Journal of Nanomedicine & Biotherapeutic Discovery
Open Access
ISSN: 2155-983X
ISSN: 2155-983X
Research Article - (2018) Volume 8, Issue 3
Plant products that are rich in antioxidant properties include grape, one of the world’s largest fruit crops and most commonly consumed fruits in the world. Grape extract comprises several bio-active compounds, mostly represented by polyphenols and phenolic acids. It could be an excellent alternative to toxic chemo drugs, with minimal side effects and economical. Grape extract exerts high antioxidant and anticancer activities against cell lines. It could be used to treat a number of cancers, including lung, colon, cervical, bladder and prostate cancers. In this paper, the antioxidant and anticancer activity of pulsed electric field (PEF) treated grape extract is investigated. HeLa cell line (cervical cancer) was used for this purpose. The results indicate more cell death due to PEF-treated extract compared to untreated one, indicating the potency of PEF-treated extract. This shows the potential of using PEF-treated grape extract as a possible anti-cancer drug.
Keywords: Anticancer activity; Antioxidant activity; Bio-active substances; Grapes; HeLa cells; Polyphenol; Pulsed electric field
Cancer is the uncontrolled growth and spread of abnormal cells and it is one of the deadliest diseases worldwide. According to Cancer Facts of 2016, Cancer is the second leading cause of death globally and is responsible for 8.8 million deaths; approximately 1 in 6 deaths is due to cancer. Around 70% of deaths from cancer occur in lowand middle-income countries [1]. According to estimates from the International Agency for Research on Cancer (IARC), in 2030, the cancer death is expected to grow up to 13 million. The most frequent cancers are colorectal, breast, prostate and lung cancers [2]. American Cancer Society estimated that the cancer incidence rate is 20% higher in men than in women. Breast cancer is the most common cancer in women and prostate cancer is more predominant cancer in men [3]. The existing cancer treatments such as surgery, radiation therapy and chemotherapy, save millions of lives, they are not serving all patients. Worldwide, millions of people die each year due to cancer. The pitiful survival rate of 5–10% for pancreatic cancer and one-year survival of glioblastoma cancer tell us that current treatments are inadequate. There are inoperable, recurrent, and radio- and chemo-resistant cancers that do not respond well to the current standard of cure. Thus, there is a critical need for safe, effective, yet affordable alternate techniques for the various types of cancers [4].
Cervical cancer is the most common cancer in Indian women and it contributes approximately 29% of all cancers in women. According to The American Cancer Society, cervical cancer has no outward symptoms in its early stages. Cervical cancer is usually treated with either radiation or chemotherapy [5]. Exposure to radiation causes damage to various cellular components with the DNA being the most critical target directly or indirectly through ionization of molecules within the cells, generating a cascade of free radicals [6]. Chemotherapy is drug therapy for cancer which works by killing the cancer cells, inhibiting them from spreading, or slowing their growth. Chemo drugs not only inhibits cancer cell proliferation but also normal cells that proliferate rapidly, such as cells in the bone marrow, oral mucosal cells and hair follicles [3]. This is the reason of several side effects that accompany chemotherapy. The use of chemical drugs causes side effects such as: fatigue, hair loss, bleeding, infection, anaemia, nausea, vomiting, constipation and diarrhoea [7]. The side effects can be minimized by the considerate choice of anticancer drug and its dose, with respect to the type of tumor, stage of cancer growth and the place of its occurrence [8]. Biomaterial based regional chemotherapy is used where local anticancer drug is directly delivered to tumor tissue to enhance chemotherapy and minimize its side-effects [7].
In recent years, the new drugs have been discovered from natural plant products and it has been proved to have the potential to cure cancer. Invent of natural anticancer drugs paved a way for cancer treatment with no or less side effects. The use of natural extracts that we use in day to day life is an excellent alternative for chemical drugs used in cancer treatment. Grape extracts rich in polyphenols has recently gained attention as an anticancer agent for cervical cancer treatment. In this paper, the potential of polyphenol as an anticancer drug is investigated. To increase the anticancer and antioxidant activity of the grape extract Pulsed Electric Field (PEF) treatment it used. PEF treatment has been proved to be a promising technology for enhancing the extraction of bioactive compounds such as antioxidants [9]. With the advent of PEF treatment, the amount of concentration of drug used for cancer treatment can be reduced. With the less amount of initial concentration of PEF treated natural grape extract, the antioxidant activity and anticancer activity can be improved. In this study, the antioxidant and anticancer activity of PEF treated and untreated grape extract against cervical cancer cell lines (HeLa cell lines) is investigated.
Grape extract was prepared from fresh grape juice using soxhlet extraction method. Obtained grape juice extract was treated by pulsed electric field to enhance the bioactive compounds present in it. The analyses were carried out according to the steps shown in Figure 1.
Figure 1: Experimental flow process: Grape extract is obtained by soxhlet extraction; Treated by pulsed electric field; Antioxidant assay (DPPH radical scavenging assay) for both untreated and PEF treated grape extract; Anticancer activity (Cytotoxicity assay/MTT assay) for both untreated and PEF treated grape extract.
Extract preparation
Fresh black grapes were purchased from local market. 500 g of the grapes were taken and then washed with tap water and then left to dry in open air away from direct sunlight to remove moisture content and then it was de-clustered. They were crushed in a juicer grinder for 2 min to obtain grape juice. The crushed black grape juice sample of 350 ml was taken and placed in the thimble of soxhlet apparatus. Solvent (300 ml ethanol) was discharged in the round bottom flask. On heating at a temperature of 70˚C to 80˚C, the solvent evaporates , rises to the condenser, where it condensed and drained back to the extractor. Once the extractor becomes full with the hot solvent, the solvent siphons down to the flask along with the extracted constituents. The recycling of the evaporated solvent is allowed to continue until the extraction was complete. The same process was repeated for green grapes.
Cell culture
A subculture of HeLa cells in Dulbecco’s Modified Eagle’s Medium (DMEM) was trypsinized separately, after discarding the culture medium. To the disaggregated cells in the flask, 25 mL of DMEM with 10% FCS was added. The cells were suspended in the medium by gentle passage with the pipette and the cells homogenized. One mL of the homogenized cell suspension was added to each well of a 24 well culture plate along with different concentration of sample extracts (0 to 250 μg/mL) and incubated at 37°C in a humidified CO2 incubator with 5% CO2 [10]. After 24 h incubation, the cells were observed under an inverted tissue culture microscope.
Electrical pulse application
BTX ECM 830 electroporator (High Voltage laboratory , College of Engineering, Anna University, Chennai) was used to generate 10 square wave unipolar pulses at 12.75 kV/cm, 100 μs pulse duration and 100 ms pulse interval. To apply electrical pulses, the extract was placed in the cuvette chamber (electrode gap=1mm) which holds approximately 100 μl of the sample. The electrodes are made up of stainless steel and the walls of the cuvette are made up of electrical insulation material Teflon. All experiments were performed in triplicates. After applying electrical pulse to the grape extract, it was transferred from cuvette to a vial and it was used for further analysis.
Antioxidant analysis
The antioxidant capacity of the samples was determined by DPPH (2,2-diphenyl-1-picryl-hydrazyl) free radical method. This method is based on the ability of the antioxidant to scavenge the DPPH cation radical. DPPH radical is stable at room temperature and produces a violet solution in ethanol. DPPH free radical is reduced in the presence of an antioxidant molecule, giving rise to colourless ethanol solution [11]. The measurement of DPPH radical scavenging activity was performed according to methodology described by Brand Williams et al. [12] 0.5 mL of sample was added to 3 mL of absolute methanol and 0.3 mL of DPPH radical solution (0.5 mM in methanol). It was incubated in dark room for 30 min at room temperature and the discoloration of DPPH was measured against the control. The color changes (from deep violet to light yellow) were read at 517 nm using spectrophotometer. The mixture of methanol and DPPH radical solution serves as blank. Free radical scavenging activity was calculated by the following formula [13].
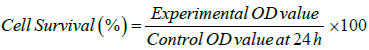 (1)
(1)
Where Ablank is absorbance of blank solution and Asample is absorbance of sample.
Cytotoxicity assay
The assay was carried out using (3-(4,5-Dimethylthiazol-2- yl)-2,5-Diphenyltetrazolium Bromide (MTT). MTT is cleaved by mitochondrial succinate dehydrogenase and reductase of viable cells, yielding a measurable purple product formazan. This formazan production is directly proportional to the viable cell number and inversely proportional to the degree of cytotoxicity. Cells were transferred into three different 96-well plates, which were cultured for 24 h. After 24 h incubation the wells were added with MTT and left for 3 h in room temperature. All contents were removed from the wells using pipette and 100 μl Sodium Dodecyl Sulfate (SDS) in DMSO were added to dissolve the formazan crystals. Optical density was read on a Lark LIPR-9608 micro plate reader, using a reference wavelength of 630 nm and a test wavelength of 570 nm [14] To calculate the experimental optical density (OD) values, OD value of blank media at 630 nm were subtracted from OD value at 570 nm. The OD values of the cells in DMEM served as the control OD. All experimental OD values were normalized with respect to the control OD value at 24 h, using the following formula.
 (2)
(2)
The grape extracts were tested for the presence of phytochemicals and their results are shown in Table 1. The grape extracts confirmed the presence of Phenol, Tannin, Flavonoid, Saponin, Anthocyanin, Catechin, Alkaloids, Anthraquinones, Carbohydrates, Terpenoids and the absence of Steroids. Total phenolic content of the grape extract was determined by Folin-Ciocalteu method. Total polyphenolic content obtained from PEF treated grape extract and untreated grape extract was 7.341 mg gallic acid/mL and 5.196 mg gallic acid/mL respectively.
| Compound | Black grape | Green grape |
|---|---|---|
| Phenol | Present | Present |
| Tannin | Present | Present |
| Flavonoid | Present | Present |
| Saponin | Present | Present |
| Anthocyanin | Present | Absent |
| Catechin | Present | Present |
| Alkaloids | Present | Present |
| Anthraquinones | Present | Present |
| Steroids | Absent | Absent |
| Carbohydrates | Present | Present |
| Terpenoids | Present | Absent |
Table 1: Phytochemical screening of black grape and green grape extracts.
Antioxidant assay
The antioxidant activity of the grape extract was determined by DPPH radical scavenging assay. Antioxidant activity depends on the ability of the sample extract to trap the DPPH radical. Higher DPPH radical scavenging ability shows that the antioxidant activity is higher in the grape extract. Antioxidant activity of PEF treated and untreated grape extract is shown in the Figure 2.
The antioxidant analysis of PEF treated and untreated grape extracts was done by varying the initial concentration of the grape extract. As the concentration of the grape extract increases from 10 μl to 50 μl, the antioxidant activity of untreated grape extract increased from 68.73% to 78.43% (+9.7%) and 69% to 82.47% (+13.47%) for PEF treated grape. For 50 μl concentration of grape extract, the antioxidant activity of untreated and PEF treated grape extract is increased from 78.43% to 82.47% (+4.04%).
Manuela Guderjan et al. reported that 11% increased antioxidant activity was obtained from the rapeseed oil when treated by PEF (at 3 kV/cm) [15]. In another study, the antioxidant capacity of the PEF treated apple juice has been discussed. Results showed that there was an insignificant difference between the control groups and the PEF treated apple juice [16]. Lamanauskas et al. [17] reported that the application of PEF treatment (5 kV/cm) to blueberries resulted in enhanced antioxidant activity (up to +16.7%). More recently, Rodriguez-Roque et al. [18] also observed an improved antioxidant capacity (up to +12.9%) of PEF treated (35 kV/cm, 1800 μs) fruit juice-based beverages compared to thermal processing (90°C, 60 s).
Cytotoxicity assay
The cytotoxicity effect of HeLa cell line against the grape extract was measured by varying the concentration of drugs for 24 hours treatment. The results of cytotoxicity obtained for different concentrations of grape extracts against HeLa cells is illustrated in Figure 3.
Figure 3: Cell viability of HeLa cells when treated with untreated grape extract and PEF treated grape extract for 24 h (for different concentrations of the grape extract) compared with control (not treated by grape extract). The results are based on the experiment performed in triplicate. Error bars are calculated using standard error.
The in-vitro cytotoxicity studies show that HeLa cells were triggered to death with the increase in sample concentration. 100% cell viability denotes that all cancer cells were live and 0% cell viability denotes that all cancer cells were dead. The cell viability of the 250 μg/ml concentration of untreated grape extract and PEF treated grape extract is 16.74% and 13.48% respectively. When compared to untreated grape extract, PEF treated grape extract kills more cancer cells. IC50 was measured to find the effectiveness of a grape extract in inhibiting cancer cells by half. The IC50 values of untreated and PEF treated grape extract was 115.105 μg/ml and 103.316 μg/ml respectively. Pulsed electric field application reduces the dosage of the chemo drugs to induce cell death in HeLa cells. After 24 h of treatment, PEF treated grape extract and untreated grape extract effectively inhibited the viability of cervical cancer cells. Morphologic changes of HeLa cells treated with untreated grape extract and PEF treated grape extract for 24 h is illustrated in Figure 4.
In general, many cancer medicines are developed from natural products. It is of practical interest to use natural products, such as grape extract to treat cancer, to reduce the toxic side-effects of typical chemo drugs, commonly administered for treating cervical and other cancers. Grape is an abundantly available fruit all over the world. Grape extract exhibits antioxidant and anticancer properties. Results of antioxidant assay showed that the radical scavenging activity of PEF treated grape extract is higher when compared to untreated grape extract. In conclusion, results of our study demonstrated that PEF treated grape extract exhibits higher antioxidant and anticancer properties than the untreated grape extract and could be used as a potent source for treating cancer cells.