Research Article - (2015) Volume 1, Issue 1
Antimicrobial Efficacy of Different Solvent Extracts of Tagetes erecta L. Flower, Alone and in Combination with Antibiotics
*Corresponding Author: Sumitra Chanda, Phytochemical, Pharmacological and Microbiological Laboratory, Department of Biosciences (UGC-CAS), Saurashtra University - Rajkot, 360 005, Gujarat, India Email:
Abstract
Bacteria have evolved numerous defences against antimicrobial agent and drug resistance in pathogen is on rise. This is due to rapid development of multi-drug resistance, limited anti-bacterial spectrum and adverse effects of available anti-microbial agents. This necessitates the search for new antimicrobials with diverse structures and novel mechanism of action. Flowers are mostly used for ornamental purposes and they are not frequently worked out hence marigold flower was selected for the study. The antimicrobial activity of different solvent (Hexane, Toluene, Ethyl acetate, Acetone, Methanol and Aqueous) extracts of marigold flowers were evaluated by agar well diffusion method against a panel of pathogenic microorganisms. The minimum inhibitory concentration (MIC) and minimum bactericidal concentration (MBC) of acetone extract and its fraction were evaluated. B. cereus and K. pneumonia were the most sensitive organisms to acetone extract and minimum inhibitory concentration (MIC) was 78 μg/ml. Synergistic effect of acetone extract and commercial antibiotics (chloroamphenicol and ceftazidime) against pathogenic bacteria was investigated. The best synergistic activity was with combination of acetone extract and ceftazidime against B. subtilis and P. aeruginosa with FIC indices 0.312 and 0.093 respectively. Only the polar solvent acetone extract showed promising antibacterial and synergistic activity. These results indicate that combination between plant extract and the antibiotics could be useful in fighting emerging drug-resistant microorganisms and choice of the solvent plays a prominent role in evaluating antimicrobial activity of medicinal plants. Flowers can be taken as an alternative source of antimicrobial agent against the human pathogens.
Keywords: Multidrug resistant; Medicinal plants; Solvents; Antimicrobial activity; Synergistic activity.
Introduction
The progressive emergence of multi-drug resistant bacteria and the infectious diseases caused by them is a major problem. Indiscriminate use of antibiotics has led to multiple resistances by pathogenic microorganisms. Clinically significant bacteria like methicillin resistant S. aureus (MRSA), vancomycin resistant enterococci and extended-spectrum β-lactamase (ESBL) producing E. coli are increasing rampantly [1,2]. Also, antibiotics sometimes cause side effects on the host like hypersensitivity, immune-suppression and allergic reactions [3]. This burning problem has generated the colossal need to investigate alternative sources of treatment with fewer side effects to combat the activities of these pathogens [4].
Synthetic antimicrobial agents are ineffective against multi-drug resistant bacteria and it is cause the global trouble for the lost of budget use for the treatment infectious diseases. So, there is urgent need to develop alternative antimicrobial drugs for the treatment of infectious diseases. One approach is use of medicinal plants screen for their possible antimicrobial properties which is novel, inexpensive and effective against pathogenic microorganism [5]. Herbal medicine is most popular for treatment of disease because it is easily available, cheap, less side effects as compared to antibiotic [6].
From ages plants have been used for curing and restoring health as they contain components of therapeutic value. Medicinal plants used in folklore remedies have drawn interest of researchers in discovering solutions to multiple drug resistance against commercial antimicrobial drugs. Many naturally occurring compounds found in plants have shown to possess antifungal, antibacterial and antiprotozoan activities and serve as a source of antimicrobial agents that could be used either systemically or locally [7,8]. The antimicrobial activity of medicinal plant extracts is very well documented [9-12]. Also the recently reported concept of drug synergism between known antimicrobial agents and bioactive plant extract can also be implemented as a new form of treatment of infectious diseases [13]. Combinations of agents with different lethal mechanisms may have synergistic or additive antimicrobial activities [14]. Thus combination therapy also helps patients with severe infections due to drug resistant pathogens [15].
Tagetes erecta, popularly known as marigold, is grown as an ornamental plant. Flowers of this plant are used loose or in garlands for social and religious purposes in Eastern countries. The flowers are usually thrown away after their religious uses. This plant belongs to the family Asteraceae (Compositae). The English name if marigold. Different parts of this plant including flower is used in folk medicine. In traditional and homeopathic medicine it has been used for skin complaints, wounds and burns, conjunctivitis and poor eyesight, menstrual irregularities, varicose veins, hemorrhoids, duodenal ulcers, etc. [16]. The flowers are especially employed to cure eye diseases, colds, conjuctivites, coughs, ulcer, bleeding piles and to purify blood [17-19].
The aim of the present study was to extract active constituents of T. erecta flowers and evaluate their antimicrobial potential. For this, six different solvents of varying polarity viz. hexane, toluene, ethyl acetate, acetone, methanol and water were used. This would help to determine the best solvent responsible for extraction of maximum active phytoconstituents which are responsible for antimicrobial efficacy of T. erecta flower. Hence in the present study, six different solvents were used for extraction and an effort was also made to understand the relationship between the plant extract obtained using different solvents and their corresponding antimicrobial activity. The extract showing best antimicrobial activity was chosen for fractionation. The MIC of crude extract and its fractions were determined. In addition to that, the synergistic effect of extract combined with commercial antibiotics against pathogenic microorganisms was investigated. To our best of knowledge so far there are no studies on the synergistic effects of marigold flower extracts with antibiotics. Thus, this study brings novel and valuable information to this area.
Materials and Methods
Chemicals
Nutrient broth, Sabouraud dextrose broth, Mueller Hinton agar no. 2, Sabouraud dextrose agar, chloramphenicol and ceftazidime were obtained from Hi-Media, Mumbai, India; Solvents hexane(HE), toluene(TE), ethyl acetate(EA), acetone(AC), methanol(ME) were obtained from Merck, India.
Plant material
Fresh flowers of marigold were collected August- 2013 from Rajkot, Gujarat, India. Flower was identified by comparison with specimens at Department of Biosciences (SU/Bio/517Thkarar), Saurashtra University, Rajkot, Gujarat, India. The flowers were washed thoroughly with tap water, petals were separated and shade dried. The dried petals were homogenized to fine powder and stored in air tight bottles which were later used for solvent extraction.
Extraction and Fractionation
The dried powder of the flower petals was extracted individually by the cold percolation method [20] using different organic solvents like hexane, toluene, ethyl acetate, acetone, methanol and water. Ten grams of dried powder was added to 100 ml of hexane in a conical flask, which was plugged with cotton wool and kept on a rotary shaker at 120 rpm for 24 h. After 24 h, the extract was filtered with 8 layers of muslin cloth and centrifuged at 5000 rpm for 10 min. Supernatant was collected and the solvent was evaporated. The residue was then added to 100 ml of solvents (toluene, ethyl acetate, acetone, methanol and water) in different conical flasks, which were plugged with cotton wool and kept on a rotary shaker at 120 rpm for 24 h. After 24 h, the extract was filtered with 8 layers of muslin cloth and centrifuged at 5000 rpm for 10 min. The supernatant was collected and the solvents were evaporated; the dry extract was stored at 4°C in airtight bottles. The extracts were weighed to obtain the extraction yield. Fractionation of acetone extract of flower was made by solvent-solvent partition method [21].
Antimicrobial susceptibility testing
Test organisms
The microorganisms used were obtained from the National Chemical Laboratory, Pune, India. The microorganisms were maintained at 4°C. The Gram-positive bacteria studied were Bacillus cereus (BC) ATCC11778, Bacillus subtilis (BS) ATCC6633, Staphylococcus aureus 1 (SA1)ATCC25923, Staphylococcus aureus 2 (SA2) ATCC29737, Staphylococcus albus (SAL) NCIM2178, Bacillus megaterium (BM) ATCC9885, Listeria monocytogenes (LM) ATCC19112 and Corynebacterium rubrum (CR) ATCC14898. The Gram-negative bacteria were Escherichia coli (EC) NCIM2931, Pseudomonas pseudoalcaligenes (PPA) ATCC17440, Pseudomonas testosterone (PT) NCIM5098, Proteus morganii (PMO) NCIM2040, Pseudomonas aeruginosa (PA) ATCC27853, Enterobacter aerogenes ATCC13048, Klebsiella pneumoniae (KP) NCIM2719 and Proteus mirabilis (PMI) NCIM2241. Yeasts were Candida albicans (CA) ATCC2091, Cryptococcus neoformans (CN) NCIM3542, Candida glabrata (CG) NCIM3448 and Candida epicola (CN) NCIM3367.
Antibiotics used in this study
The antibiotics ampicillin (AMP10μg/disc), chloramphenicol (CH30 μg/disc), tetracycline (TE30 μg/disc), penicillin-g (P100 unit), gentamicin (GEN10 μg/ml), polymyxin –B (PB100 unit), cephalothin (CEP30 μg/ml), amoxyclav (AMC30 μg/ml), amphotericin (AMP100 unit) and nystatin (NS100 units/disc) were used for antimicrobial susceptibility test. The antibiotics chloramphenicol and ceftazidime were used for MIC study. All antibiotics were purchased from Hi-Media Laboratory Pvt. Ltd., (Mumbai, India).
Screening for antimicrobial activity (agar well diffusion assay)
In vitro antimicrobial activity of all different extracts of T. erecta was determined by standard agar well diffusion assay [49,50]. Mueller Hinton agar and Sabouraud dextrose agar media was used for antibacterial and antifungal activity respectively. Molten Mueller Hinton agar/ Sabouraud dextrose agar (40-42°C) were seeded with 200 μl of inoculum (1 × 108 cfu/ml) and poured into Petri dishes. The media were allowed to solidify and wells were prepared in the seeded agar plates with the help of a cup borer (8.5 mm). Different extracts were dissolved in 100% DMSO at a concentration of 20 mg/ml, from this 100μl of different extracts were added into the sterile 8.5 mm diameter well. The plates were incubated at 37°C and 28°C for 24 and 48 h for bacteria and fungi, respectively. DMSO was used as a negative control. Antibacterial activity was assayed by measuring the diameter of the zone of inhibition formed around the well in millimetres. The experiment was done in triplicate and the average values were calculated for antibacterial activity.
Determination of minimum inhibitory concentration (MIC) and minimum bactericidal concentration (MBC)
To determine MIC of acetone extract and its fractions, the broth micro-dilution method was performed [22] with some modifications. The inoculums of the test bacteria were prepared using the colony suspension method [23]. Ninety-six-well culture plates (Tarsons Products Pvt. Ltd.) were used, and serial two-fold dilutions of the extracts (39-1250μg/ml) were dispensed into the plate wells. Two-fold dilutions of chloramphenicol and ceftazidime (1 to32 μg/ml) were used as a positive control. The volume of dispensing extract was 20μl per well along with 150μl of Mueller Hinton Broth. 30μl of bacterial culture at a density of 6×105 CFU/ml was added to the wells. Three control wells were maintained for each test batch; the positive control (antibiotic, Mueller-Hinton broth and test organism) and sterility control (Mueller-Hinton broth and DMSO) and negative control (Mueller-Hinton broth, test organism and DMSO).The plates were incubated at 37 °C for 24 h. The bacterial activity in the test wells was detected by adding 40 μL of 0.2 mg/ml of 2-(4-Iodo phenyl)-3-(4-nitro phenyl)5-phenyltetrazolium chloride (I.N.T.) (Himedia, India) solution dissolved in sterile distilled water to each well [51]. The plates were incubated for further 30 min, and estimated visually for any change in color to pink indicating reduction of the dye due to bacterial growth. The lowest concentration (highest dilution) of the plant extract required to inhibit visible growth of the tested microorganism was designated as the MIC.
For determination of minimum bactericidal concentration (MBC), wells showing no growth as well as from the lowest concentration showing growth in the MIC assay for all the samples were chosen. Bacterial cells from the MIC test plate were sub-cultured on freshly prepared solid nutrient agar by making streaks on the surface of the agar. The plates were incubated at 37°C for 24 h overnight. Plates that did not show growth were considered to be the MBC for the extract or drug used [24]. Using the values of MIC in micro broth dilution assay method, the MIC index values (MBC/MIC) for both the extracts and standard control drug were calculated against the test strains.
Combinations of extracts and antibiotics
Acetone extract and antibiotics (Chloramphenicol and Ceftazidime) were mixed at ratios of 1:1 and was tested for MICs which were determined by micro well dilution method as described above. The fractional inhibitory concentration (ΣFIC) index was calculated by adding the FIC values of antimicrobial compounds (FICA+FICB). The FICA and FICB values represent the lowest concentration of antibiotics and extract respectively that caused the inhibition of bacterial growth in the combination tests. Each of the combinations was calculated according to the following formula:
ΣFICI= FICA+FICB
FICA= (MICA combination/ MICA alone)
FICB= (MICB combination/ MICB alone)
The results were interpreted as follows: FICI ≤ 0.5 synergistic, 0.5 < FICI < 1 partially synergistic, FICI = 1 additive, 1 < FICI ≤ 4 indifferent and FICI > 4 antagonistic [14].
Qualitative phytochemical analysis
The crude powder of leaf and stem was subjected to qualitative phytochemical analysis [25,26]. The phytochemicals analysed were alkaloids, flavonoids, tannins, phlobatanins, triterpenes, steroids, saponinis and cardiac glycosides.
Statistical analysis
All experiments were repeated at least three times. Results are reported as Mean ± SEM (Standard Error of Mean).
Results and Discussion
Extractive yield
The extractive yield of T. erecta flower using different solvents is presented in Figure 1. The extractive yield was maximum in aqueous extract followed by methanol extract. The extraction ability of different solvents from flower for recovering extractable components followed the order: aqueous > methanol > hexane >acetone > ethyl acetate > toluene. Aqueous extract was superior in their ability to extract phytoconstituents from T. erecta (18.11%). Extraction with toluene existing the least yield (0.96%). Methanol and acetone both are polar solvents but methanol had more extractive yield than acetone. Non polar solvent hexane had more extractive yield than polar solvent acetone while semi polar solvents ethyl acetate and toluene had minimum extractive yield. It can be concluded that polar compounds are more than non polar compounds. Significant differences of extractive yield among different solvents might be attributed to the varied polarity of the solvents. The choice of solvent has a great influence on the extraction yield but it does not imply that the solvent which had maximum yield will show maximum activity under investigation Effect of extraction solvent on antimicrobial and antioxidant activity of medicinal plant extracts is reported by other researchers [27,29].
Antimicrobial activity
Since last few years, there has been a dramatic rise in search for natural products with antimicrobial properties because they offer a hope to find new drugs or drug leads which have a promising antimicrobial activity with lesser side effects to human beings. It is all the more worthwhile to evaluate flowers, peels, fruit rind, seeds, etc which are normally thrown away into the environment. In the present study, T. erecta flowers were extracted with hexane, toluene, ethyl acetate, acetone, methanol and water. Antimicrobial potentiality of the extracts was investigated against some 8 Gram positive bacteria, 8 Gram negative bacteria and 4 fungal strains.
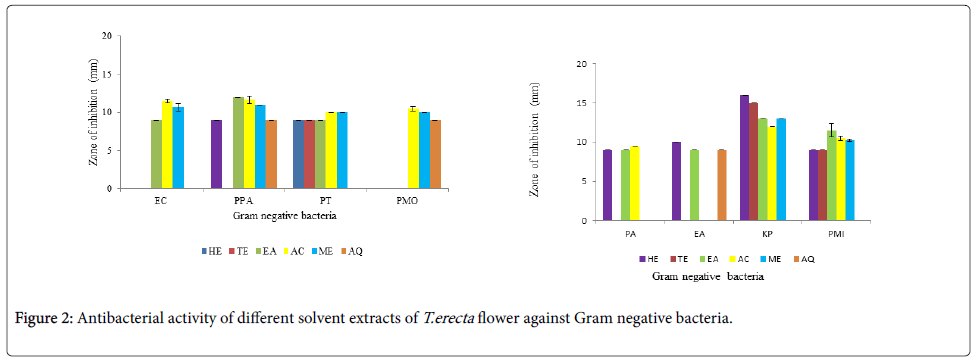
The antibacterial activity of different solvent extracts against Gram negative bacteria is shown in Figure 2a and 2b. A distinct effect of influence of polarity of solvent was envisaged. Aqueous extract showed activity only against E. aerogenes , P. pseudoalcaligenes and P. morganii. The non polar solvent hexane inhibited 6 Gram negative bacteria while semi polar solvents toluene and ethyl acetate inhibited 3 and 7 organisms respectively. Maximum inhibition was shown by both polar solvents acetone and methanol. They could inhibit almost all the Gram negative bacterial strains. Acetone extract did not inhibit E. aerogenes while methanol extract did not inhibit P. aerogenosa and E. aerogenes. Highest antibacterial activity was seen against K. pneumoniae by all the 5 organic solvents, maximum being by hexane extract. A similar trend was observed against P. mirabilis and P. testosterone. The non polar solvent hexane extract showed maximum activity against K. pneumonia. The antibacterial activity depends not only on the polarity of the solvent but also on the bacterial strain involved. There are various reports that antibacterial activity depends on the solvent used, structure of the compound in the extracts and the strain under investigation [30]. Different organic solvents extracts have different phytoconstituents in different amounts and that is why there is differential inhibition of the bacteria.
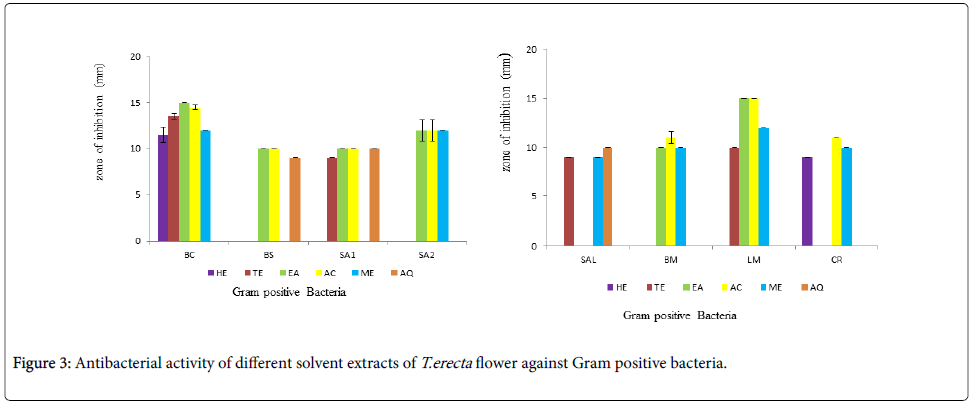
The antibacterial activity of different solvent extracts against Gram positive bacteria is shown in Figure 3a and 3b. Non polar solvent hexane showed antibacterial activity against only B. cereus and C. rubrum while semi polar solvents toluene and ethyl acetate showed antibacterial activity against 4 and 6 Gram positive bacteria respectively. Ethyl acetate and acetone extracts showed highest antibacterial activity against B. cereus and L. monocytogenes. The polar solvent acetone and methanol showed antibacterial activity against 7 and 6 Gram positive bacteria respectively. They could inhibit almost all the Gram positive bacterial strains. Acetone extract did not inhibit S. albus while methanol extracts did not inhibit B. subtilis and S. aureus 1. Aqueous extract showed activity only against B. subtilis, S. aureus 1 and S. albus.
Out of the 8 Gram positive and 8 Gram negative strains studied, B. cereus and K. pneumoniae were inhibited by all the solvent extracts except aqueous extract. Amongst Gram positive bacteria, highest antibacterial activity was seen against B. cereus followed by S. aureus and L. monocytogenes; while amongst Gram negative strains, highest antibacterial activity was seen against K. pneumoniae followed by P. pseudoalcaligenes. Solomon-Wisdom et al., [31] reported highest antibacterial activity of A. muricata (L) leaf extract against K. pneumoniae. Amongst all the six solvents used, acetone extract showed best activity against both Gram negative and positive bacteria. [32]. Thus a broad spectrum of antibacterial activity is envisaged; as also reported by Gehrke et al., [33] for Schinus lentiscifolius extracts.
In antifungal study, yeast strains showed little antifungal activity was shown in Figure 4. Acetone and ethyl acetate extract showed activity against all the four yeasts while toluene extract showed antifungal activity only against C. neoformans and C. glabrata (Figure 4). Other solvent like hexane, methanol and aqueous did not show any antifungal activity. Majority of the test organisms were susceptible to acetone extract with zone of inhibition ranging from 10 mm to 16 mm.
All 6 extracts were compared with 10 standard antibiotics, the results of which are presented in Table 2. The antimicrobial activity of some of the solvent extracts was comparable with that of standard antibiotics. K. pneumoniae was more inhibited by hexane and toluene extracts as compared to standard antibiotics polmyxin-B and gentamicin. L. monocytogenes was more inhibited by ethyl acetate and acetone extracts as compared to all 10 standard antibiotics. Tongpoothorn et al., [52] found that Jatropha curcas extracts showed lower antibacterial activity than that of gentamicin. Whereas different solvent extracts of T. erecta flower showed better activity than gentamicin against all tested bacterial strains.
When inhibition of various solvent extracts is considered, both polar solvents acetone and methanol showed maximum inhibition (87.5 and 75% respectively) (Table 4) while semi polar solvents ethyl acetate and toluene inhibited 81% and 43 % of the bacterial strain studied. The non polar solvent hexane inhibited only 50 % and aqueous extract inhibited only 37.5% of the bacteria. Hexane, methanol and aqueous extract did not show any antifungal activity, while toluene inhibited 25% of fungal strain and ethyl acetate and acetone inhibited 100 % of the fungal strains studied. Thus, a clear effect of the polarity of the solvents was envisaged. It also suggests that extracting solvent plays a crucial role for evaluating the antimicrobial activity of medicinal plants.
Qualitative phytochemical analysis revealed the presence of alkaloids, flavonoids, tannins, triterpenes and cardiac glycosides in T. erecta flowers (Table 1). Gayle et al., [34] and Hadden et al., [35] had also reported that T. erecta flowers are rich in flavonoids, alkaloids and glycosides. Plants rich in phytoconstituents like alkaloids, flavonoids, tannins, terpenoids and steroids have antibacterial properties [36,37]. In addition, secondary metabolites such as tannins and other compounds of phenolic nature are also classified as active antimicrobial compounds [38]. Alkaloid enriched extract from Prosopis juliflora pods showed good antibacterial activity [39]. Therefore, it can be suggested that the antibacterial activity observed with various solvent extracts of T. erecta in the present study may be because of the various phytoconstituents present in them.
| Test | Result |
|---|---|
| Flavonoids | ++++ |
| Tannins | +++ |
| Phlobatanins | - |
| Triterpenes | +++ |
| Steroids | - |
| Saponins | - |
| Cardiac glycoside | +++ |
| Meyer’s | - |
| Dragondroff | ++++ |
| Wagner’s | +++ |
| Legal’s | - |
Phytochemicals present in less (+), moderate (+++) and high (++++) amount; absent (-)
Table 1: Phytochemicals test of T. erecta flower powder.
| Zone of inhibition (mm) | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Antibiotics | Gram positive bacteria | Gram negative bacteria | Fungi | ||||||||||||||||
| BC | BS | SA1 | SA2 | SAL | BM | LM | CR | EC | PMO | PA | EA | KP | PMI | CA | CG | CN | CE | ||
| AMP10 | 9 | 39 | 0 | 23 | 0 | 0 | 0 | 25 | 0 | 22 | 14 | 0 | 20 | 9 | ND | ND | ND | ND | |
| TE30 | 14 | 20 | 19 | 22 | 19 | 19 | 0 | 0 | 16 | 22 | 0 | 25 | 24 | 20 | ND | ND | ND | ND | |
| CH30 | 14 | 24 | 15 | 18 | 15 | 0 | 0 | 15 | 19 | 23 | 0 | 23 | 21 | 0 | ND | ND | ND | ND | |
| P100 | 0 | 0 | 33 | 31 | 33 | 0 | 0 | 9 | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | |
| GEN10 | 13 | 12 | 12 | 9 | 12 | 14 | 13 | 11 | 12 | 17 | 14 | 15 | 14 | 12 | ND | ND | ND | ND | |
| PB100 | ND | ND | ND | ND | ND | ND | ND | ND | 7 | 8 | 8 | 9 | 8 | 7 | ND | ND | ND | ND | |
| CEP30 | 0 | 22 | 12 | 27 | 12 | 0 | 0 | 24 | 0 | 27 | 0 | 0 | 21 | 23 | ND | ND | ND | ND | |
| AMC10 | 0 | 8 | 26 | 26 | 26 | 0 | 0 | 11 | 0 | 25 | 0 | 9 | 19 | 8 | ND | ND | ND | ND | |
| NS100 | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | 18 | 18 | 22 | 22 | |
| AMP100 | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | 10 | 10 | 11 | 16 | |
ND = Not Done
Table 2: Antimicrobial activity using standard antibiotics.
Determination of MIC and MBC
Minimum inhibitory concentration refers to the lowest concentration of the antimicrobial agent which is required for the inhibition of visible growth of the tested microorganism [40]. MIC values were observed using INT dye on a 96 well micro-titre plate. The MBC is interpreted as the lowest concentration that can completely kill the microorganisms.
The MIC and MBC values of acetone extract and its fractions were tested for its antibacterial activity at various concentrations against bacterial strains that exhibited positive results in antimicrobial activity test. The MIC and MBC values were evaluated against 5 Gram positive bacteria (B. cereus, B. subtilis , S. aureus2 , S. albus , L. monocytogenes) and 5 Gram negative bacteria (P. Pseudoalcaligenes , P. morganii , P. aeruginosa , K. pneumoniae , P. mirabilis). The values are presented in Table 3a and 3b.
| Organism | PA | KP | PPA | PMI | PMO | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| MIC | MBC | MIC | MBC | MIC | MBC | MIC | MBC | MIC | MBC | |
| Acetone extract | 62 | >1250 | 78 | 312 | 312 | >1250 | 1250 | >1250 | 1250 | >1250 |
| Fraction 1 | 62 | 1250 | 156 | >1250 | 625 | 1250 | >1250 | >1250 | 1250 | >1250 |
| Fraction 2 | 156 | 620 | 78 | 620 | 625 | >1250 | 1250 | >1250 | 625 | 1250 |
| Chloramphenicol | 8 | >32 | 4 | 32 | 8 | 8 | 32 | 32 | 4 | >32 |
| Ceftazidime | 32 | >32 | 4 | >32 | >32 | >32 | >32 | >32 | 16 | >32 |
Pseudomonas pseudoalcaligenes (PPA), Proteus morganii (PMO) Pseudomonas aeruginosa (PA), Klebsiellapneumoniae (KP) Proteus mirabilis (PMI)
Table 3a: MIC and MBC values (μg/ml) of T. erecta flower extracts against Gram negative bacteria compared with chloramphenicol and ceftazidime.
| Organism | BC | BS | SA2 | SAL | LM | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| MIC | MBC | MIC | MBC | MIC | MBC | MIC | MBC | MIC | MBC | ||
| Acetone extract | 78 | 312 | 1250 | >1250 | 625 | >1250 | 312 | >1250 | 156 | >1250 | |
| Fraction 1 | 156 | 312 | 312 | >1250 | 1250 | 1250 | 625 | >1250 | 312 | >1250 | |
| Fraction 2 | 78 | 312 | 625 | >1250 | 625 | >1250 | 625 | >1250 | 312 | >1250 | |
| Chloramphenicol | 4 | >32 | 4 | 4 | 2 | >32 | 4 | 32 | 4 | 16 | |
| Ceftazidime | 32 | >32 | 8 | 8 | 4 | >32 | 2 | 16 | 32 | >32 | |
Bacillus cereus (BC), Bacillus subtilis (BS), Staphylococcus aureus 2 (SA2), Staphylococcus albus (SAL), Listeria monocytogenes (LM).
Table 3b: MIC and MBC values (μg/ml) of T. erecta flower extracts against Gram positive bacteria compared with chloramphenicol and ceftazidime.
For both Gram negative and Gram positive bacteria, MIC and MBC values varied from 78 to> 1250 μg/ ml and 312 to > 1250 μg/ml respectively. K. pneumoniae and B. cereus were the most susceptible Gram negative and positive pathogens to acetone extract and its fraction 2 (MIC: 78 μg/ml and MBC: 312 μg/ ml). For the standard antibiotics (CH and CF) MIC and MBC ranged from 4 to > 32 and 8 to > 32 μg/ ml respectively. The results indicated that fraction 2 showed significant antibacterial activity than crude acetone extract and fraction 1.
The MIC values of Gram negative bacteria were P. aeruginosa (156 μg/ml) in fraction 1 and 2, P. pseudoalcaligenes (312 μg/ml) in acetone extract, P. morganii (625 μg/ml) in fraction 2, P. mirabilis (1250 μg/ml) in acetone extract. The MBC values recorded were in the range between 625 μg/ml to >1250 μg/ml. The moderate antibacterial activity was seen against Gram positive bacteria B. subtilis (312 μg/ml) in fraction 1, S. aureus 2 (625 μg/ml) in acetone extract and its fraction 2, L. monocytogenes (156 μg/ml) in acetone extract, S. albus (312 μg/ml) acetone extract. The MBC values recorded were in range between 1250 μg/ml to >1250 μg/ml. Londonkar et al., [3] and Jamal et al., [41] reported lower MIC values for leaves of T. angustifolia Linn and N. oleander against pathogenic microorganisms respectively. The minimum inhibitory concentration (MIC) results of acetone extract of T. erecta flower were in agreement with the observations recorded in antimicrobial activity by agar well method as describe above.
MBC/MIC ratio of ≤ 4 is indicative of a bactericidal nature of the test sample [42]. In this study bactericidal effect was shown by crude acetone extract against B. cereus and K. pneumoniae, by fraction 1 against B. cereus , B. subtilis , C. rubrum , P. pseudoalcaligenes and by fraction 2 against B. cereus , B. subtilis and P. morgani
The results showed that acetone extract of T. erecta flower possessed considerable in vitro antimicrobial activity against many of the microorganisms involved in the pathogenesis of the human infections. The demonstration of antimicrobial activity against both Gram positive and Gram negative bacteria is indicative of the presence of broad spectrum antibiotic compounds as also reported by Jai N et al., [43] and Shohayeb et al., [44] . The MIC and MBC values are useful as guideline to the choice of appropriate and effective concentrations for therapeutic substances.
Synergistic activity
Synergistic activity of acetone extract of T. erecta flower with different standard antibiotics chloramphenicol and ceftazidime and against bacteria is shown in Table 5a and 5b. The combination of acetone extract and ceftazidime manifested synergistic effect on growth of B. subtilis and P. aeruginosa with FIC indices 0.312 and 0.093 respectively (Table 4a). This suggests the potential of acetone extract of this flower to improve the performance of antibiotics. Partial synergistic effect was observed against S. albus with FIC index 0.6. The combination showed an additive/indifferent effect against remaining bacterial strains. On the other hand, the combination of acetone extract and chloramphenicol manifested antagonistic effect against P. aerogenosa while indifferent effect was observed against all other bacterial strains (Table 4b).
| Solvents | % Inhibition of microorganism | |
|---|---|---|
| Bacteria | Fungi | |
| Hexane | 50 .00% | 0 % |
| Toluene | 43.75 % | 25 % |
| Ethyl acetate | 81.25 % | 100 % |
| Acetone | 87.5 0% | 100 % |
| Methanol | 75.00 % | 0 % |
| Aqueous | 37.5 0% | 0 % |
Table 4: Percentage inhibition of microorganisms by solvent extracts of T. erecta flowers.
| Microorganism | Extract and antibiotics (Alone) | Extract and antibiotics (Combination) | FIC | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MIC | MBC | MIC | MBC | MIC | MBC | ||||||||||
| FA | CF | FA | CF | FA | CF | FA | CF | FA | CF | ΣFIC | FA | CF | ΣFIC | ||
| Gram positive bacteria | BC | 78 | 32 | 312 | >32 | 312 | 8 | >1250 | >1250 | 4 | 0.25 | 4.25 | ND | ND | ND |
| BS | 1250 | 8 | >1250 | 16 | 78 | 2 | >1250 | >1250 | 0.0625 | 0.25 | 0.312 | ND | ND | ND | |
| SA2 | 620 | 4 | >1250 | >32 | 78 | 2 | >1250 | >1250 | ND | ND | ND | ND | ND | ND | |
| SAL | 312 | 2 | >1250 | 16 | 39 | 1 | >1250 | >1250 | 0.125 | 0.5 | 0.625 | ND | ND | ND | |
| LM | 156 | 32 | >1250 | >32 | 39 | 1 | >1250 | >1250 | ND | ND | ND | ND | ND | ND | |
| Gram negative bacteria | PA | 620 | 32 | >1250 | >32 | 39 | 1 | >1250 | >1250 | 0.062 | 0.0313 | 0.093 | ND | ND | ND |
| PMI | 1250 | 32 | >1250 | >32 | 1250 | 32 | >1250 | >1250 | 1 | 1 | 2 | ND | ND | ND | |
| PPA | 312 | >32 | >1250 | >32 | 78 | 2 | >1250 | >1250 | 0.25 | ND | ND | ND | ND | ND | |
| KP | 78 | 4 | 312 | >32 | 312 | 8 | >1250 | >1250 | 4 | 0.25 | 4.25 | ND | ND | ND | |
| PMO | 1250 | 16 | >1250 | >32 | 1250 | 32 | >1250 | >1250 | 1 | 1 | 2 | ND | ND | ND | |
Table 5a: Synergistic effect of T. erecta flower acetone extract (FA) with ceftazidime (CF)
| Microorganism | Extract and antibiotics (Alone) | Extract and antibiotics (Combination) | FIC | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MIC | MBC | MIC | MBC | MIC | MBC | ||||||||||
| FA | CH | FA | CH | FA | CH | FA | CH | FA | CH | ΣFIC | FA | CH | ΣFIC | ||
| Gram positive bacteria | BC | 78 | 4 | 625 | >32 | 156 | 4 | >1250 | >1250 | 2 | 1 | 3 | ND | ND | ND |
| BS | 1250 | 4 | >1250 | 8 | 156 | 4 | >1250 | >1250 | 0.12 | 1 | 1.01 | ND | ND | ND | |
| SA2 | 620 | 2 | >1250 | >32 | 78 | 2 | >1250 | >1250 | ND | ND | ND | ND | ND | ND | |
| SAL | 312 | 4 | >1250 | 32 | 156 | 4 | >1250 | >1250 | 0.5 | 1 | 1.5 | ND | ND | ND | |
| LM | 156 | 4 | >1250 | 16 | 156 | 4 | >1250 | >1250 | ND | ND | ND | ND | ND | ND | |
| Gram negative bacteria | PA | 620 | 8 | >1250 | >32 | 1250 | 32 | >1250 | >1250 | 2 | 4 | 6 | ND | ND | ND |
| PMI | 1250 | 8 | >1250 | >32 | 625 | 6 | >1250 | >1250 | 0.5 | 0.75 | 1.25 | ND | ND | ND | |
| PPA | 312 | >1250 | 16 | 156 | 4 | >1250 | >1250 | 0.5 | 0.5 | 1 | ND | ND | ND | ||
| KP | 78 | 4 | 312 | 32 | 156 | 4 | >1250 | >1250 | 2 | 1 | 3 | ND | ND | ND | |
| PMO | 1250 | 4 | >1250 | >32 | 312 | 8 | >1250 | >1250 | 0.24 | 2 | 2.24 | ND | ND | ND | |
All values are expressed in μg/ml; ΣFIC (Fractional Inhibitory Concentration Index) = FICA + FICB; FICA = (MICA combination / MICA alone); FICB = (MICB combination / MICB alone); Results interpreted as follows: ≤ 0.5 = synergistic; >0.5 to 0.75 = partially synergistic; 0.76 to 1.0 = additive; >1.0 to 4.0 = indifferent and > 4.0 = antagonistic; ND = Note determined because of high MIC value >1250 μg/ml.
Bacillus cereus (BC), Bacillus subtilis (BS), Staphylococcus aureus 2 (SA2), Staphylococcus albus (SAL), Listeria monocytogenes (LM), Pseudomonas pseudoalcaligenes (PPA), Proteus morganii (PMO) Pseudomonas aeruginosa (PA), Klebsiellapneumoniae (KP) Proteus mirabilis (PMI)
Table 5b: Synergistic effect of T. erecta flower acetone extract (FA) with chloramphenicol (CH).
The synergy between acetone extract and ceftazidime showed a higher decrease in MIC and a strong bactericidal activity. These results indicated that combination between acetone extract and ceftazidime could be useful in fighting emerging drug-resistant microorganisms. Similar synergistic effect of T. catappa extract and Eucalyptus camaldulensis extract with different antibiotics is reported by Rakholiya and Chanda [45] and Pereiraa et al., [46] respectively. The synergy detected in this study as enumerated suggests that plant crude extracts bring together a number of compounds that can enhance the activity of different antibiotics, as also reported by Wadhwa et al., [47]. Acetone extracts showed a good synergistic antimicrobial activity which is due to presence of the various phytoconstituents in the extracts. Romagnoli et al., [48] identified the various compound present in Tagetes patula L. essential oil by gas chromatography. The results showed that two most abundant component present in the oil is piperitone and piperitonone which is responsible for antifungal activity.
Conclusion
In the pursuit of searching for new antibiotics, the role of T. erecta flower cannot be negated as that is evident with the present results. Generally, in case of plant extracts, good antimicrobial activity is seen only against Gram positive bacteria. But T. erecta flower extracts showed broad spectrum activity, inhibiting Gram negative bacteria as well, which is a point worth noting. To meet the daunting challenge of drug resistance, the synergistic activity shown by acetone extract is valuable. The results indicate that combination between plant extract and the antibiotics could be useful in fighting emerging drug-resistant microorganisms and choice of the solvent plays a prominent role in evaluating antimicrobial activity of medicinal plants. Flowers can be taken as an alternative source of antimicrobial agent against the human pathogens. The future plan of work would be to figure out active chemical classes of molecules responsible for antimicrobial activity.
Acknowledgements
The authors thank Department of Biosciences (UGC-CAS) for providing excellent research facilities. One of the authors Ms. Hemali Padalia is thankful to UGC-CAS, New Delhi, India for providing Junior Research Fellowship.
References
- Pitout JD, Hanson ND, Church DL, Laupland KB (2004) Population-based laboratory surveillance for Escherichia coli producing extended spectrumbeta-lactamases: importance of community isolates with blaCTX-M genes. Clin Infect Dis. 2004;38:1736-41.
- Stevenson KB (2005) Methicillin-resistant Staphylococcus aureusandvancomycin-resistant enterococci in rural communities, WesternUnited States. Emerg Infect Dis 11: 895-903.
- Londonkar RL, Kattegouga UM, Shivsharanappa K, Hanchinalmath JV (2013) Phytochemical screening and in vitro antimicrobial activity of Typhaangustifolia Linn leaves extract against pathogenic gram negative microorganisms. J Pharm Res 6: 280-283.
- Khanahmadi M, Rezazadeh SH, Taran M (2010) In vitro antimicrobial and antioxidant properties of SmyrniumcordifoliumBoiss. (Umbelliferae) extract. Asian J Plant Sci 9: 99-103.
- Punopas K, Eumkeb G, Chitsomboon B, Nakkiew P (2003) The study of antibacterial activity of some medicinal plants in lamiaceae family. Suranaree J SciTechnol 11: 52-59.
- Karmegam N, Karappusamy S, Prakash M, Jayakamar M, Rajasekar K (2008) Antibacterial potency and synergistic effect of certain plant extracts against food borne diaraheagenic bacteria. Int J Biomed PharmaSci 2: 88-93.
- Heinrich M, Barnes J, Gibbons S, Williamson EM (2004) Fundamentals of Pharmacognosy and Phytotherapy. Churchill Livingstone, Edinburgh pp. 4–7.
- Thabti I, Elfalleh W, Tlili N, Ziadi M, Campos MG, et al., (2014) Phenols, flavonoids, and antioxidant and antibacterial activity of leaves and stem bark of Morus species. Int J Food Properties 17: 842–854.
- Raja RD, Jeeva S, Prakash JW, Antonisamy JM, Irudayarai V (2011) Antibacterial activity of selected ethnomedicinal plants from south india. Asian Pac J Trop Med 4: 375-378.
- Vijayarathna S, Zakaria Z, Chen Y, Latha LY, Kanwar JR et al., (2012) The antimicrobial efficacy of Elaeisguineensis: characterization, in vitro and in vivo studies. Molecules. 17: 4860-77.
- Tekwu EM, Pieme AC, Beng VP. Investigation of antimicrobial activity of some cameroonian medicinal plant extracts against bacteria and yeast with gastrointestinal relevance. J Ethanopharmacol. 2012;142(1) 265-73.
- Menpara D and Chanda S (2014) Phytochemical and phamacognosticevalution of leaves of Pongaminpinnata L. (Fabaceae). PhcogCommun. 4: 3-7.
- Chanda S, Rakholiya K, Dholakia K, Baravalia Y (2013) Antimicrobial, antioxidant and synergistic property of two nutraceutical plants: TerminaliacatappaL. and ColocasiaesculentumL. Turk J Biol 37: 81-91.
- Hyejung H, Kim J, Bang J, Kim H, Beuchat LR, et al., (2013) Combined effects of plant extracts in inhibiting the growth of Bacillus cereus in reconstituted infant rice cereal. Int J Food Microbiol 2160: 260–266.
- Dawis MA, Isenberg HD, France KA, Jenkins SG (2003) In vitro activity of gatifloxacin alone and in combination with cefepime, meropenem, piperacillin and gentamicin against multidrug resistant organisms. J AntimicrobChemother 51: 1203-1211.
- Wichtl M (1994) Herbal drugs and phytopharmaceuticals. Stuttgart: Medpharm Scientific Publisher, pp. 446.
- Manjunath BL (1969) The Wealth of India, Raw material. CSIR, New Delhi.
- Kirtikar KR, Basu BD (1994) In: Indian medicinal plants. Allahabad: Lalit Mohan Basu India, pp. 1385-1386-1987.
- Ghani A (2003) Medicinal plants of Bangladesh: chemical constituents and uses. Dhaka. In: Asiatic Society of Bangladesh (2 Edn.), Bangladesh.
- Parekh J, Chanda S (2007) In vitro antibacterial activity of the crude methanol extract of WoodfordiafruticosaKurz. flower (Lythraceae). Braz J Microbiol38: 204-207.
- Tang J, Meng X, Liu H, Zhao J, Zhou L, et al., (2010) Antimicrobial activity of Sphingolipids isolated from the stems of Cucumber (Cucumissativus L). Molecules 15: 9288-9297.
- Edziri H, Ammar S, Souad L, Mahjoub MA, Mastouri M, et al., (2012) In vitro evaluation of antimicrobial and antioxidant activities of some Tunisian vegetables. South Afr J Bot78: 252–256.
- European Committee for Antimicrobial Susceptibility Testing (EUCAST) (2003) Determination of minimum inhibitory concentrations (MICs) of antibacterial agents by broth dilution. Clin J Microbiol Infect 9: 1-7.
- Akinyemi KO, Oladapo O, Okwara CE, Ibe CC, Fasure KA (2005) Screening of crude extracts of six medicinal plants used in South-West Nigerian unorthodox medicine for anti-methicillin resistant Staphylococcus aureus activity. BMC Comp Altern Med 5: 6.
- Harborne JB (1973) Phytochemical methods. (2nd Edn.) Chapman & Hall, London.
- Menpara D, Desai D, Rathod T, Chanda S (2014) Evaluation of nutraceutical bottle gourd (Lagenariasiceraria) as a potential source of natural antimicrobial agent. Am J Phytomed Clinical Therapeut. 2: 375-389.
- Kchaou W, Abbès F, Blecker C, Atti H, Besbes S (2013) Effects of extraction solvents on phenolic contents and antioxidant activities of Tunisian date varieties (Phoenix dactylifera L.). Indus Crops Prod 45: 262– 269.
- Rakholiya KD, Kaneria MJ and Chanda SV (2014) Mango pulp: A potential source of natural antioxidant and antimicrobial agent. In: Medicinal Plants: Phytochemistry, Pharmacology and Therapeutics. Ed. Gupta VK, Daya Publishing House, New Delhi 3: 253-284.
- Norziah M H, Fezea FA, Bhat R, Ahmad M (2015) Effect of extraction solvents on antioxidant and antimicrobial properties of fenugreek seeds (Trigonellafoenum-graecum L). Int Food Res J 22: 1261-1271.
- Nair R, Shah A, Baluja S, and Chanda S (2006) Synthesis and antibacterial activity of some Schiff base complexes. J Serb Chem Soc. 71: 733-744.
- Solomon-Wisdom GO, Ugoh SC, Mohammed B (2014) Phytochemical screening and antimicrobial activities of Annonamuricata (L) leaf extract. Am J Bio Chem Pharm Sci. 2: 01-07.
- Parekh J, Karathia N, Chanda S (2006) Evaluation of antibacterial activity and phytochemical analysis of Bauhinia variegataL. bark. Afr J Biomedical Res 9: 53-56.
- Gehrke IT, Neto AT, Pedroso M, Mostardeiro CP et al., (2013) Antimicrobial activity of Schinuslentiscifolius (Anacardiaceae). J Ethnopharmacol 148: 486-91.
- Gayle KG, Tung-Shan C, Philip T (1986) Quantitative analysis of lutein esters in marigold flowers (Tageteserecta) by high performance liquid chromatography. J Food Sci 51: 1093-4.
- Hadden LW (1999) Carotenoid composition of marigold (Tageteserecta) flower extract used as nutritional supplement. J Agric Food Chem 47: 4189-94.
- Costa EV, Cruz PE, Lourenço CC, Moraes VR, Nogueira PC, et al., (2013) Antioxidant and antimicrobial activities of aporphinoids and other alkaloids from the bark of Annonasalzmannii A. DC. (Annonaceae). Nat Prod Res 27: 1002–1006.
- Ngoci NS, Ramadhan M, Ngari MS, Leonard OP (2014) Screening for antimicrobial activity of Cissampelospareira L. methanol root extract. Eur J Med Plant 4: 45.
- Sacchetti G, Maietti S, Muzzoli M, Scaglianti M, Manfredini S et al., (2005) Comparative evaluation of 11 essential oils of different origin as functional antioxidant, antiradicals and antimicrobials in foods. Food Chem. 91: 621-632.
- Dos Santos ET, Pereira ML, Da Silva CF, Souza-Neta LC, Geris R, et al., (2013) Antibacterial activity of the alkaloid-enriched extract from Prosopisjuliflora pods and its influence on in Vitro ruminal Digestion. Int J MolSci 14: 8496-8516.
- Sharma A, Gupta S, Sarethy IP, Dang S, Gabrani R. Green tea extract possible mechanism and antibacterial activity on skin pathogen. Food Chem. 2012;135:672-675.
- Jamal MA, Rahman S, Ialam MA, Karim MR, Alam MS, et al., (2012) Mimimum inhibitory concentraction analysis of Nerium oleander against bacterial pathogens. Asian Pac J Trop Biomed 3: 1664 -1666.
- Teke GN, Kuiate JR, Kuete V, Teponno RB, TapondjouLA,et al., (2011) Bio guided is plation of potential antimicrobial and antioxidant agents from the stem bark of Trilepisiummadagascariense. South Afr J Bot 77: 319-327.
- Jain R, Katare N, Kumar V, Samanta AK, Goswami Set al., (2012) In Vitro Anti bacterial potential of different extracts of Tageteserecta and Tagetespatula. J Nat Sci Res 2.
- Shohayeb M, Hameed ES, Bazaid SA, Maghrabi I (2014) Antibacterial and antifungal activity of Rosa damascena MILL. essential oil, different extracts of rose petals. Global J Pharma. 8: 01-07
- Rakholiya K, Chanda S (2012) In vitro interaction of certain antimicrobial agents in combination with plant extracts against some pathogenic bacterial strains. Asian Pacific J Trop Biomed. 2: 1466-1470.
- Pereira V, Dias C, Vasconcelos MC, Rosa E, Saavedra MJ (2014) Antibacterial activity and synergistic effects between Eucalyptus globulusleaf residues (essential oils and extracts) and antibiotics against several isolates of respiratory tractinfections (Pseudomonas aeruginosa). Indus Crop Prod 52: 1– 7.
- Wadhwa S, Bairagi M, Bhatt G, Panday M, Porwal A (2010) Antimicrobial activity of essential oils of Trachyspermumammi. Int J Pharm Biol Arch 1: 131-133.
- Romagnolli C, Bruni B, Andreotti E, Rai MK, Vicentini CB (2005) Chemical characterization and antifungal activity of essential oil of capitula from wild indianTagetesputala L. Protoplasma. 225: 57-65.
- Perez C, Paul M, Bazerque P (1990) An antibiotic assay by the agar well diffusion method. ActaBiologiae et Med Experimentalis 15: 113–115.
- Kaneria M, Chanda S (2013) The effect of sequential fractionation technique on the various efficacies of pomegranate (PunicagranatumL.). Food Anal Methods 6: 164-175.
- Frey FM and Meyers R. Antibacterial activity of traditional medicinal plants used by Haudenosaunee peoples of New York State. BMC Comp Alt Med. 2011;10:64.
- Tongpoothorn W, Chanthai S, Sriuttha K, Ruangviriyachai C (2012) Bioactive properties and chemical constituents of methanolic extract and its fraction from Jatrophacurcas oil. Indus Crop Prod. 36: 437-444.
- Kaneria M, Bapodara M, Chanda S (2012) Effect of extraction techniques and solvents on antioxidant activity of pomegranate (Punicagranatum L.) leaf and stem. Food Anal Method 5: 396-404.
- Anwar F, Kalsoom U, Sultana B, Mushtaq M, Mehmood T et al., (2013) Effect of drying method and extraction solvent on the total phenolics and antioxidant activity of cauliflower (Brassica oleracea L.) extract. Int Food Res J 20: 653-659.
Copyright: © 2015 Padalia H, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.