Jatropha curcas against intermediate snail hosts and larvae of schistosomes. Trop Med Int Health 5: 423-430.
- Carcia RP, Lawas MVP (1990) Note: Potential plant extracts for the control of Azolla Fungal Pathogens. Philipp AgricSci 73: 343-348.
- Goel G, Makkar HP, Francis G, Becker K (2007) Phorbol esters: structure, biological activity, and toxicity in animals. Int J Toxicol 26: 279-288.
- Gübitz G (1999) Exploitation of the tropical oil seed plant Jatropha curcas L. BioresourTechnol 67: 73-82.
- Haas W, Sterk H, Mittelbach M (2002) Novel 12-deoxy-16-hydroxyphorbol diesters isolated from the seed oil of Jatropha curcas. J Nat Prod 65: 1434-1440.
- Naengchomnong W, Thebtaranonth Y, Wiriyachitra P, Okamoto KT, J Clardy (1986) Isolation and structure determination of four novel diterpenes from Jatropha curcus. Tetrahedron Lett 27: 2439-2442.
- Ravindranath N, Ravinder Reddy M, Ramesh C, Ramu R, Prabhakar A, et al. (2004) New lathyrane and podocarpanediterpenoids from Jatropha curcas. Chem Pharm Bull (Tokyo) 52: 608-611.
- Aitken A (1986) The biochemical mechanism of action of phorbol esters. In: Naturally occurring phorbol esters. CRC Press 271-288.
- Devappa, Rakshit K, Makkar HP, Becker K (2012) Jatropha, challenges for a new energy crop. Plant Food Hum Nutr, Springer Publisher, USA.
- Adekunle AA, Ikumapayi AM (2006) Antifungal property and phytochemical screening of the crude extracts of Funtumiaelastica and Mallotusoppositifolius. West Ind Med J 55: 219-223.
- Da Silva SL, Chaar JD, Damico DCS, Figueiredo PDS, Yano T (2008) Antimicrobial activity of ethanol extract from leaves of Caseariasylvestris. Pharm Biol 46: 347-251.
- Lee JC, Kim HR, Kim J, Jang YS (2002) Antioxidant property of an ethanol extract of the stem of OpuntiaFicus-indicaVar. saboten. J Agric Food Chem 50: 6490-6496.
- Aladag H, Ercisli S, Yesil DZ, Gormez A, Yesil M (2009) Antifungal activity of green tea leaves (Camellia sinensis L.) sampled in different harvest time. Pharmacogn Mag 5: 437.
- Safaei-Ghomi J, Ahd AA (2010) Antimicrobial and antifungal properties of the essential oil and methanol extracts of Eucalyptus largiflorens and Eucalyptus intertexta. Pharmacogn Mag 6: 172-175.
- Bhandari DK, Nath G, Ray AB, Tewari PV (2000) Antimicrobial activity of crude extracts from Berberisasiatica stem bark. Pharm Biol 38: 254-257.
- Singh RK, Nath G (1999) Antimicrobial activity of Elaeocarpussphaericus. Phytother Res 13: 448-450.
- El Sohafy SM, Metwally AM, Omar AA, Harraz FM (2010) Phytochemical investigation and antimicrobial activity of PsidiumGuajava L. leaves. Pharmacogn Mag 6: 212.
- Adeniyi BA, Odufowora RA (2000) In-Vitro Antimicrobial properties of Aspilla Africana (Compositae). Afr J Biomed Res 3: 167-170.
- Griess P (1879) BemerkungenZu Der Abhandlung Der HH. Weselsky Und Benedikt, UeberEinigeAzoverbindungen. BerDtschChemGes 12: 426-428.
- Hassan MM, Oyewale AO, Amupitan JO, Abduallahi MS, Okonkwo EM (2004) Preliminary phytochemical and antibacterial investigation of crude extracts of the root bark of DetariumMicrocarpum. J ChemSoc Nigeria 29: 26-29.
- Usman H, Osuji JC (2007) Phytochemical and In Vitro antimicrobial assay of the leaf extract of Newbouldialaevis.” Afr J Tradit Complement Altern Med 4: 476-480.
- Shimada T (2006) Salivary proteins as a defense against dietary tannins. J ChemEcol 32: 1149-1163.
- Aliyu AB, Musa AM, J Oshanimi, Ibrahim HA, Oyewale AO (2008) Phytochemical analyses and mineral elements composition of some medicinal plants of Northern Nigeria. Nigerian J Pharm Sci 7: 119-125.
- Kapil A, Sharma S (1994) Anti-Complement activity of oleanolic acid: an inhibitor of C3-convertase of the classical complement pathway. J Pharm Pharmacol 46: 922-923.
- Mujumdar AM, Misar AV, Salaskar MV, Upadhye AS (2001) Antidiarrhoeal effect of isolated fraction (JC) of Jatropha curcas roots in mice. J Nat Remedies 1: 89-93.
- Aiyelaagbe OO, Adeniyi BA, Fatunsin OF, Arimah BA (2007) In Vitro antimicrobial activity and phytochemical analysis of Jatropha Curcas roots. Int J Pharmacol 3: 106-110.
- Kowalski, Radoslaw, Kedzia B (2007) Antibacterial activity of Silphiumperfoliatum extracts. Pharm Biol 45: 494-500.
- Askew EW (2002) Work at high altitude and oxidative stress: antioxidant nutrients. Toxicology 180: 107-119.
- Radák Z. (1998) The effect of high altitude and caloric restriction on reactive carbonyl derivatives and activity of glutamine synthetase in rat brain. Life sci 62: 1317-1322.
- Manach C, Scalbert A, Morand C, Rémésy C, Jiménez L (2004) Polyphenols: food sources and bioavailability. Am J ClinNutr 79: 727-747.
- Pasha Z (2007) Preconditioning enhances cell survival and differentiation of stem cells during transplantation in infarcted myocardium. Cardiovasc Res 77: 134-142.
- Middleton E Jr, Kandaswami C, Theoharides TC (2000) The effects of plant flavonoids on mammalian cells: implications for inflammation, heart disease, and cancer. Pharmacol Rev 52: 673-751.
- Pandey N, Tripathi YB (2010) Antioxidant activity of tuberosin isolated from Pueraria tuberose Linn. J Inflamm (Lond) 7: 47.
- Tiwari OP, Tripathi YB (2007) Antioxidant properties of different fractions of Vitexnegundo Linn. Food Chemistry 100: 1170-1176.
- Tripathi, Yamini B, Upadhyay AK (2002) Effect of the alcohol extract of the seeds of MucunaPruriens on free radicals and oxidative stress in albino rats. Phytother Res 16: 534-538.
- Tripathi YB, Upadhyay AK, Chaturvedi P (2001) Antioxidant property of Smilex china Linn. Indian J ExpBiol 39: 1176-1179.
- Tripathi YB, Singh AV (2001) Effect of Semecarpusanacardium nuts on lipid peroxidation. Indian J ExpBiol 39: 798-801.
- Tripathi YB, Sharma M (1999) The interaction of Rubiacordifolia with iron redox status: a mechanistic aspect in free radical reactions. Phytomedicine 6: 51-57.
Copyright: © Tripathi YB, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.