Advanced Techniques in Biology & Medicine
Open Access
ISSN: 2379-1764
ISSN: 2379-1764
Mini Review - (2019)Volume 7, Issue 2
It is certain that tumor vasculature plays a crucial role in tumor growth and metastasis with unique physiological features. Up to date, rational design of tumor therapeutics targeting tumor blood vessels has become a consensus strategy. However, the current small molecular anti-vascular drugs are always accompanied by toxicity or side effects. It is still necessary to develop novel medication with high efficacy and low toxicity. Gadofullerene (Gd@C82), a novel star material, has been exploited in many different biomedical fields after functionalized. Specially, the highly efficient antitumor effects of functionalized Gd@C82 were explored extensively by suppressing tumor angiogenesis or cutting off the existing tumor vasculature under radiofrequency (RF) irradiation. And studies demonstrated that no detectable toxicity was observed with functionalized Gd@C82 usage. This paper gives an overview of the antineoplastic activities of functionalized Gd@C82 nanomaterials via targeting tumor vasculature.
Functionalized Gd@C82; Antineoplastic activities; Targeting tumor vasculature
Tumor vasculature is the foundation of tumor development, growth and metastatic dissemination, as tumor blood vessels could provide a steady supply of nutrients and oxygen for the rapid propagation of tumor cells [1,2]. In the absence of adequate vasculature, tumors will undergo growth inhibition and cell death [3]. Therefore, tumor blood vessels could be a promising cancer therapeutic target. In recent years, there are a large number of investigational drugs associated with tumor vascular-targeting agents (VTAs) appeared, some could inhibit the formation of new vessels, called tumor angiogenesis inhibitors (TAIs); and some could occlude the pre-existing tumor blood vessels, called vascular disrupting agents (VDAs) [4-6]. These VTAs possess much more advantages than conventional cancer chemotherapy, such as low toxicity, inaccessibility to drug resistance and wide application for different tumors [7].
With the rapid development of nanotechnology, engineered nanoparticles, including carbon nanomaterials, are emerging as excellent platforms for biomedical application, including effective cancer therapy [8-10]. Fullerenes, a unique class of carbon allotropes, have attracted a deal of attention and been studied intensively since their discovery because of the superior physiochemical properties
derived from their unique structures and rich modification [11,12]. Gadofullerene as an endohedral metallofullere developed recently for biomedical applications after functionalized with various functional groups like hydroxy, carboxyl, amino acid and so on [13,14]. Specially, hydroxylated Gd@C82 is the most common derivative of gadofullerene with superior water-solubility and biocompatibility. And it has inspired considerable investigations on tumor angiogenesis inhibition and tumor vascular disruption by Gd@C82(OH)n nanoparticles and (Gd@C82)m(OH)n nanocrystals, respectively [15]. The current review examines the highly effective antitumor activities of functionalized Gd@C82 via inhibiting angiogenesis and disrupting tumor vasculature, as a promising tumor vascular targeting agent with high biocompatibility and biosafety.
Fullerene is a new kind of carbon allotrope with closed spherical or ellipsoidal cage structure, composed by many different numbers of carbon atoms [16]. The structures of fullerenes are featured by symmetric cages with either 5- or 6-membered rings on the surface [17]. Metallofullerene is formed when many metal atoms are put inside fullerenes [13]. Derived from the unique structures, fullerenes/metallofullerenes possess unique properties, such as the excellent stability, the large surface area, the unsaturated double bands on the surface and the special surface easy to be functionalized [18]. It has been reported that fullerenes could be modified by physical modification with polyethylene glycol (PEG), polyvinylpyrrolidone (PVP), cyclodextrin and so on, as well as chemical reactions by functional groups [14,19]. Functionalized fullerenes have attracted wide attention in the field of biomedicine. Importantly, Gd@C82 is a common metallofullerene, consisting of a core of gadolinium (Gd) atom and a closed outer sheath of 82 carbon atoms [13]. After modified by hydroxy, carboxyl, amino acid and so on, Gd@C82 could be a promising anti-tumor vascular agent via inhibiting angiogenesis or disrupting pre-existing tumor blood vessels (Figure 1).
Figure 1: The different antineoplastic mechanisms of functionalized Gd@C.
Considering the essential of neovasculature for tumor growth, survival and metastasis, angiogenesis inhibition is emerging as a potential antitumor strategy [20]. In terms of the process of angiogenesis, TAIs always work by inhibiting angiogenic stimulators, endothelial proliferation, proteolysis of extracellular matrix (ECM) and integrins [21].
In 2005, the functionalized Gd@C82 nanoparticles, Gd@C82(OH)22 (f-NPs), were synthesized via a liquid-liquid reaction, which could prevent tumor growth in 22 hepatocellular carcinoma [22]. The f-NPs, whose size is approximately 70 nm, consist of an innermost core of gadolinium (Gd) atom, an outer carbon cage, and 22 outermost hydroxyl groups (Figure 2a and 2b) [23]. And this kind of functionalized Gd@C82 nanoparticles showed high efficacy of anti-angiogenesis. As shown in Figure 2c, f-NPs could inhibit tumor angiogenesis by the simultaneous downregulation of more than 10 angiogenic factors, resulting in significantly reduced microvessel density and insufficient tumor blood perfusion, and thus, yielding a marked tumor growth inhibition with a tumor inhibitory rate much higher than the common tumor chemotherapeutic drug paclitaxel [23]. Meanwhile, the normal vessels were not interfered by f-NPs treatment without detectable abnormalities such as disfigurements, gaps, or breaches [23]. What’s more, Gd@ C82(OH)22 could also inhibit tumor angiogenesis by impairing matrix metalloproteinase (MMPs) activities as activated MMPs (specifically, MMP-2 and MMP-9) are responsible for proteolysis of the ECM, which is a prerequisite for angiogenesis [24,25]. It was validated that Gd@C82(OH)22 could not only depress MMPs expressions but also inhibit their activities by indirectly interacting with MMPs by a special binding mode, and thus suppress tumor growth (Figure 2d) [26]. All these findings indicted that Gd@ C82(OH)22 nanoparticles showed highly efficient antineoplastic effects, resulting from the inhibition of angiogenic factors and the suppression of MMPs activities.
Figure 2: The antitumor effects of Gd@C82(OH)22 (f-NPs) via inhibiting tumor angiogenesis. (a) The synthesis and structure of f-NPs. (b) SEM image of f-NPs. (c) The downregulation of angiogenic factors and anti-angiogenesis effects of the f-NPs. (d) Inhibition of fullerene on MMP-2 and MMP-9 in tumor tissues and tumor growth [23,26].
In contrast to normal vessels, tumor vasculatures possess peculiar pathophysiological features, including chaotic architecture, high vascular permeability, non-uniform surface markers and the lack of lymphatic drainage [27,28]. These characters, known as the enhanced permeability and retention (EPR) effects, provide us the opportunities to disrupt the unmatured tumor vasculature, and thus lead to tumor ischemic necrosis. Accordingly, some VDAs like CA4P, ZD6126 and DMXAA have been clinically applied, but they also show serious side-effects such as tumor pain and cardiotoxicity [29].
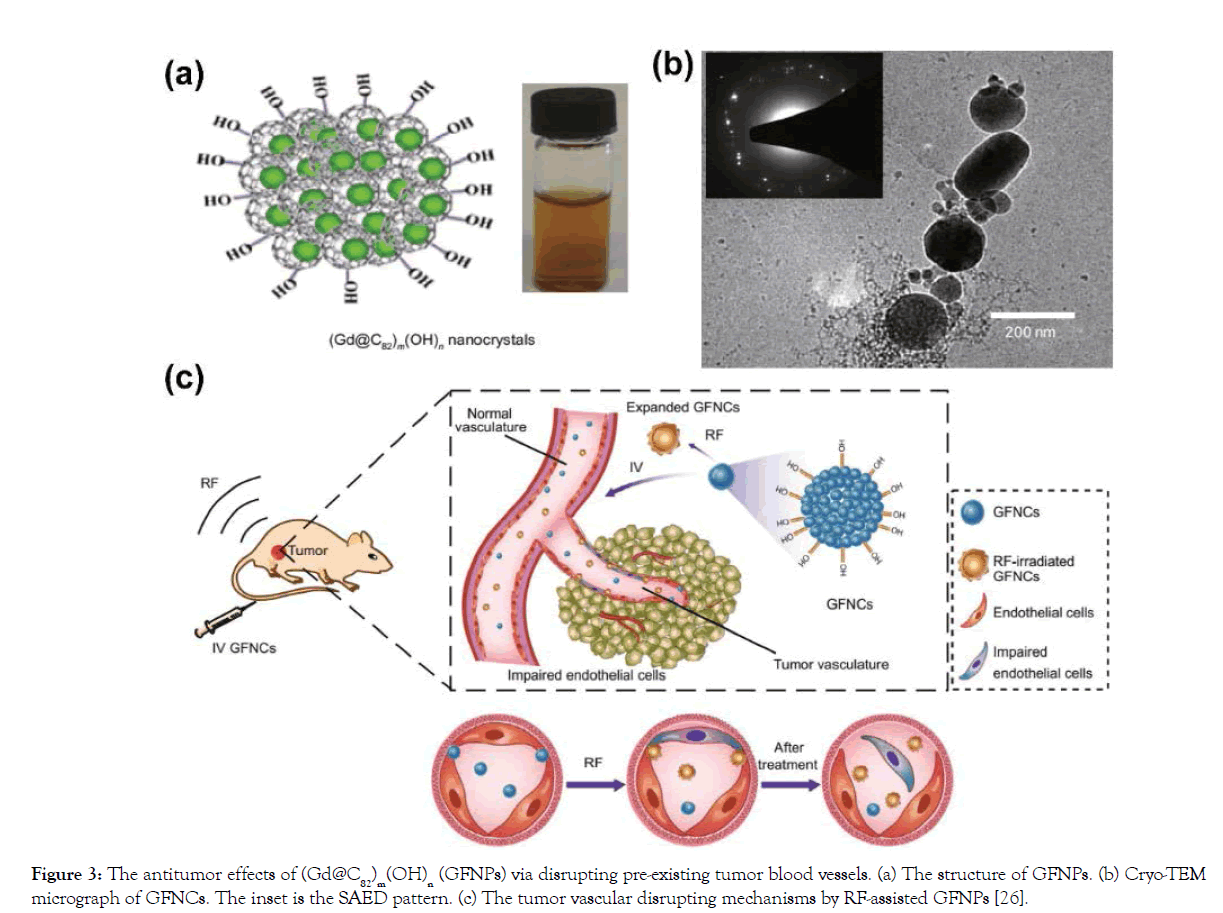
Figure 3: The antitumor effects of (Gd@C82)m(OH)n (GFNPs) via disrupting pre-existing tumor blood vessels. (a) The structure of GFNPs. (b) Cryo-TEM micrograph of GFNCs. The inset is the SAED pattern. (c) The tumor vascular disrupting mechanisms by RF-assisted GFNPs [26].
Importantly, a new kind of functionalized Gd@C82 nanocrystals, (Gd@C82)m(OH)n (GFNCs), were synthesized via a novel solidliquid reaction. Different from the previous hydroxylated Gd@C82 nanoparticles, GFNCs consist of a cluster of Gd@C82 crystal and further modification by outer functional groups, and the diameter is about 140 nm (Figure 3a and 3b). What is more, GFNCs exhibit superior properties such as expansion by phase transition, size controllability and so on [14]. Based on these characterizations of GFNCs, a new anticancer technique was presented in 2015, which could disrupt abnormal tumor blood vessels under the assistance of radiofrequency (RF) [30]. This opens the gates to realize disruption of tumor vasculatures by functionalized gadofullerene. Under RF assistance, this nanocrystal shows excellent anticancer activities in different kind of tumors like H22, 4T1, HepG2 and so on [30,31]. After RF-assisted GFNCs treatment, the tumor was observed to induce a rapid and extensive necrosis, and finally serious inner collapses occurred in 24 h [30]. Calculated by hematoxylin-eosin (HE) staining of tumor tissues, the tumor inhibition rate was up to 88.7% [31]. The disruption of tumor blood vessels was investigated in detail. To real-time monitor tumor vascular disruption during the treatment by RF-assisted GFNCs, dorsal skin flap chamber (DSFC) model was developed, by which the vascular structure and blood flow could be observed visually [32]. Tumor blood vessels were broken and hemorrhaged 20 minutes after treatment, leading to remarkable collapse and fragmentation of the whole tumor vascular net. As time goes by, the areas of bleeding were continually increased, the shape of the vessels became unrecognizable from DSFC window, and last the vessels got fuzzy and invisible. However, normal vessels remained undamaged after treated by RF-assisted GFNCs [32].
Besides, dynamic contrast enhanced (DCE)-MRI, a “gold standard” method to evaluate functional characteristics of the vascular net, was performed. The blood perfusion and the Ktrans values (the rate of transfer of the contrast agent from the blood to the interstitial space) of tumors were greatly and persistently reduced after treatment, indicating the considerable destruction of capillary function of the tumor [32]. Furthermore, it was reported that VE-cadherin, the specific transmembrane adhesion proteins in endothelial cells located at intercellular junctions of tumor blood vessels, was down-regulated by RF-assisted GFNCs, indicating the disruption of tumor vascular endothelium [31]. Overall, the mechanisms of tumor vascular disruption by RF-assisted GFNCs were investigated profoundly as shown in Figure 3c [30]. After iv injection, GFNCs would rapidly flow along the normal blood vessels and penetrate the leaks of tumor vascular endothelial cells. With the properties of size-expansion by a phase transition, GFNCs would undergo explosive volume expansion and the possible rotation when the RF is applied. And thus, the networks of endothelia cells could be destroyed and abscised from the basilemma.
With further research, a new β-alanine functionalized gadofullerene nanocrystals, (Gd@C82)m(Ala)n, were developed and applied to vascular disruption [33]. Compared with GFNCs, this new nanoparticle presented superior physicochemical properties, which could improve the antitumor effects as vascular disrupting agents. The technique based on RF-assisted functionalized gadofullerene as a new tumor vascular disrupting strategy brings a new dawn for cancer treatment.
Although the potential applications of functionalized Gd@C82 nanomaterials have been exploited in antitumor therapy as vascular targeting agents, it should also not be neglected in considering their biocompatibility and toxicity for clinical application. Numerous experiments have been explored to evaluate the low cytotoxicity of functionalized Gd@C82 nanomaterials either in vitro or in vivo, which may be derived from the water-soluble modifications on the surface of carbon cage [34]. Recently, studies revealed that functionalized Gd@C82 nanomaterials showed no reduction of cell abilities, but promotion of cell proliferation, such as immune cells (B cells, T cells or macrophages) [35], hepatoma cells (HepG2) [31], human umbilical vein endothelial cells (HUVECs) [31], human epidermal keratinocytes alpha (HEKα) [36], human embryonic lung fibroblast cells (MRC-5) [37], A549 and brain capillary endothelial cells (rBCECs) [38] and so forth.
Similarly, the toxicities in vivo were also studied by many researchers. After uptake of Gd@C82(OH)22, tumor-bearing mice remained normal body weight compared with mice in saline groups, suggesting no detectable toxicity [39]. To evaluate the liver and kidney function, the common indicators such as alanine aminotransferase (ALT), aspartate aminotransferase (AST), blood urea nitrogen (BUN), alkaline phosphatase (ALP) and creatinine (Cr) were monitored and tended to levels in healthy mice, indicating no toxicity of functionalized Gd@C82 nanomaterials [23,33]. Furthermore, the nanomaterials could be mainly delivered to liver, spleen, kidney and bone of mice; however, no abnormal pathological changes were observed in these organs and tissues [30]. Notably, functionalized Gd@C82 nanomaterials could restore the damaged liver, kidney and bone barrow induced by radiation or chemotherapeutic agents [8,40]. In addition, studies using the nematode Caenorhabditis elegans (C. elegans) as a model revealed that Gd@C82(OH)22 nanoparticles exhibited no detectable toxicity on growth, reproductivity and lifespan of C. elegans [41]. All these studies demonstrated that functionalized Gd@C82 nanomaterials exhibits high biosafety, thus laying the foundations for further biomedical application.
In short, functionalized Gd@C82 nanomaterials exhibit highpotential in treating tumor effectively and rapidly via targeting tumor blood vessels. In particular, Gd@C82(OH)n nanoparticles could inhibit angiogenesis not only by down-regulating many angiogenic factors but also by suppressing the expressions and activities of MMPs. Differently, (Gd@C82)m(OH)n nanocrystals could disrupt the pre-existing tumor blood vessels with the help of RF, deriving from their size-expansion when passing through tumor vascular endothelial cells. And no disruption of normal blood vessels occurred after injecting Gd@C82 nanomaterials. Importantly, no detectable toxicity is observed in vitro or in vivo. With high-efficacy and low toxicity, functionalized Gd@C82 nanomaterials could be a promising antineoplastic agent in clinic.
This work is supported by the National Major Scientific Instruments and Equipments Development Project (ZDYZ2015-2).
Citation: Li X, Zhen M, Wang C (2019) Antineoplastic Activities of Functionalized Gd@C82 Nanomaterials via Targeting Tumor Vasculature. Adv Tech Biol Med 7:267.
Received: 11-Mar-2019 Accepted: 08-May-2019 Published: 15-May-2019
Copyright: © 2019 Li X, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.