Journal of Agricultural Science and Food Research
Open Access
ISSN: 2593-9173
ISSN: 2593-9173
Research Article - (2018) Volume 9, Issue 2
Research of antioxidant activity of syrup purple sweet potatoes (Ipomeae batatas L.) had been done. The research is conducted in Agriculture Laboratory of University of Quality and Laboratory of Pharmacy, University of North Sumatra, for three months, October-December 2014. Purpose of the research is to know whether the temperature of liquidation has an impact on degree of anthocyanin syrup in purple sweet potatoes. Testing of antioxidants activity is done by using DPPH method. Results of the research shows, liquidation temperatures have significantly declined the degree of anthocyanin. Degree of anthocyanin in liquidation temperature of 80°C is 112.310 mg/100 g, in liquidation temperature of 95°C is 108.806 mg/100 g, and in liquidation temperature of 110°C is 107.876 mg/100 g. Antioxidant activity of syrup declines when liquidation temperatures escalates. Values of IC 50 in each of syrups are S1 (liquidation temperature 80°C=129.87 mg/ml); S2 (liquidation temperature 95°C=109.16 mg/ml); and S2 (liquidation temperature 110°C=55.29 mg/ml).
Keywords: Liquidation temperature; Anthocyanin; Antioxidant activities
Development of foods security in Indonesia has inscribed in Law No. 7 in 1996 about foods. The security of food is defined as effort to warrant food availability for all household, enough in amount, qualify and safety, secure to consume, posses, and buyable of every individual. Until recently mandate of the law is not completely done yet.
One of the approaches to support the law mandate is diversification of foods. Syrup of purple sweet potatoes shown be able to act as an antioxidant to the experiment animals with reduction of blood MDA (malondialdehyde) and heart tissues that is an indicator of peroxidation fat. This object is related to pigment alignment of larger amount of anthocyanin and as an antioxidant. The average of anthocyanin in purple sweet potatoes is 110 mg/100 g to 210 mg/100 g.
Anthocyanin has often been used as a natural dye, especially beverages. Besides for natural foods dye, anthocyanin is also believed has rule in biological system include tying the free radical scavenging, cardio protective capacity and the rule to trial initiation chemical reaction that cause carcinogenesis [1,2].
Turker and Erdogdu pointed out, temperature and pH have effects to efficiency of the anthocyanin extracts and its diffusion coefficient. When the pH is low, coefficient distribution is high, and the temperature is also high. However, anthocyanin is a phenolic unstable and easily damaged by heating, thus effecting to declination of its bioactivity. According to Revilla, impact of the temperature become insignificant by adding of HCL to protractor that used to extract; because HCL has greater impact than the temperature [3,4].
One of the tests to determine of antioxidant activity of absorber free radicals is the method of DPPH (1,1 Diphenyl-2-picrylhydrazil). The method of DPPH will give information of fusion reactivity that confirmed by a stable radical. DPPH has ability to absorb free radical optimally in long surf of 517 nm and its color is purple. Absorber of free radical causing the electron pairing thus causing to loss of color that equivalent with the amount of electron taken to form electron pair [5].
The research conducted on Agriculture Laboratory of University of Quality and the Laboratory of Pharmacy, University of North Sumatra, for three months, October-December 2014. The materials used in this research are four months aged purple sweet potatoes, enzyme α- amylase, enzyme amyl glycosidase, active carbon, HCl 0.1 N, NaOH 0.1 N, H2SO4 6 N, sterilize water, indicator amylin 1% solution Luff Schoorl, 30% solution of KI, thick of sulphuric acid, Na Thiosulfate 0.1 N, solution of Pb acetate, 5% solution of NaHPO4, DPPH, BHT. All the chemical materials are classified as pure analytical (pa).
The main equipment used in this research are water bath shaker, oven, rotary evaporator, magnetic stirrer, pair of scale analysis, hand refract to meter, thermometer, spectrophotometer, vacuum filter, kitchen equipment and glasses.
The purple sweet potatoes were cleaned, shelled, and blended as juice, then filtered by blacu cloth. The dreg of the first straining was filtered again using sterile aqua, and blended, and filtered again using the same blacu cloth. The process results are deposit essence of wet starch. The wet starch then dried using vacuum evaporator until it is arid. Starch of sweet potatoes were weight as much as 100 g, then add sterilize aqua 1000 ml to arrangement of starch suspension and adjust its pH between 5.2-5.6 by adding NaOH. The suspension added with enzyme α-amylase dosage of 0.65 kg/ton of starch, which is 0.1 ml. Liquid it by heating the suspense in various temperature, 80°C, 95°C, 110°C, respectively for 120 minutes. During the process, the liquid was shaken by magnetic stirrer. Solution was cooled until the temperature is 60°C and the pH was adjusted to 4.0-4.5 by adding HCL. The solution of dextrin is then with enzyme α-amylase 0.2 ml with dosage of 0.40-0.80 kg/ton starch. The next step is sugaring process. Keep the temperature in 60°C for 24 hours by using water bath shaker. Glucose syrup solution is then purified by adding active carbon 2% of dry weight of starch in temperature 80°C in 10 minutes. The liquid is then filtered again using vacuum rotary evaporator until it is concentrated with the degree solid of syrup is 70° Brix [6-10].
Chemical analyses
Anthocyanin grade (method of Lees and Francis): The sample weighing is ± 10 g, added of HCl 1% that has dissolved in 50 ml of methanol and stored for 8 hours. Next the solution is filtered and diluted until the volume is 100 ml using measuring retort. We have taken 1 ml of diluted liquid and added 9 ml buffer acetic, till pH 4.5 is attained. We also took 1 ml of dilute liquid and adding by 9 ml buffer HCl-KCl till pH 9 is attained. Value of absorbency measured in wave length of 700 nm using spectrophotometer.
The method of DPPH of antioxidants activity test
Creating of curve calibration of DPPH: The test starts by creating standard curve for solution of DPPH i.e., 1 mg of DPPH poured in to 25 ml of measuring retort and fermented in methanol. The solution of DPPH has 40 ppm of its concentrations, and then diluted in 10 ml capacity of measuring retort until the concentration are 5, 10, 20, and 25 ppm. The value of absorbency measured in length of wave 515 nm.
Measuring antioxidant activity of samples: To test antioxidant activity of sweet potatoes syrup, 5 ml each of sweet potato poured in to 25 ml capacity of measuring retort and adding by watering (water or ethanol) up to limit spot. While for DPPH, 0.001 g poured in to 50 ml capacity of measuring retort and adding by solution of methanol. The next step is 4 ml each of sweet potato syrup and 2 ml of DPPH poured in to vial bottle, shaken for 30 minutes and measured its absorbance using UV-Vis shimadzu 1240 in length of wave 515.5 nm. Each of the samples is measured triplo [10-12].
The obtained data is used to account concentration remain of DPPH (DPPH concentration has added by sample). Antioxidant activity accounted using the formula:
Antioxidant activity=(DPPH first)-(DPPH remains)/(DPPH first) × 100%
Explaining:
(DPPH first): Absorbance DPPH before reacted by sample
(DPPH remains): Absorbance DPPH after reacted by sample
Data analyses: The research used complete random design nonfactorial. Treatments of hydroxylation starch symbolized as follows:
S1: hydroxylation temperature 80°C
S2: hydroxylation temperature 95°C
S3: hydroxylation temperature 110°C
Data analyzed using ANOVA and continued by DMRT test when there is a significant difference between the treatments. Data analyzed using software of SPSS version 20.0.
Anthocyanin
Anthocyanin is unstably compound and easily damage. Antioxidant can be perceived in absorbing of length wave around 500 nm. In this research the maximum absorb is in length of wave 515.5 nm (Figure 1).
Results analyzes of variance of anthocyanin to the treatments of liquidation temperature shown, the temperature of liquidation has significantly impact (p<0.05) (Table 1). At temperature of liquidation 80°C anthocyanin degree of average is 112.3100 mg/100 g sample. In the higher temperature of liquidation, degree of anthocyanin is more declining. In temperature of 95°C, degree of anthocyanin is 108.8067 mg/100 g sample and in temperature of 110°C the anthocyanin degree is 107.8767 mg/100 g sample. The significant difference of anthocyanin in this research occurs among the treatments of liquidation temperature.
| Liquidation temperature (°C) | Anthocyanin Degree (mg/100 g) |
|---|---|
| 80 | 112.3100 ± 0.2253 c |
| 90 | 108.8067 ± 0.4272 b |
| 110 | 107.8767 ± 0.2107 a |
Table 1: Effect of Liquidation Temperature to the Average of Anthocyanin Degree. Explaining: The same letter in the same rows shown is not have significant difference among the treatments in α=0.05.
Turker and Erdogdu pointed out that temperature pH has impact to efficiency of anthocyanin extract and the coefficient of its diffusion. When the pH is low, the coefficient of distribution is a higher. However, the anthocyanin is an unstable phenolic compound and be easy to eradicate by heating, thus have consequences to decline of its bioactivity. The temperature that is a very high can be increasing the degree of anthocyanin degradation. Temperature and pH are interconnecting. When the temperature is high in pH 2-4, it can be inducted abolish of the structure. The impact of heating can be abolishing almost 50% of anthocyanin degree such as frying, steams, and others food processing.
According to Revilla, impact of temperature is insignificant by adding HCL in solution that used for extraction, because the impact of HCL is greater than temperature. Using of HCl 1% in extract of anthocyanin will cause parts of hydration or even entire of anthocyanin that acetylation influencing it’s absorb in the body. Anthocyanin is more stable in acid circumstance than in bases one [13-16].
Besides of temperature stability, anthocyanin is also impacted of light, enzyme, oxygenation, different structure in anthocyanin, and concentration of anthocyanin. The declining of anthocyanin in syrup which made in different liquidation temperature is performing in Figure 2.
Antioxidant activity
Antioxidant activity tested with it ability as constraint unrestricted radical (peroxide) of DPPH compounds. The syrup that is different concentration liquidated in temperature of 80°C in DPPH which the concentration of 40 ppm. Results testing of absorbing in length wave of 515.5 nm shown that when the syrup concentrate is higher, the percentage of constraints are rising, as presented in Table 2 [17-21].
| Samples | Absorbance (A) | Average | % of Inhibition | |||||
|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | ||||||
| DPPH | 40 ppm | S1 | 80 mg/l | 0.801 | 0.8 | 0.8 | 0.8 | 28.51 |
| DPPH | 40 ppm | S1 | 100 mg/l | 0.679 | 0.679 | 0.679 | 0.679 | 39.32 |
| DPPH | 40 ppm | S1 | 120 mg/I | 0.6 | 0.568 | 0.568 | 0.579 | 49.24 |
| DPPH | 40 ppm | S1 | 140 mg/l | 0.543 | 0.543 | 0.543 | 0.543 | 51.47 |
| DPPH | 40 ppm | SI | 160 mg/l | 0.426 | 0.425 | 0.426 | 0.426 | 61.93 |
Table 2: Constraining Activity of Peroxide in Liquidation temperature of 80°C absorbance (A).
Results of regression analyze of percentage (%) inhibition and syrup concentration S1 presented in linear formula 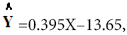 as shown in Figure 3.
as shown in Figure 3.
The percentage (%) of concentration of free radical inhibition is 50% (IC 50). The value of IC 50 syrup made of liquidation temperature of 80°C is 129.87 mg/ml. The syrup that is different concentration liquidated in temperature of 95°C in DPPH which the concentration of 40 ppm. The test result of absorbance in length wave of 515.5 nm shown that when the concentration of syrup is higher will be increasing percentage of constraint (Table 3).
| Samples | Absorbance (A) | Average | Percentage (%) of inhibition | ||
|---|---|---|---|---|---|
| 1 | 2 | 3 | |||
| DPPH 40 ppm S2 40 mg/l | 0.865 | 0.864 | 0.865 | 0.865 | 22.69 |
| DPPH 40 ppm S2 50 mg/l | 0.765 | 0.765 | 0.765 | 0.765 | 31.64 |
| DPPH 40 ppm S2 60 mg/l | 0.717 | 0.718 | 0.716 | 0.717 | 35.92 |
| DPPH 40 ppm S2 70 mg/I | 0.637 | 0.634 | 0.638 | 0.636 | 43.16 |
| DPPH 40 ppm S2 80 mg/l | 0.543 | 0.543 | 0.543 | 0.543 | 51.47 |
Table 3: Constraint Activity of Peroxide in liquidation temperature of 95°C.
Results of regression analyze of percentage (%) inhibition and syrup concentration presented in linear formulation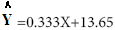 (Figure 4).
(Figure 4).
From the formula can be counted of percentage (%) of concentration inhibition to the free radical is 50% (IC 50). The value IC 50 of syrup was made by liquidation temperature of 95°C is 109.16 mg/ml. The syrup that is different concentration liquidated in temperature of 110°C in DPPH which the concentration of 40 ppm. Results test of absorbance on length wave is 515.5 nm shown that when the syrup concentration is higher, the percentage of constraining is also increase (Table 4).
| Samples | Absorbance (A) | Average | Percentage (%) of Inhibition | ||
|---|---|---|---|---|---|
| 1 | 2 | 3 | |||
| DPPH 40 ppm S3 30 mg/l | 0.721 | 0.721 | 0.072 | 0.505 | 35.57 |
| DPPH 40 ppm S3 40 mg/l | 0.674 | 0.674 | 0.674 | 0.674 | 39.77 |
| DPPH 40 ppm S3 50 mg/l | 0.533 | 0.531 | 0.532 | 0.532 | 52.46 |
| DPPH 40 ppm S3 60 mg/I | 0.436 | 0.485 | 0.485 | 0.485 | 56.66 |
| DPPH 40 ppm S3 70 mg/l | 0.361 | 0.362 | 0.362 | 0.362 | 67.65 |
| DPPH 40 ppm S3 80 mg/l | 0.721 | 0.721 | 0.072 | 0.505 | 35.57 |
Table 4: Constraining Activity of Peroxide on Liquidation temperature of 110°C.
The results of regression analyze of percentage (%) Inhibition and syrup concentration is presented in linear formula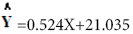 (Figure 5).
(Figure 5).
From the formula in Figure 5 can be accounted percentage (%) concentration of inhibition to free radical is 50% (IC 50). The value of IC 50 of syrup which made in liquidation temperature of 110°C is 55.29 mg/ml. Meanwhile, from the results of regression percentage (%) inhibition (constraints) of compound free radical of DPPH and the syrup concentration which made in different liquidation temperature would be obtained value IC 50 (Figure 6).
• Liquidation temperature significantly impacts declination of anthocyanin degree.
• Antioxidant activity of the syrup declines when the liquidation temperature increases.
• The values of IC 50 of each syrup are Sl (liquidation temperature 80°C=129.87 mg/ml); S2 (liquidation temperature 95°C=109.16 mg/ml); S2 (liquidation temperature 110°C=55.29 mg/ml).
The next research needed is to make sure whether the syrup is functional in the body of animal.
Thank you to Head of Bukit Barisan Simalem Foundation who let us use laboratory and the facilities to accomplish this research. We also thank to Prof. Dr. Erna Frida, M.Si for the supporting during the research. Special thanks to Murni Situmorang, SS who has reviewed and translated this manuscript until it is published.