Journal of Horticulture
Open Access
ISSN: 2376-0354
ISSN: 2376-0354
Research Article - (2015) Volume 2, Issue 1
Nowadays it is known that resistance to antibiotics is often caused by biofilm formation of the microbial pathogen. The aim of this study is to evaluate the activity of Indonesian medicinal plants extracts on planktonic growth and biofilm of two bacteria species. Fifty-four (54) ethanol extracts were obtained from a variety of known Indonesian medicinal plants. The growth inhibitory concentration (MIC), effects on biofilm formation and biofilm breakdown, and biofilm architecture in the absence and presence of the extracts by confocal laser-scanning microscopy were performed with Pseudomonas aeruginosa PAO1 and Staphylococcus aureus Cowan I. The extracts showed an inhibitory effect on planktonic growth of these bacteria and also on their biofilm formation. At a concentration as low as 0.12 mg/mL, biofilm formation of P. aeruginosa PAO1 and S. aureus Cowan I is inhibited by 5 plant ethanol extracts Kaempferia rotunda L., Caesalpinia sappan L., Cinnamomum burmanii Nees ex Bl., C. sintoc L., and Nymphaea nouchali Burm.f. The limited bacteriostatic activity was evident. The results obtained clearly indicate the extracts obtained are interesting sources of putative antibiofilm agents. This research can contribute to the development of new strategies to prevent and treat biofilm infections.
<Keywords: Staphylococcus aureus Cowan I; Antibiofilm agents; Biofilm architecture
In former times, it was thought that microorganisms are freefloating single-celled (planktonic) organisms. They rapidly multiply and are living an individualistic lifestyle in nutrient rich media. However, in nature most microorganisms mostly live together in large numbers, attached to a surface, forming structured layers. This feature is known as a biofilm. A biofilm community can be formed by a single kind of microorganism, but in nature biofilms can also consist of mixtures of many species of bacteria, as well as fungi, algae, yeasts, protozoa etc. [1,2].
Biofilms of infectious microorganisms play an important role in human health [3] and because of their resistance to detergents and antimicrobial agents they are difficult to treat. The National Institute of Health (NIH) estimates that biofilms are involved in at least more than 65% of nosocomial infections and up to 75% of microbial infections occurring in the human body [4]. Biofilms of infectious microorganisms are also formed on medical instruments and implants such as catheters, artificial heart valves, contact lenses and artificial joints, putting patients at risk for local and systemic infectious [5,6]. In addition, the prevalence of microbial resistance to many commonly used antibiotics tends to increase these days. These findings enlarge the need for new antimicrobial compounds.
Since ancient times, man has used plants for healing, although incapable to find a rational explanation for their curing effects.According to the World Health Organization, the use of traditional medicine (TM) continues to play an important role in health care. In many parts of the world, it is the preferred form of health care. About 80% of people in developing countries, especially in rural areas, use TM as the primary source of medicine [7]. There are approximately 500.000 plant species occurring worldwide, and less than 1% has been screened for biologically active compounds [8]. The Indonesian Country Study on Biodiversity [9] places the number of species of flowering plants in Indonesia between 25.000 and 30.000. Of the total flora of Indonesia, 10% is expected to have pharmaceutical potential. There is a large variety of plants that are used as medicine [10].
Previously, antibiotic discovery and characterization has been performed mostly with planktonic bacteria. Therefore it can be predicted that compounds that are suitable to inhibit biofilm formation still need to be discovered. Up to now, only a few compounds, isolated from natural products with activity against microbial biofilm formation have been reported [11]. Eugenol isolated from clove showed inhibition of Candida albicans biofilm formation [12,13]. Aeromonas hydrophylla biofilm formation is inhibited by vanilin [14]. Usnic acid, a secondary lichen metabolite, is also capable to inhibit Pseudomonas aeruginosa biofilm formation [15]. In this study, we screened extracts of Indonesian medicinal plants with respect to their capacity to inhibit biofilm formation and or to breakdown the biofilms of two known human opportunistic pathogens, the Gram negative strain Pseudomonas aeruginosa PAO1 and the Gram positive strain Staphylococcus aureus Cowan I. P. aeruginosa and S. aureus are bacteria that cause nosocomial infections worldwide and can form biofilms which play an important role in various acute infections.
The plants investigated in this paper were those predicted and known to have antimicrobial properties based on the studied and local uses of the plants. The results of this screening may provide a powerful tool in the discovery of a successful treatment for biofilm infections.
Plant material and extraction
Indonesian medicinal plants were collected from Yogyakarta, Indonesia and its surroundings on the basis of ethnopharmacological information during January – May 2009. The plant materials were identified and authenticated by Djoko Santosa, M.Sc, Department of Pharmaceutical Biology, Faculty of Pharmacy, Gadjah Mada University, Yogyakarta, Indonesia. The voucher specimens were preserved at Department of Pharmaceutical Biology, Faculty of Pharmacy, Gadjah Mada University, Yogyakarta, Indonesia for further reference.
Plants samples (Table 1) were washed, cut into small pieces and oven dried (40°C) for 48-72 hours. The dried plant materials were ground into a fine powder. The pulverized materials were extracted by maceration using Petroleum Ether (PE) in a ratio of 1 g (plant material): 10 mL PE to remove the lipids. The plant material of which lipids have previously been removed were again extracted with 70% ethanol (EtOH) using a ratio of 1 g (plant material) : 10 mL (EtOH) to obtain crude ethanol extract. Furthermore, extracts were dried and concentrated under reduced pressure using a rotary evaporator. Stock solutions (100 mg/mL) of crude ethanol extract in dimethyl sulfoxide (DMSO) were prepared, filter-sterilized (0.2 μm) and stored at 4°C.
| Reference | Family | Binomial name | Local name | Part tested | Indication |
|---|---|---|---|---|---|
| STP001 | Zingiberaceae | Curcuma xanthorrhiza Roxb. | Temu lawak | Rhizome | kidney pain, back pains, asthma, headache, cold, ulcer, stomach pain, constipation, Chicken pox, sprue, acne |
| STP002 | Zingiberaceae | C. heyneanaVal. & v.Zijp | Temu giring | Rhizome | acne, scar, scabies, chickenpox |
| STP003 | Zingiberaceae | C. aeruginosa Roxb. | Temu hitam | Rhizome | launched a dirty bleeding after childbirth, skin diseases: scabies, rashes, and ulcers; abdominal pains (colic), sprue, cough, antihelmintics |
| STP006 | Zingiberaceae | Zingiber officinaleRoxb. | Jahe | Rhizome | Cough, Heartburn, Flatulence, Itching, Injury, headache, Cold, immunostimulant |
| STP007 | Zingiberaceae | Zingiber officinale Roscoe (var. rubrum Theilade) | Jahe merah | Rhizome | Antihistamine, cold, antirheumatism, cough, aphrodisiac, asthma, immunostimulant |
| STP004 | Zingiberaceae | C. domesticaL. | Kunyit | Rhizome | Diabetes mellitus, Typhoid, Dysentery, stomach pains during menstruation, immunostimulant |
| STP011 | Zingiberaceae | Kaempferia galangaL. | Kencur | Rhizome | For toothache, headache, cold, rheumatism, dyspepsia, stomachic, inflammation, cough, carminative, tonic and stimulant. |
| STP013 | Zingiberaceae | Boesenbergia pandurata(Roxb.) Schlecht. | Temu kunci | Rhizome | indigestion, abdominal pain, abdominal colic |
| STP005 | Zingiberaceae | C. manggaVal. & v.Zijp | Temu mangga | Rhizome | anticancer |
| STP012 | Zingiberaceae | Kaempferia rotundaL. | Temu putri (Temu putih) | Rhizome | scabies, dermatitis, flatulence, and other disorders of the digestive tract |
| STP014 | Zingiberaceae | Languas galanga(L.) Stuntz. | Lengkuas | Rhizome | Skin disease: Pityriasis versicolor, ringworm, scabies, sores, ulcers; rheumatism, headache, chest pain, improves digestion |
| STP008 | Zingiberaceae | Z. aromaticumVal. | Lempuyang wangi | Rhizome | asthma, stimulate appetite, reduce pain, blood cleanser, birth control, cramps relief, arthritis, cough, cholera, anemia, malaria, neurological diseases, abdominal pain |
| STP009 | Zingiberaceae | Z. zerumbet(L.)J.E. Smith | Lempuyang Gajah | Rhizome | Dysentery, skin disease, appetite enhancer, carminative, ulcus pepticum |
| STP015 | Zingiberaceae | Elettaria cardamomum(L.) Maton | Kapulaga | Fruit | cough, osteoporosis, |
| STP018 | Asteraceae | Cosmos caudatus H.B.K. | Kenikir | Leaves | Appetite enhancer, bone reinforcement and insect repellent |
| STP016 | Asteraceae | Sonchus arvensis L. | Tempuyung | Leaves | gall stones, dysentery, hemorrhoids, appendicitis, mastitis, abscess, hypertension, burns, bruise |
| STP019 | Asteraceae | Pluchea indica(L.) Less. | Beluntas | Leaves | Eliminate body odor, Digestive disorders, Scabies, rheumatitis, back pain (Lumbago) |
| STP017 | Asteraceae | Elephantophus scaberL. | Tapak liman | Leaves | Influenza, fever, tonsils, Sore throat, Sore eyes; Dysentery, diarrhea, snake bites, cough, ulcer, leucorrhea |
| STP020 | Asteraceae | Blumea balsamifera(L.) DC. | Sembung | Leaves | treat colds and mild hypertension, diuretic, astringent, expectorant, anti-diarrhea and anti-spasm |
| STP021 | Myrtaceae | Psidium guajavaL. | Jambu biji | Leaves | Diabetes mellitus, Maag, Diarrhea, Sprue, wound |
| STP022 | Myrtaceae | Syzygium aromaticum(Linn.) Merr. | Cengkeh | Flower | For toothache, carminative, stomachic, antiseptic anticarcinogenic, Cholera, dysentery, measles |
| STP024 | Apiaceae | Apium graveolens L. | Seledri | Leaves | Hypertension, eye pain, rheumatic |
| STP026 | Apiaceae | Foeniculum vulgareMill. | Adas | Fruit | Abdominal pain (heartburn), abdominal bloating, nausea, vomiting, diarrhea, jaundice (jaundice), cough, asthma, menstrual pain, insomnia, colic |
| STP027 | Piperaceae | Piper betleL. | Sirih | Leaves | Eczema, itchy skin, acne, Bleeding gums, Nosebleed, bronchitis, cough, Sprue, Leucorrhea, syphilis, Allergy, diarrhea, Toothache |
| STP029 | Piperaceae | P. retrofractumVahl. | Cabe jawa | Fruit | Stomach cramps, vomiting, flatulence, colic, dysentery, diarrhea, headache, toothache, cough, fever |
| STP040 | Combretaceae | Terminalia catappaL. | Ketapang | Leaves | Hipertension, malaria, rheumatism, thrush, diarrhea |
| STP041 | Meliaceae | Azadirachta indicaA.Juss | Mimba | Leaves | Appetite enhancer, dysentery, ulcers, malaria, anti-bacterial, eczema, scabies, stomach pain, reducing a fever. |
| STP039 | Oxallidaceae | Averrhoa bilimbiL. | Belimbing | Leaves | Cough, ulcers, stomach pain, Mumps (Parotitis), Rheumatism, toothache, Acne, hypertension |
| STP031 | Rutaceae | Citrus aurantifoliaSwingle | Jeruk nipis | Leaves | Tonsils, Malaria, Ambeien, influenza, cough, constipation, dysentery, Nausea, Fatigue |
| STP032 | Leguminosae | Tamarindus indicaL. | Asam jawa | Leaves | Asthma, cough, fever, rheumatism, abdominal pain, morbili, Allergy, Sprue, ulcers, eczema |
| STP034 | Leguminosae | Caesalpinia sappanL. | Secang | Bark | Diarrhea, dysentery, tuberculosis, wounds, syphilis, malaria, tetanus, tumors, inflammation of the mucous membranes |
| STP042 | Leguminosae | Sesbania grandifloraL.PERS var. Rubra | Turi | Leaves | Snake bite, antiulcer, Headache, swellings, anemia, bronchitis, pains, liver disorders, laxative, analgesic, fever (anti piretik), astringent, tonic, anti-tumour |
| STP033 | Poaceae | Andropogon citratus(DC.)Stapf. | Sereh | Leaves | Cough, for mouthwash and body warmers, appetite enhancer, postpartum treatment, fever and cramps relief |
| STP044 | Verbenaceae | Clerodendron serratum(L.) Spreng. | Senggugu | Leaves; Stem | cough, asthma, bruises, rheumatism, bronchitis, Stomach edema, antihelmintics, malaria |
| STP043 | Euphorbiaceae | Sauropus androgynus (L.) Merr. | Katu | Leaves | Fever, breastfeeding, Leprosy |
| STP045 | Acanthaceae | Andrographis paniculata (Burm.f) Nees. | Sambiloto | Leaves | Hepatitis, dysentery, typhoid, diarrhea, influenza, tonsillitis, malaria, pneumonia, bronchitis, toothache, fever, gonorrhea, Diabetes, cough (pertussis), asthma, hypertension |
| STP035 | Myristicaceae | Myristica fragransHoutt. | Pala | Seeds | Dysentery, Maag, Diarrhea, vomiting, Nausea, rheumatism |
| STP036 | Sterculiaceae | Guazuma ulmifoliaLmk. | Jati belanda | Leaves | Abdominal pain, flatulence, bronchitis |
| STP046 | Labiatae | Ocimum basilicumL. | Kemangi | Leaves | Acne, for disease in kidneys, bladder and urethra, antidepressant, fever, cough |
| STP047 | Lamiaceae | Orthosiphon stamineusBenth. | Kumis kucing (Remujung) | Leaves | diabetes, kidney and urinary disorders problems, high blood pressure and bone or muscle pain, diarrhea, leucorrhea, diuretic, anti-hypertension, nti-inflammatory |
| STP048 | Lamiaceae | Coleus scutellaroides(L.) Benth. | Iler | Leaves | emenagog, appetite stimulant, antitoxic, antiseptic, , ulcers and vermicide, gastritis |
| STP038 | Basselaceae | Anredera scandens(L.) Moq. | Binahong | Leaves | incision healing, typhoid, gastroenteritis, gout, dysentery, hypertension, diabetes melitus |
| STP037 | Thymelaeaceae | Phaleria macrocarpa(Scheff.) Boerl. | Mahkota dewa | Leaves | Dysentery, Psoriasis, acne, Skin diseases, anticancer |
| STP050 | Lauraceae | Litsea cubeba(Lours.) Pers. | Krangean | Bark | Antiinsect, improve digestion and to promote a restful sleep |
| STP051 | Lauraceae | Cinnamomum burmaniiNees ex Bl. | Kayu manis | Bark | stomach ulcers, nausea, vomiting, flatulence |
| STP052 | Lauraceae | C. sintocBl. | Sintok | Bark | Vermicida, antiacne, flatulence, fever |
| STP049 | Menispermeaceae | Tinospora tuberculataBeumee. | Bratawali | Leaves | Scabies, fever and diuretic |
| STP054 | Rubiaceae | Paederia foetidaL. | Daun kentut (Sembukan) | Leaves | Ulcers, skin infections, diuretic ,Colic, malnutrition, icteric hepatitis, whooping cough, leucopenia, pruritus, neurodermatitis, antiinflammation |
| STP055 | Melastomataceae | Melastoma polyanthumBl. | Cincau pohon | Leaves | Hipertension |
| STP056 | Annonaceae | Stelechocarpus burahol(Blume) Hook F. & Thomson | Kepel | Leaves | Pregnancy prevention, kidney inflammation, diuretic |
| STP058 | Acanthaceae | Stachytarpheta mutabilis(Jacq.) Vahl. | Keji beling | Leaves | Eliminate kidney stones |
| STP059 | Apocynaceae | Alyxia stellateRoem & Schult. | Pulasari | Bark | whooping cough, flatulence, leucorrheae, thrush, reduce fever and stop bleeding |
| STP060 | Apocynaceae | Parameria laevigata(A.Juss.) Moldenke | Kayu rapat | Bark | Antiulcer, antidiarrheal, to treat wounds, Reduce cholesterol, anti-inflammation, anti-oxidant, dysentery, Antipyretics, Desinfectan. |
| STP061 | Nymphaeaceae | Nymphaea nouchaliBurm.f. | Teratai merah | Flower | Malabsorbtion, antidhiarrhea, spermatorrhea, dysentery, impetigo, leucorrhea, impetigo |
Table 1: Indonesian medicinal plants tested for antibiofilm activity. *Reference is the Indonesian medicinal plants voucher number deposited at Department of Pharmaceutical Biology, Faculty of Pharmacy, Gadjah Mada University, Yogyakarta, Indonesia; local name is local Indonesian name, indication is/are the usage of the plant for medical application(s) according to Indonesia’s National Health Department.
Determination of growth inhibitory concentration (MIC)
Pseudomonas aeruginosa PAO1 and Staphylococcus aureus Cowan I were grown on LB agar plates at 28°C and 37°C, respectively. A single colony was inoculated in 5 ml LB broth. After overnight growth the OD600 was set to 0.01 (107 CFU/mL). Cells were incubated for 2 hours and the final OD600 was diluted to 105 CFU/mL. Inhibiting concentration of extracts were determined by the microtiter broth method in sterile flat-bottom 96-well polystyrene plates using Mueller- Hinton broth medium (Difco). Experiments were perfomed according to the Clinical and Laboratory Standards Institute (CSLI) guidelines [16], with concentration ranging from 0.06 to 1 mg/mL. Controls were: media control, infected untreated control (100% growth), DMSO as vehicle control, and streptomycin as positive control. All tests were performed in triplicate. Culture plates were incubated overnight at 37°C for S. aureus Cowan I and 28°C for P. aeruginosa PAO1. Optical density readings were obtained by using plate read outs at 595 nm.
Growth reduction was calculated as % of inhibition by using the formula mentioned below. The % of inhibition of replicate tests was used to determine the final MIC50 values. The concentration at which the extract depleted the growth of bacterial by at least 50% was labeled as the MIC50.
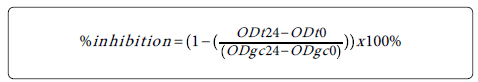
ODt24 = optical density (595 nm) of the test well at 24 h postinoculation; ODt0: optical density (595 nm) of the test well at 0 h postinoculation; ODgc24: optical density (595 nm) of the growth control well at 24 h post-inoculation; ODgc0: optical density (595 nm) of the growth control well at 0 h post-inoculation [17].
Effect on biofilm formation and biofilm breakdown
To test for the inhibition activity of plant extract on biofilm formation, a PVC (polyvinyl chloride) flexible U bottom 96 wells plates were used (Falcon 3911, Becton Dickinson, Franklin Lakes, NY. To determine biofilm formation inhibition and biofilm breakdown activity, extracts at sub inhibitory concentration (1/2 of MIC50) ranging from 0.03-0.5 mg/mL were used to ensure a non-toxic concentration. Negative controls (cells + media : TSB for S. aureus Cowan I and M63 supplemented with 20% casamino acid, 20% glucose and 1mM MgSO4 for P. aeruginosa PAO1), positive control (cells + media + streptomycin), vehicle controls (cells + media +DMSO), and media controls were included. For the positive controls concentrations of 128-512 μg/mL streptomycin were used, prepared by serial dilution techniques. Blanks undergo the same treatment as samples, but without incubation. All tests were performed in triplicate.
Plates were incubated for 24 h at 28&°C for P. aeruginosa and 48 h at 37°C for S. aureus . After 24-48 h incubation, the content of the well was aspired, rinsed 3 times with distilled water, and dried at room temperature for 10 min. Then, 125 μL of 1% crystal violet stain was added to the wells for staining for 15 min. The excess stain was rinsed off with tap water and 200 μL metanol was added to the wells, and transferred to a flat-bottom 96-well plates. Optical density readings were obtained by a plate reader at 595 nm. Biofilm formation inhibition was calculated as % of inhibition by using the formula mentioned below. The % of inhibition of replicate tests was used to determine the final IC50 values. The concentration at which the extract depleted the bacterial biofilm by at least 50% was labeled as the IC50.
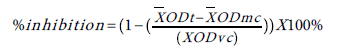
ODt= optical density (595 nm) of the test well; ODmc: optical density (595 nm) of the media control well; ODvc: optical density (595 nm) of the vehicle control well [17,18].
The efficacy of plant extract on established biofilm (biofilm breakdown) was also studied, as described by Nostro et al. [19] with some modifications. Biofilms were grown on 96-well plates for 24-48 h. At post-inoculation time, planktonic cells and media were removed and fresh media was added together with the test extract. Plates were placed back into the incubator for 24 h. The staining methods have been described above. Percentage of inhibition was calculated, as described before.
Biofilm architecture
Confocal laser-scanning microscopy (CLSM) was used to study the structure of the P. aeruginosa PAO1 and S. aureus Cowan I biofilms [20]. Bacterial biofilms were grown under static conditions on glass slides in sterile tubes. To examine effect of extract on inhibiting biofilm formation, fifteen ml of LB media in a sterile tube with or without plant ethanol extract was inoculated with the different bacteria to an OD600 of 0.1 from overnight grown LB cultures. Glass slides were submerged in this suspension and tubes were incubated for 24 h or 48h at 28 °C or 37 °C. For analysis the effect of extract in breaking down the biofilm, bacterial biofilm were grown under static conditions on glass slides in sterile tubes for 24 h or 48 h at 28 °C or 37°C. Following the incubation period, the suspensions of bacteria were removed and glass slides were rinsed with 0.15 M phosphate-buffered saline (PBS, pH 7.0) to remove unattached cells. Fifteen ml of LB media with or without plant ethanolic extract were poured into the tubes, and the tubes then incubated for another 24 h at 28 °C or 37°C.
Prior to CLSM analysis, glass slides were rinsed with 0.15 M phosphate-buffered saline (PBS, pH 7.0) to remove unattached cells. After a washing with PBS, the bacterial biofilm on the cover-glass slide was incubated for 15 min with 1.5 μL of 3.34 mM SYTO9 in anhydrous DMSO to stain the living organisms, and with 1.5 μL of 20 mM Propidium Iodide (PI) in anhydrous DMSO to stain the dead organisms. SYTO9 penetrates intact bacterial membranes (life) and stains the cells green; while PI penetrates only cells with damaged membranes (dead) and stains the cells red. The life organisms, freshly cultured and subsequently harvested, were used for control staining. Cells killed by heating in 100°C were used for control PI staining. Stained biofilms were observed with a Carl Zeiss LSM 5 Exciter Laser Scanning Confocal Microscope (Leica Microsystems, Germany). A 40× and 63x oil immersion objective was used with 488 nm Ar laser excitation and 500–640 nm band pass emission setting. The images were subsequently analysed using the freely available image processing software imageJ version 1.46 (Rasband, National Institutes of Health (NIH), Bethesda, Maryland, USA : http://rsb.info.nih.gov/ij/) including the LSM reader plugin to open LSM5 formatted image stack created by the microscope software. The images' scale bar used to calibrate the ImageJ area measurement algorithm. The observations were made in triplicates and representative images are presented here [21].
The image obtained has 2 channel (red and green) and was convert into a composite image with: Image>Color>Make composite. By default, it will assign red to channel #1, green to #2. Brightness and contrast levels were then adjusted to give the best differentiation between the live (green) and dead (red) areas. The scale bar was determined with : Analyze > Tools > Scale bar. Estimated 3D surface plot was obtained using : Plugins>3D>Interactive 3D Surface Plot. Data containing arrays of the type (x, y, z) where x and y are the coordinates of the pixel positioning and the luminance of an image is interpreted as height for the plot (z): http://rsbweb.nih.gov/ij/plugins/surface-plot-3d.html.
Statistical analysis
The data from the assay were compared using one-way analysis of variance (ANOVA) followed by student’s t test. Pearson’s correlation coefficient has been also used to measure the correlation between biofilm growth and planktonic growth of cultures. All calculations were carried out using SPSS 19 software. A P value of 0.05 or less was considered to be statistically significant.
Preparation of ethanol extract from 54 Indonesian plants
During this study, fifty four plants were collected in Yogyakarta, Indonesia and its surroundings. The ethanol extracts of these plants were obtained as described in Material and Methods. Briefly, plant materials of which lipids were previously been removedere macerated in an ethanol solution (70%). Crude ethanol extracts were obtained after filtration and the evaporation of this solution [22,23].
Effects of ethanol extracts on planktonic growth, biofilm formation and biofilm breakdown of P. aeruginosa PAO1 and S. aureus Cowan I
The scientific, family, and local names of the 54 Indonesian plant samples as well as their common medical usage are presented in Table 1. Plant extracts assayed in this research were selected based on their anti-bacterial activity that was reported in the literature [24]. The maximum concentration of 1 mg/mL of plant ethanol extracts for testing was chosen based on the previous study by Rios and Recio [25] who reported that extracts should be avoided exhibiting MIC values higher than 1 mg/mL or isolated compounds exhibiting MIC values higher than 0.1 mg/mL.
As shown in Table 2, most of the crude extracts used in this study have limited antibacterial activity against planktonic growth of P. aeruginosa PAO1 and S. aureus Cowan I. The lowest concentration of plant ethanol extract to inhibit growth of the bacterial tested shown by C. xanthorrhiza and M. fragrans which give 50% growth inhibition of P. aeruginosa PAO1 at concentration of 0.25 mg/mL, whereas C. xanthorrhiza and M. fragrans ethanol extract showed a MIC50 against the growth of S. aureus Cowan I at concentration of 0.12 mg/mL. In addition to testing of the plant extracts for inhibition of planktonic growth we also have investigated their effect on biofilm formation and biofilm breakdown. Crystal violet staining has been widely adopted by microbiologist to investigate biofilm formation and attachment of microorganisms to diverse surfaces. This staining method is inexpensive, relatively quick, and adaptable for use in high-throughput screening with microtiter plates [26].
| Plant | Antibacterial activity (MIC50) in μg/mL | Antibiofilm formation activity (IC50) in mg/mL | Biofilm breakdown activity (IC50) in mg/mL | |||
|---|---|---|---|---|---|---|
| P.aeruginosa PAO1 | S. aureus Cowan I | P.aeruginosa PAO1 | S. aureus Cowan I | P.aeruginosa AO1 | S. aureus Cowan I | |
| Curcuma xanthorrhiza Roxb. | 0.25 | 0.125 | - | - | ||
| C. heyneana Val. & v.Zijp | - | 0.5 | - | 0.5 | - | - |
| C. aeruginosa Roxb. | - | 0.25 | 0.25 | - | - | - |
| Zingiber officinale Roxb. | - | 0.5 | 0.25 | - | - | - |
| Z. officinale Roscoe var. rubrum Theilade | - | 0.5 | 0.25 | - | - | - |
| C. domestica L. | - | - | - | - | - | - |
| Kaempferia galanga L. | - | 0.5 | 0.25 | - | - | - |
| Boesenbergia pandurata (Roxb.) Schlecht. | - | - | - | - | - | - |
| C. mangga Val.&v.Zijp | - | 1 | - | 0.5 | - | - |
| Kaempferia rotunda L. | - | 0.5 | 0.125 | 0.125 | 0.5 | 0.5 |
| Languas galanga (L.) Stuntz. | 0.5 | 0.5 | - | - | - | - |
| Z. aromaticum Val. | - | - | - | - | ||
| Z. zerumbet (L.)J.E. Smith | - | - | - | 0.25 | - | - |
| Elettaria cardamomum (L.) Maton | - | - | - | 0.5 | - | - |
| Cosmos caudatus H.B.K | - | - | - | 0.5 | - | - |
| Sonchus arvensis L. | - | - | - | - | - | - |
| Pluchea indica (L.) Less. | - | - | 0.5 | - | - | - |
| ElephantophusscaberL. | - | 1 | - | - | - | - |
| Blumea balsamifera (L.) DC. | - | - | 0.5 | - | - | - |
| Psidium guajava L. | 1 | 1 | - | - | - | - |
| Syzygium aromaticum (Linn.) Merr. | - | - | - | - | - | - |
| Apium graveolens L. | - | - | - | 0.5 | - | - |
| Foeniculum vulgare Mill. | - | - | - | - | - | - |
| Piper betle L. | 1 | 0.5 | 0.5 | 0.25 | - | 0.5 |
| P. retrofractum Vahl. | 1 | 0.25 | 0.5 | - | - | - |
| Terminalia catappa L. | - | 1 | - | - | - | - |
| Azadirachta indica A.Juss | 1 | 1 | - | - | - | - |
| Averrhoa bilimbi L. | - | - | - | - | - | - |
| Citrus aurantifolia Swingle | - | - | - | - | - | - |
| Tamarindus indica L. | - | - | - | 0.5 | - | - |
| Caesalpinia sappan L. | 0.5 | 0.25 | 0.125 | 0.125 | 0.5 | 0.5 |
| Sesbania grandiflora L. PERS Leaves |
- | - | - | 0.5 | - | - |
| Andropogon citratus (DC.)Stapf. | - | - | - | - | - | - |
| Clerodendron serratum (L.) Spreng (Leaves) | 1 | 1 | - | - | - | - |
| Sauropus androgynus (L.) Merr. | - | - | 0.5 | 0.25 | - | - |
| Andrographis paniculata (Burm.f) Nees | - | - | - | - | - | - |
| Myristica fragrans Houtt. | 0.25 | 0.125 | - | - | - | - |
| Guazuma ulmifolia Lmk. | - | - | - | - | - | - |
| Ocimum basilicum L. | - | - | - | 0.5 | - | - |
| Orthosiphon stamineus Bth. | - | - | - | 0.5 | - | - |
| Coleus scutellaroides (L.) Benth. | - | - | - | - | - | - |
| Anredera scandens (L.) Moq. | - | - | - | - | - | - |
| Phaleria macrocarpa (Scheff.) Boerl. | - | - | - | - | - | - |
| Litsea cubeba (Lours.) Pers. | - | 1 | - | - | - | - |
| Cinnamomum burmanii Nees ex Bl. | 1 | 1 | 0.125 | 0.125 | - | 0.5 |
| C. sintoc L. | 1 | 0.5 | 0.125 | 0.125 | - | - |
| Tinospora tuberculata Beumee | - | - | - | - | - | - |
| Paederia foetida L. | - | - | - | - | - | - |
| Melastoma polyanthum Bl. | - | - | - | 0.5 | - | - |
| Stelechocarpus burahol (Blume) Hook F & Thomson | - | - | - | - | - | - |
| Stachytarpheta mutabilis (Jacq.) Vahl | - | - | - | - | - | - |
| Alyxia stellate Roem & Schult | - | - | - | - | - | - |
| Parameria laevigata (A.Juss) Moldenke | - | - | - | - | - | - |
| NymphaeanouchaliBurm.f. | 0.5 | 0.5 | 0.125 | 0.125 | 0.5 | 0.5 |
Table 2: Effects of ethanol extracts on planktonic growth, biofilm formation and biofilm breakdown of P. aeruginosa PAO1 and S. aureus Cowan I.
Using the crystal violet method, we have found that the inhibition of biofilm formation and biofilm breakdown by plant ethanol extract was dose dependent (1 and 2) in both P. aeruginosa PAO1 and S. aureus Cowan I. Plant ethanol extract concentration of 0.12 mg/mL is the lowest concentration which show 50% inhibition on P. aeruginosa biofilm formation (Table 2). Only five of the 54 extracts tested inhibit ≥ 50% of P. aeruginosa PAO1 biofilm formation at that concentration (Figure 1A). Ethanol extract of N. nouchali at concentration of 0.12 mg/mL inhibit P. aeruginosa biofilm formation as much as 54.7 ± 0.2%. 51.1 ± 0.5% and 53.3 ± 0.5% inhibition of P. aeruginosa biofilm formation were obtained by ethanol extract of C. sappan and C. burmanii respectively, and 51.0 ± 0.5% and 53.0 ± 0.2% by K. rotunda and C. sintoc respectively. In addition, 4 of 54 extracts show 50% inhibition on P. aeruginosa biofilm formation at an extract concentration of 0.25 mg/mL and 6 extracts at an extract concentration of 0.5 mg/mL (Table 2).
Figure 1: Percentage of inhibition in planktonic growth and biofilm formation of A) P. aeruginosa PAO1 or B) S. aureus Cowan I by plant ethanol extracts at different concentrations. (a) K. rotunda , (b) C. sappan , (c) N. nouchali , (d) C. burmanii , (e) C. sintoc . The standard deviation in the percentages are indicated by bar.
The lowest concentration which shows 50% of biofilm breakdown of P. aeruginosa (Table 2) is 0.5 mg/mL and only three of 54 extracts shows that activity. Nymphaea nouchali extract at a concentration of 0.5 mg/mL shows as much as 52.8 ± 0.2% degradation of the P. aeruginosa PAO1 preformed biofilm, and ethanol extracts of C. sappan and K. rotunda shows 52.1 ± 0.5% and 50.6 ± 0.5% degradation respectively (2A).
The lowest concentration of ethanol extracts which causes 50% inhibition on S. aureus Cowan I biofilm formation was also 0.12 mg/mL. As much as 51.0 ± 0.6% inhibition of S. aureus biofilm formation was observed by incubation with K. rotunda ethanol extract at concentration of 0.12 mg/mL. At the same concentration, N. nouchali , C. sappan , C . burmanii and C. cintoc cause 53.4 ± 0.5%, 52.5 ± 0.5%, 51.0 ± 0.5% and 50.6 ± 0.5% inhibitions of S. aureus biofilm formation ( 1B). In addition, 4 of the 54 extracts show 50% inhibitions on P. aeruginosa biofilm formation at an extract concentration of 0.25 mg/ml and 10 extracts at an extract concentration of 0.5 mg/mL (Table 2).
Similar as for breakdown of preformed biofilm of P. aeruginosa , only 5 of the 54 ethanol extracts caused 50% breakdown of preformed biofilm of S. aureus Cowan I at an extract concentration of 0.5 mg/mL.
In the presence of the ethanol extract of C. sappan at a concentration of 0.5 mg/ml, preformed biofilm of S. aureus was decreased as much as 53.8 ± 0.4%. At the same concentration, C. burmanii , P. betle , K. rotunda and N. nouchali show the capability to degrade S. aureus biofilm as much as 50.0 ± 0.2%, 51.1 ± 0.9%, 50.1 ± 0.3% and 52.8 ± 0.3%, respectively (2B).
Statistical test using Pearson’s correlation coefficient was carried out to obtain information whether there is significant correlation between the inhibition of bacterial growth and the inhibition of bacterial biofilm formation by the plant extract tested. The result showed that there is significance correlation (P value<0.05 or 0.01) between the activity of K. rotunda, C. sappan, C. burmanii, C. sintoc and N. nouchali ethanol extract in inhibiting the growth and biofilm formation of the bacterials tested.
Qualitative analysis of P. aeruginosa and S. aureus biofilm
The activity of the extracts on the biofilm formation inhibition and biofilm breakdown was analysed by confocal laser scanning microscope (CLSM), along with LIVE/DEAD staining as described in Material and Methods. Examples of estimated 3D surface plot of the biofilm are shown in s 3 and 4.
Qualitative analysis of biofilm structure by CLSM indicated an evident disruption of the biofilm structure resulting from exposure to plant extract (Figures 3 and 4). Viability staining using LIVE/DEAD staining showed that both life and dead cells were present in the analyzed biofilms. The control cells fluorescened green indicating that the cells were alive, embedded in a polysaccharide matrix that stimulates cell clustering.
Figure 3: a. Biofilm inhibition activity of C. burmanii ethanol extract against P. aeruginosa PAO1, and b. biofilm inhibition activity of N. nouchali ethanol extract against S. aureus Cowan I. 1 and 3 : projected upper view of the biofilm, 2 and 4 : estimated three-dimensional surface plot of the biofilm refers to the total area in the x-y-z dimension, where x and y are the coordinates of the pixel positioning and z is the intensity collected using ImageJ. Extract concentration from 0.5 mg/mL – 0.03 mg/mL. Negative control is P. aeruginosa PAO1 and S. aureus Cowan I biofilm without extract.
Figure 4: a Biofilm breakdown activity of K. rotunda ethanol extract against P. aeruginosa PAO1, and b. biofilm breakdown activity of P. betle ethanol extract against S. aureus Cowan I. 1&3 : projected upper view of the biofilm, 2&4 : estimated three-dimensional surface plot of the biofilm refers to the total area in the x-y-z dimension, where x and y are the coordinates of the pixel positioning and z is the intensity collected using ImageJ. Extract concentration from 0.5 mg/mL – 0.03 mg/mL. Negative control is P. aeruginosa PAO1 and S. aureus Cowan I biofilm without extract.
Ethanolic extracts from K. rotunda , C. sappan , C. burmanii , C. sintoc , and N. nouchali at concentration of 0.12 mg/mL significantly reduced P. aeruginosa PAO1 and S. aureus Cowan I initial biofilm formation compared to the negative control (biofilm cells without addition of plant extract) which is densely packed (Figures 3 and 4). The initial biofilm formation inhibition by plant ethanolic extracts was found to be concentration dependent. The presence of 0.25 mg/mL extract resulted in loss of aggregates structures. The cells were found scattered individually along the substratum (Figure 3).
At concentration of 0.5 mg/ml, the ethanol extracts from C. sappan , K. rotunda and N. nouchali showed capability in reducing preformed biofilm of both bacterial tested even more than at a concentration of 0.25 mg/mL (Figure 4). The preformed biofilm of S. aureus Cowan I was also disrupted by ethanol extract at concentration of 0.5 mg/mL. The biofilm exposed to the plant extracts were disrupted, leaving small aggregates which are remained attached to the substrate compare to the densely packed cells in biofilm control (without the presence of extract) (Figure 4).
CLSM images showed that plant ethanol extracts tested significantly prevent the formation of biofilm at concentration of 0.12 mg/mL. Compared to the cells in the control which is formed cells clusters and attached to the substratum, the amount of cells in the clusters, embedded in the EPS matrix was diminished with the presence of plant extract. It seems that bacterial growth was inhibited before the cells were able to promote attachment on the surface. However the result showed that activity on biofilm breakdown was more difficult to achieve than inhibition in cell attachment. The concentration of plant extract needed to disrupt preformed biofilm was higher (0.5 mg/mL) than the concentration needed to inhibit the initial attachment. It is evident that cells in a biofilm are more resistant to antimicrobial agents compare to free floating cells [27]
The cell attachment is the initial stage in biofilm formation following formation of film consists of nutrients, organic and inorganic molecules adsorbed on a surface (surface conditioning). The surface conditioning is important for the growth of cells and often creates a favorable environment for bacterial attachment, which in turn promotes cell adhesion to surfaces which subsequently leads to infections [18]. It can therefore be postulated that the presence of plant extracts in growth media produced an unfavorable condition that could inhibit cell attachment or reduce the surface adhesion [28,29].
The reduced susceptibility of bacteria in a biofilm is thought to be due to a combination of several factors. The presence of extracellular polymer substances (EPS) containing mainly polysaccharides, proteins and nucleic acids and other compounds that surrounds biofilm cells contribute to the antimicrobial resistance properties of biofilms by impeding the mass transport of antibiotics through the biofilm [5]. The antimicrobial agent is adsorbed onto the EPS and effectively diluted before it reaches the individual bacterial cells in the biofilm [30]. Killing by many antimicrobial agents is growth dependent by targeting macromolecular synthesis. Reduction in oxygen and nutrients availability in biofilm leads to cell growth limitation and bacterial macromolecular synthesis is arrested. This among others makes the bacterial cells in the biofilm less susceptible to antimicrobial agents [27,31].
Our study suggests that the inhibition activity of the plant ethanol extract of bacterial biofilm formation and the disperse existing of biofilms appears to be coupled with biocidal/biostatic activity. These results are helpful for designing novel biofilm inhibitors and developing more effective therapeutic methods.
The activity of K. rotunda, C. burmanii, C. sappan, C. sintoc and N. nouchali ethanol extracts to inhibit P. aeruginosa PAO1 and S. aureus Cowan I initial biofilm formation and degradation of formed biofilm has not been reported previously. It has been reported that Kaempferia rotunda contains flavonoids, crotepoxide, chalcones, quercetin, flavonols, β-sitosterol, stigmasterol, benzoic acid, syringic acid, protocatechuic acid and some hydrocarbons. The abundant presence of flavonoids in this plant has interpreted as the involvement in antioxidant mechanisms as a prime role [32]. Resins, tannin and essential oils which contains cinnamaldehyde, cinnamyl acetate, eugenol and anethole are present in C. burmanii bark. Other chemical components of the essential oil include ethyl cinnamate, betacaryophyllene, linalool and methyl chavicol. Eugenol oil that can be used as an ingredient in cosmetics is also present in C. sintoc bark [33]. Especially cinnamaldehyde and eugenol are proved to be active against many pathogenic bacteria, and fungi [34-36].
Phytochemical investigations on heartwood and other parts of C. sappan (sappan wood), also chave resulted in reports of various compounds including triterpenoids, lipids, amino acids, flavonoids and phenolic compounds such as 4-O-methylsappanol, protosappanin A,18 protosappanin B, protosappanin E, brazilin, brazilein, caesalpin J, brazilide A, neosappanone A, caesalpin P, sappanchalcone, 3- deoxysappanone, 7,3′,4′-trihydroxy-3-benzyl-2H-chromene [37,38]. From Brazilin it is known that it has antibacterial activity and has the potency to be developed into an antibiotic [39].
N. nouchali (red and blue water lily), synonym N. stellata Willd flowers, contain quercetin, luteolin, isoquercitrin, kaempferol, galuteolin, and alkaloids. The seeds are rich in starch, and also contain raffinose, proteins, fats, carbohydrates, calcium, phosphorus, iron, nuciferine, oxoushinsunine, N-norarmepavine. The rhizome contains starch, protein, asparagine, and vitamin C. It also contains catechol, dgallocatechol, neochlorogenic acid, leucocyanidin, leucodelphinidin, and peroxidase. The roots contain tannic and asparagine. The leaves of this plant contain roemerine, nuciferine, nornuciferine, armepavine, pronuciferine, N-nornuciferine, DN-methylcoclaurine, anonaine, liriodenine, quercetin, isoquercitrin, nelumboside, citric acid, tartaric acid, malic acid, gluconic acid, oxalic acid, succinic acid, and tannin. It has been found that oxoushinsunine, found on the seed coat, suppress the development of throat cancer while the seeds and stalks have efficacy as anti-hypertension [9,40].
Biofilm formation can be controlled by quorum sensing, a bacterial communication system which causes a rapidly and coordinately change of expression pattern in the bacterial population in response to population density. The fact that in sub-MIC concentration, the K. rotunda, C. sappan, C. burmanii, C. sintoc and N. nouchali ethanolic extracts are capable to disturb biofilm formation and biofilm breakdown suggests that this disturbance may has been caused by the presence of compounds inhibiting quorum sensing. Similarly, Rasmussen et al. [41] reported that carrot, garlic, habanera and water lily produce compounds that interfere with bacterial quorum sensing. Further studies need to be performed to confirm whether the antibiofilm activity from these extracts is due to quorum sensing inhibition.
Assignment of the active compound to one of these groups is often the first step in determining the identity of the compound. Characterization of the active antibiofilm compound(s) is needed to gain a deeper understanding of the active compounds that affect the biofilm formation of P. aeruginosa PAO1 and S. aureus Cowan I and to develop a possible antibiofilm therapeutic.
We gratefully acknowledge the funds support of this research by the Indonesian Directorate General for Higher Education. We thank Gerda Lammers (Institute Biology Leiden, Leiden University) for technical assistance in CLSM and Djoko Santosa, M.Sc (Pharmacognosy Laboratory, Faculty of Pharmacy, Gadjah Mada University) for the plants taxonomy identification and authentication.