Medicinal & Aromatic Plants
Open Access
ISSN: 2167-0412
ISSN: 2167-0412
Research Article - (2023)Volume 12, Issue 1
This present study was to investigate the invitro antioxidant and anti-inflammatory activity of aqueous extract of Nilavembu Kudineer Choornam (NKC). Phytochemical screening that used in the present study was a conventional method. The antioxidant activity was evaluated by various antioxidant assays, including DPPH, Nitric oxide (NO) Hydrogen peroxide (H2O2) scavenging and Reducing Power method. The antioxidant activities were compared to an antioxidant Ascorbic acid. The Nilavembu kudineer choornam (NKC) were extracted using water solvent into different concentration (50 mg/mLto 250 mg/mL). Diclofenac sodium was used as the standard. Anti-inflammatory assays were performed by the human red blood cell (HRBC) membrane stabilization method, antiproteinase activity and anti-lipoxygenase activity. Phytochemical screening showed the presence of Alkaloids, Flavonoids, Tannins, Steroids, Glycosides, Triterpenoids, Saponins and Phenol. Nilavembu kudineer choornam (NKC) showed a significant antioxidant and anti-inflammatory activity. The highest protection capability possessed by the extract of Nilavembu kudineer choornam (NKC) in human red blood cell (HRBC) membrane stabilization method, antiproteinase activity and anti-lipoxygenase activity was at a concentration of 250 mg/mL. The findings of the present study suggested that Nilavembu kudineer choornam (NKC) could be a potential natural source of antioxidants, anti-inflammatory activities by in vitro assays and could have greater importance as therapeutic agent in preventing or slowing oxidative stress related degenerative diseases.
Nilavembu kudineer choornam (NKC); DPPH; NO; H2O2; Antiproteinase activity; Anti-lipoxygenase activity; Diclofenac sodium.
Inflammation is defined as a part of complex biological response of vascular tissues to harmful stimuli, such as pathogens, damaged cells or irritants. It is characterized by redness, edema, fever, pain, and loss of function. Inflammation may be associated with general “flu-like” symptoms such as fever, fatigue, cold, loss of appetite and muscular stiffness. The migration of leukocytes from the venous systems to the location of injury and therefore the release of cytokines are known to play an important role within the inflammatory response. These chemicals released increase blood flow to the area, resulting in redness and warmth. Some of the chemicals cause leakage of fluid into the tissues resulting in swelling. Hence, the inflammatory process may stimulate nerves, cause pain, swelling and redness [1].
Inflammation are often classified as either acute or chronic. Acute inflammation is a short-termprocess, usually appearing within a few minutes or hours and ceasing upon the removal of injurious stimulus. Chronic inflammation ensues when the persistence (due to non-degradable pathogens) of injurious agents (foreign bodies) leads to a progressive shift in the type of cells present at the site of inflammation and it may last for many days, months or even years. Chronic inflammation is characterized by dominating presence of macrophages in the injured tissue. Although, these cells are powerful defensive agents in the body, the toxins they release (including reactive oxygen species) are injurious to the organisms’ own tissues as well as invading agents. Consequently, chronic inflammation is always accompanied by tissue destruction. One of the features of inflammation is increased oxygenation of arachidonic acid which is metabolized by two enzymic pathways: The 5- lipooxygenase and the Cyclooxygenase (CO) pathways leading to production of mediators of inflammation; leukotrienes and prostaglandins respectively [2-4].
Anti-inflammatory agents exert their effects through a variety of mechanisms, including inhibition of cotton pellet granulation, uncoupling of oxidative phosphorylation, inhibition of denaturation of protein, stimulation and inactivation of adenosine triphosphate phosphatase, erythrocyte membrane stabilization, lysosomal membrane stabilization, fibrinolytic assay, proteinase inhibition and inhibition of some enzymes that are involved in inflammation. Available nonsteroidal anti-inflammatory drugs, glucocorticoids, and disease-modifying anti-rheumatic drugs provide only symptomatic reliefs and are associated with severe side effects. Hence, there is an urge to develop drugs with negligible risks. Herbal medicine contains many phytoconstituents and has potential medicinal values with fewer side effects [5,6].
NKC composition is equal proportion of heartwood of Santalum album Linn. (Santalaceae), rhizomes nigrum Linn (Piperaceae), whole plant of Andrographis paniculata (Burm) Wall, ex Nees (Acanthaceae), tubers of Cyperus rotundus Linn. (Cyperaceae), roots of Vetiveria zizanioides (Linn) Nash. (Poaceae), whole plant of Hedyotis corymbosa (Linn.) Lam. (Rubiaceae), root of Plectranthus vettiveroides (Linn.) Nash. (Lamiaceae), whole plant of Trichosanthes cucumerina Linn. (Curcurbitaceae) and rhizomes of Zingiber officinale Rosc. (Zingiberaceae).Kudineer (decoction) is the common name given to the Siddha formulation in which the whole plant (s) or particular part of plant (s) is ground into coarse powder, called as Kudineer Choornam (coarse powder for preparation of decoction). All these plants are used traditionally in the treatment of fever, inflammation, arthralgia, arthritis, gastric ulcer, jaundice and general debility conditions [7].
The present study aimed to investigate the in vitro antioxidant and anti-inflammatory activity of aqueous extract of nilavembu kudineer choornam [8].
Collection of Nilavembu kudineer choornam (NKC)
1 Kg of Nilavembu Kudineer Choornam (NKC) was purchased from the locally available store at Thanjavur. The purchased nilavembu kudinneer powder (1 kg) was taken in an aspirator bottle and extracted successively by cold maceration technique with for 6 days. At the top of extraction, the extracts were filtered using paper and were concentrated employing a rotary vacuum evaporator [9].
Chemicals
1, 1- diphenyl-2-picrylhydrazyl and a couple of, 2’-azino-bis (3 ethylbenzthiazoline-6 sulfonic acid) (Aldrich), Ascorbic acid (SD Fine Chemicals Ltd.). Phenylhydrazine, Deoxy- D-ribose, Trichloro ethanoic acid (TCA), Thio malonylurea (TBA), Sodium nitroprusside, orthophosphoric acid, Ophenanthroline, Sulfanilamide, Naphylethylenediamine dihydrochloride, Ferric chloride, Sodium dithionite, dextrose, sodium citrate, acid, NaCl, DMSO, Tris HCl buffer, voltaren, 70% acid, lipoxidase and lenoleic acid. All chemicals used were of analytical grade [10].
Phytochemical Screening
Phytochemical analysis was done to research the presence of phytochemicals found in aqueous extracts of Nilavembu Kudineer Choornam (NKC).
In vitro anti-Oxidant activity
DPPH Radical Scavenging Activity: DPPH radical scavenging activity radical scavenging potentials of the extracts were tested against a methanolic solution of α,α-diphenyl-βpicryl hydrazyl (DPPH). 10 μg to 50 μg /mL of extract , 10 μg to 50 μg/mL of vitamin C as standards in 500 μL ethanol were taken and added with 5 mL of 100 μM DPPH in methanol. The mixture was allowed to face at temperature for 20 minutes. The control was prepared as above without extract. The readings were read at 517 nm using methanol as blank. The absorbance of control was first noted at 517 nm. The change in absorbance of the samples was measured. Scavenging activity is expressed because the inhibition percentage calculated using the subsequent equation: % Anti radical activity={(Control Abs.-Sample Abs.)/Control Abs.} x 100. Each experiment was administered in triplicate and results are expressed as mean deviation antiradical activity ± SD.
Hydroxyl Radical Scavenging Activity
The reaction volume contains different concentrations of NKC (from 10 μg to 50 μg) or vitamin C, which was employed as positive control (10 μg to 50 μg), 2.8 mM deoxy-D-ribose, and 0.2 mM phenylhydrazine. The mixture was incubated for two hours at 37°C during a water bath hydroxyl scavenging was measured by TBARS method to every tube, 1 mL of TCA (2.8%, %w/v), containing 1% TBA, was added. Further on, the test tubes were heated during a water bath for 30 min. and cooled at temperature. A mix prepared as above without deoxy-D- ribose served as blank. Absorbance was read at 532 nm. hydroxyl scavenging activity was calculated with the subsequent equation: percentage hydroxyl scavenging activity={(C-S)/C} X 100, Where C is that the absorbance of the control and S is that the absorbance of the sample. All experiments were performed in triplicate and therefore the results are expressed as mean ± SD.
Nitric Oxide Scavenging Activity: Nitric oxide scavenging activity scavenging of NO decided using Sodium Nitroprusside (SNP) as NO donor. SNP (10 mM) in phosphate buffered saline was mixed with different concentrations of extract (10 μg to 50μg /mL) in water and incubated at 25°C for 180 min. Vitamin C, rather than extract was used as positive control within the concentration of 10 μg to 50 μg μg/ mL. Further an equal volume of the griess reagent (1% sulfanilamide, 0.1% naphyl ethylenediamine dihydrochloride and three phosphoric acid) was added to the incubated solution. The absorbance was immediately measured at 546 nm. Scavenging activity was calculated with the subsequent equation: NO scavenging activity = {(Abs of Control - Abs of sample)/Abs of Control} x 100. All experiments were performed in triplicate. The results are expressed as mean deviation NO scavenging activity ± SD.
Reducing Power Activity
The reaction mixture containing o-phenanthroline (0.5 mg), 0.2 mM ferric chloride, NKC (10 to 50 μg) or vitamin C (10 to 50 μg), previously dissolved in ethanol was made up to a final volume of 5 mL. Then it had been incubated at temperature for 15–20 min. In another set, 0.3 mM sodium dithionite was added rather than the extract. Altogether cases, the absorbance was read at 510 nm. Within the case of sodium dithionite, the absorbance was considered as 100% reduction of all ferric ions present. All experiments were administered in triplicate, and results are expressed as mean ± SD
In vitro anti-inflammatory activity
Preparation of Red Blood Cells (RBCs) Suspension
Fresh whole human blood (10 ml) was collected and transferred to the heparin zed centrifuged tubes. The tubes were centrifuged at 3000 rpm for 10 min and were washed 3 times with equal volume of normal saline. The volume of the blood was measured and reconstituted as 10% v/v suspension with normal saline.
The Human Red Blood Corpuscle (HRBC) membrane stabilization method
The method was used for the present study following the methodology of Leelaprakash and Dass with some modification. The blood was collected from a healthy human volunteer who had not taken any NSAIDs for 2 weeks prior to the experiment and mixed with equal volume of Alsever solution (2 % dextrose, 0.8 % sodium citrate, 0.5 % acid and 0.42 % NaCl) and centrifuged at 3.000 rpm. The packed cells were washed with iso saline and 10 % suspension was made. Different concentration of extracts was prepared (50, 100, 150, 200 and 250 μg/ml) using DMSO and to each concentration, 1 ml of phosphate buffer, 2 ml hypo saline and 0.5 ml of HRBC suspension were added. It was incubated at 37°C for 30 minutes and centrifuged at 3.000 rpm for 20 minutes and the hemoglobin content of the supernatant solution was estimated by spectrophotometric at 560 nm. Diclofenac sodium was used as a standard drug and a control was prepared by omitting the extracts.
Percentage Protection (%)=100–[(optical density (OD) of sample/OD of control) X 100]
In vitro antiproteinase activity
The antiproteinase activity was performed according to Oyedepo and Femurewa with minor modification. The reaction mixture (2 ml) was containing 0.06 mg trypsin, 20 mM Tris HCl buffer (pH 7.4) and 1 ml test sample of different concentrations (50-250 μg/ml). The mixture was incubated for 20 minutes. After incubation 2 ml of 70% perchloric acid was added to arrest the reaction. Cloudy suspension was centrifuged, and therefore the absorbance of the supernatant was read at 210 nm against buffer as blank. The experiment was performed as triplicate. The percentage inhibition of proteinase inhibitory activity was calculated.
Percentage inhibition=Control -Test /Control × 100
Anti-lipoxygenase activity
Anti-Lipoxygenase activity was considered using linolic acid as substrate and lipoxidase as enzyme. Test samples were dissolved in 0.25 ml of 2 M borate buffer pH 9.0 and additional 0.25 ml of lipoxidase enzyme solution (20,000U/ml) is added and kept warm for 5 min at 25°C. After which, 1.0 ml of lenoleic acid solution (0.6mM) was added, mixed well and absorbance was measured at 234 nm. Diclofenac sodium was used as reference standard.
The percent inhibition was computed from the subsequent equation,
% Inhibition=[{Abs control-Abs sample}/Abs control] x 100
Statistical analysis
All data are presented as the mean ± standard deviation for the all in vitro assays tested, and each analysis was done in triplicate.
Phytochemical analysis
In this study, phytochemical screening revealed that the aqueous extract of nilavembu kudineer choornam contained phenols, flavonoids, tannins, alkaloids and saponins. Research has shown that flavonoids, saponins and tannins have been accounted for their anti¬inflammatory activities and are capable of significantly interference with inflammatory mediators (Table 1).
| Phytochemical | Test Result |
|---|---|
| Alkaloids | + |
| Flavonoids | + |
| Tannins | + |
| Steroids | + |
| Glycosides | + |
| Triterpenoids | + |
| Saponins | + |
| Phenol | + |
Table1: Phytochemical screening of Nilavembu Kudineer Choornam (NKC).
In vitro antioxidant activity
As shown in Table 1, NKC strongly scavenged, in a dose dependent manner, the DPPH radical with an IC50 value of 28.72 μg/mL. IC50 values of ascorbic acid were found to be 25.70 μg/mL respectively.
DPPH is a relatively stable free radical. The assay is based on the measurement of the scavenging ability of antioxidants towards the stable radical DPPH. From our study it may be postulated that NKC reduces the radical to the corresponding hydrazine when it reacts with the hydrogen donors in the antioxidant principles. DPPH radicals react with suitable reducing agents, the electrons become paired off and the solution loses color stoichometrically depending on the number of electrons taken up (Table 2).
| Concentration in μg/mL | % Free radical | % Free radical |
|---|---|---|
| scavenging of NKC | scavenging of Ascorbic acid | |
| 10 | 21.62 | 23.43 |
| 20 | 40.3 | 47.5 |
| 30 | 52.51 | 64.45 |
| 40 | 70.81 | 75.6 |
| 50 | 82.86 | 91.19 |
| IC50 | 28.72 | 25.7 |
Table 2: Free radical scavenging activity of NKC and Ascorbic acid in DPPH method.
Nitric oxide scavenging activity
NKC moderately inhibited NO in a dose-dependent fashion (Table 3). IC50 value was determined as 33.24 μg/mL, which is compared with ascorbic acid (31.15 μg/mL).
| Concentration in μg/mL | % Nitric Oxide scavenging of NKC | % Nitric Oxide scavenging of Ascorbic acid |
|---|---|---|
| 10 | 32.1 | 37.81 |
| 20 | 43.71 | 43.34 |
| 30 | 48.66 | 53.4 |
| 40 | 53.6 | 59.69 |
| 50 | 69.45 | 71.85 |
| IC50 | 33.24 | 31.15 |
Table 3: Nitric oxide radical activity of Nilavembu kudineer choornam (NKC) and Ascorbic Acid.
Nitric oxide is a free radical produced in mammalian cells, involved in the regulation of various physiological processes. However, excess production of NO is associated with several diseases. In our study the nitrite produced by the incubation of solutions of sodium nitroprusside in standard phosphate buffer at 25ºC was reduced by NKC. This may be due to the antioxidant principles in the extract which compete with oxygen to react with nitric oxide and thus inhibits the generation of nitrite.
Hydroxyl radical scavenging activity
NKC scavenged the hydroxyl radicals strongly with an IC50 value of 30.49 μg/mL. IC50 value of Ascorbic acid is 26.90 μg/mL. Results are shown in Table 4.
| Concentration in μg/mL | % Hydroxyl radical scavenging activity of NKC | % Hydroxyl radical scavenging activity of Ascorbic acid |
|---|---|---|
| 10 | 25.43 | 37.72 |
| 20 | 43.22 | 51.76 |
| 30 | 56.47 | 65.43 |
| 40 | 65.61 | 72.91 |
| 50 | 71.6 | 78.63 |
| IC50 | 30.49 | 26.9 |
Table 4: Hydroxyl radical scavenging activity of Nilavembu kudineer choornam (NKC) and Ascorbic Acid.
The hydroxyl is a particularly reactive radical formed in biological systems and has been implicated as a highly damaging species in radical pathology, capable of damaging almost every molecule found in living cell. This radical has the capacity to join nucleotide in DNA and cause strand breakage which contributes to carcinogenesis, mutagenesis and cytotoxicity. In addition, this species is considered to be one of the quick initiator of lipid peroxidation process, abstracting hydrogen atom form the unsaturated fatty acids. NKC has found to scavenge OH radicals but not as strongly as Ascorbic Acid. Thus NKC may be useful as an antioxidant and may prevent damages that arise from OH radicals by scavenging them. This OH radical capacity of NKC may be due flavonoids as found present in NKC by qualitative chemical tests. It is found that flavonoids are potent OH radical scavenging agents.
Reducing power activity: Reducing power is related to antioxidant activity and should function a big reflection of the antioxidant activity. Compounds with reducing power indicate that they're electron donors and may reduce the oxidized intermediates of lipid peroxidation processes, in order that they will act as primary and secondary antioxidants. In this assay, the yellow colour of the test solution changes to varied reminder green and blue counting on the reducing power of every compound. Presence of reducers causes the conversion of the Fe3+/ferricyanide complex used in this method to the ferrous form. By measuring the formation of pearl’s prussian blue at 700 nm, it is possible to determine the concentration of Fe3+ ion. Higher absorbance of the reaction mixture indicates higher reductive potential. The reducing capacity of a compound may serve as a significant indicator of its potential antioxidant activity. As illustrated in the Table 5, NKC has shown IC50 value of 28.60 μg. IC50 of ascorbic acid is 27.31 μg /ml.
| Concentration in μg/mL | % Reducing Power activity of NKC | % Reducing Power activity of Ascorbic acid |
|---|---|---|
| 10 | 21.56 | 26.44 |
| 20 | 42.49 | 47.46 |
| 30 | 58.46 | 63.28 |
| 40 | 71.85 | 72.81 |
| 50 | 78.49 | 80.86 |
| IC50 | 28.6 | 27.31 |
Table 5: Reducing Power activity of Nilavembu kudineer choornam (NKC) and Ascorbic Acid
In vitro anti-inflammatory activity
The Human Red Blood Corpuscle (HRBC) membrane stabilization method
In the present study, it can be seen that each concentration of NKC could provide protection against stabilization of human red blood cell membrane. We found that the highest protection capability possessed by NKC was at concentration 250 μg / ml (Figure 1).
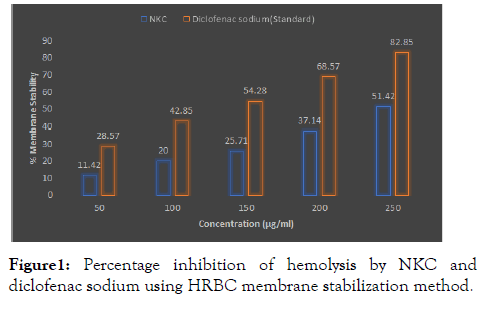
Figure 1: Percentage inhibition of hemolysis by NKC and diclofenac sodium using HRBC membrane stabilization method.
The HRBC membrane stabilization has been used as a method to study the invitro anti-inflammatory activity because the erythrocyte membrane is analogous to the lysosomal membrane and its stabilization implies that the extract may well stabilize lysosomal membranes. Stabilization of lysosomal is vital in limiting the inflammatory response by preventing the discharge of lysosomal constituents of activated neutrophil, like bacterial enzymes and proteases, which causes further tissue inflammation and damage upon extra cellular release. The lysosomal enzymes released during inflammation produce a various disorders. The extra cellular activity of these enzymes are said to be related to acute or chronic inflammation. The nonsteroidal drugs act either by inhibiting these lysosomal enzymes or by stabilizing the lysosomal membrane. Aitadafoun have indicated some plant extracts which show membrane stabilizing properties, and that they possess interfering activity with the first phase of the inflammatory mediators’ release, namely, the prevention of phospholipases release that trigger the formation of inflammatory mediators. Earlier studies have reported plant extracts possessing phytochemicals with anti-inflammatory properties may as well have antioxidant activities against chain reactions triggered by reactive oxygen species associated with inflammation.
Proteinase inhibitory activity
The Nilavembu Kudineer Choornam (NKC) exhibited significant antiproteinase activity from different parts. The maximum inhibition was observed NKC extract (56.41 %). The standard (82.05 %) drug showed the maximum proteinase inhibitory action (Figure 2).
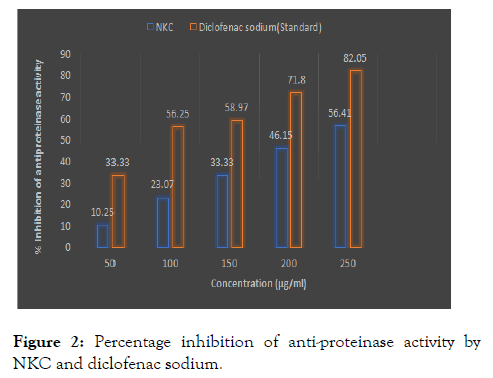
Figure 2: Percentage inhibition of anti-proteinase activity by NKC and diclofenac sodium.
Proteinases have been implicated in arthritic reactions. Neutrophils are known to be a source of proteinase which carries in their lysosomal granules many serine proteinases. It was previously reported that leukocytes proteinase play important role in the development of tissue damage during in inflammatory reactions and significant level of protection was provided by proteinase inhibitors. Recent studies have shown that a lot of flavonoids and related polyphenols contributed significantly to the antioxidant and anti-inflammatory activities of the many plants. Hence, the presence of bioactive compounds in the Nilavembu Kudineer Choornam (NKC) may contribute to its antioxidant and anti-inflammatory activity.
Anti-lipoxygenase activity
Aqueous extract of NKU has been checked at 50, 100, 150, 200 and 250 μ/ml. From these result the strongest inhibition was obtained at concentration 250 mg/ml. The NKC showed 64.7 % and standard Diclofenac showed a 91.17 % inhibition at a degree of 250 μ/ml (Figure 3).
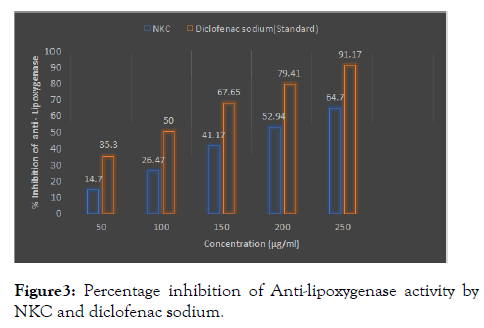
Figure 3: Percentage inhibition of Anti-lipoxygenase activity by NKC and diclofenac sodium.
The establishment of latest in vitro test systems has stimulated the screening of plants getting to find leads for the event of latest drugs. The plant lipoxygenase pathway is in many respects like the ‘arachidonic acid cascades’ in animals. For this reason, the in vitro inhibition of lipoxygenase constitutes an honest model for the screening of plants with antiinflammatory potential.
Lipoxygenases are the key enzymes within the biosynthesis of leukotrienes. Leukotrienes play a crucial role in several inflammatory diseases, like arthritis, asthma, cancer, and allergic diseases. The mechanism of anti-inflammation may involve a series of events during which the metabolism of arachidonic acid plays a crucial role. During this process, arachidonic acid is cleaved from the membrane phospholipids upon appropriate stimulation of neutrophils, and may be converted to leukotrienes and prostaglandins through lipoxygenase and cyclooxygenase pathways, respectively.
Lipoxygenase catalyzes deoxygenation of polyunsaturated fatty acids to supply cis, trans-conjugated diene hydroperoxides, like leukotrienes, which are essential mediators during a sort of inflammatory events. Previous studies have shown that polyphenols may block or interfere with the cascade process of arachidonic acid metabolism by inhibiting lipoxygenase activity and, also, they'll function scavengers of varied reactive free radicals which are produced during arachidonic acid metabolism. The results obtained from our studies on NKU have shown a possible anti-inflammatory activity. The NKU inhibited the lipoxygenase enzyme activity. This means that NKC is more useful in studies of inflammation and in various related physiological studies, aging and diseases like cancer, nervous disorder etc.
In the present study, results indicate that the aqueous extracts of Nilavembu kudineer choornam (NKC) possess anti-oxidant and anti-inflammatory properties. These activities could also be thanks to the strong occurrence of Phytochemicals compounds like alkaloids, flavonoids, tannins, steroids and phenols. The NKC function radical inhibitors or scavenger or acting possibly as primary oxidants and inhibited the human red blood corpuscle (HRBC) membrane stabilization method, antiproteinase and Anti-lipoxygenase activity. This study gives on concept the compound of the Nilavembu kudineer choornam (NKC) are often used as lead compound for designing a potent anti-inflammatory which may be used for treatment of varied diseases like cancer, nervous disorder, aging and inflammation.
The authors wish to thank the management of American University of Phnom Penh Mukuba University, Zambia and Govt. Arts College (Autonomous) Kumbakonam, Tamil Nadu, India for providing infrastructure and support for the research work.
Citation: Pakkirisamy M, Daniel N, Mani J (2022) Antioxidant and Anti-inflammatory Activity of Nilavembu kudineer choornam: In vitro Evaluation. Med Aromat Plants 10: 060
Received: 11-Aug-2022, Manuscript No. MAP-22-11334; Editor assigned: 15-Aug-2022, Pre QC No. MAP-22-11334; Reviewed: 31-Aug-2022, QC No. MAP-22-11334; Revised: 12-Sep-2022, Manuscript No. MAP-22-11334; Accepted: 20-Sep-2022 Published: 20-Sep-2022 , DOI: 10.35284/2471-9315.22.11.440
Copyright: © 2022 Pakkirisamy M, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.