Medicinal & Aromatic Plants
Open Access
ISSN: 2167-0412
ISSN: 2167-0412
Research Article - (2017) Volume 6, Issue 1
Fever is a symptom that is associated with pathological processes in animal body. It is mostly presented in a number of infections and diseases. Although, fever is utilized by the immune system as a means of defense against infections and diseases, it lowers the quality of life. Since fever brings discomfort to the victims it requires medication. Synthetic drugs that are used to manage fever are inaccessible and are associated with adverse effects. Herbal medicines are deemed to be safe, have good efficacy and are assumed to have fewer side effects. C. abyssinica has been used traditionally by Kallenjin community to manage fever among other ailments. However, ethnomedicinal use of the C. abyssinica lacks scientific validation. The study was designed to determine the antipyretic potential of dichloromethane root extracts of C. abyssinica on turpentine-induced pyrexia in Wistar albino rat. Thirty Wistar albino rats aged 8-9 weeks and weighing 120-140 g were grouped into normal group, positive control, negative control and three experimental groups. The laboratory animals were administered with the dichloromethane root extract at 50, 100 and 150 mg/kg body weight. The dichloromethane root extract reduced the elevated rectal temperature between 0.68 and 3.34%, while the reference drug reduced between 3.32 and 4.96%. Qualitative phytochemical screening of the root extract showed the presences of various phytochemicals compounds associated with antipyretic activities. The present study demonstrated antipyretic potential of C. abyssinica in Wistar albino rats and thus the traditional use of the C. abyssinica was scientifically confirmed.
Keywords: C. abyssinica; Fever; Antipyretic; OVLT; Albino rats
Fever means the body temperature is above normal and fever is a symptom, not a disease. It is a mechanism used by immune system to fight or defend against infections. Fever turns on the body’s immune system and helps fight infections [1]. Fever or pyrexia [2] is a common clinical sign that is characterized by raise in body temperature beyond the normal range of (36.5°C -37.5°C) brought about by raise in the body temperature regulatory set point [3]. Regulation of temperature is in the hypothalamus. Fever is triggered by substances called ‘pyrogen’ and usually induces the synthesis of prostaglandin E2 (PGE2) [4] which are major mediators of fever. Febrile response or fever involves innate immune system activation via Toll-like receptor 4 (TLR-4) leading to production of pyrogenic cytokines such as; (IL)-1β, IL-6, and tumor necrosis factor (TNF-α). These pyrogenic cytokines act on an area of the brain known as the Organum vasculum of the laminae terminalis (OVLT) and eventually leading to the release of PGE2 via activation of cyclo-oxygenase 2 enzyme (COX-2) [5]. Prostaglandin E2 acts on the hypothalamus to generate systemic response to the rest of the body, causing heat- creating effects to match the new temperature. The hypothalamus works like a thermostat in many situations [4]. Fever is associated with sickness behavior, such as depression, anorexia, sleepiness, lethargy, inability to concentrate and hyperalgesia [6].
A number of different microorganisms and other substances can cause fever and are collectively termed as pyrogens. Pyrogens are classified into either exogenous or endogenous pyrogens [7]. Products released by bacterial cell membranes such as lipopolysaccharide, toxins and breakdown of protein products in an organism body can cause the set point of the hypothalamic thermostat to increase [8]. In essence, endogenous pyrogens are cytokines, molecules associated with innate immune system. Phagocytic cells produce this molecules that causes increase in thermoregulatory set-point and this includes; interleukin 1α and interleukin 1β [9].
To manage fever, nonsteroidal anti-inflammatory drugs (NSAIDS) such as aspirin are prescribed [10]. Antipyretic drugs such as aspirin, exhibit their antipyretic activity by inhibiting cyclo-oxygenase enzyme (COX). Inhibition of cyclooxygenase enzyme results in blockage of synthesis of prostaglandins consequently the levels of PGE2 in the hypothalamic region is also reduced [11]. NSAIDs also act by suppressing the production of pyrogenic cytokines such as TNF-α and IL-β [12]. However, this synthetic drugs manifest a lot of side effects after long term use for example gastric irritation, ulceration, prolonged bleeding, renal failure, interstitial corrosion, and pruritis [13].
Though medicinal plants from immemorial time have been used as a source of antipyretic agent to manage fever, emergence of synthetic drugs resulted in neglect of their use. However, due to its availability, low cost and fewer side effects herbal medicine is gaining its position [14]. Herbal medicines from medicinal plants have been utilized by societies as the principal source of curing a number of diseases [15]. Therefore, medicinal plants are assuming important role in their wellbeing [16]. C. abyssinica has been used traditionally by the Kallenjin community to treat several ailments such as; malaria, colds, fever, skin diseases, chest problems, cancer and infertility in humans [17].
Though there are some etnobotanical studies that have been conducted on C. abyssinica, no study has been carried out on its antipyretic potential. Therefore, this study aimed at providing information on antipyretic activity of C. abyssinica as a potential candidate in development of antipyretic drug from plant based origin.
Collection and preparation of plant materials
Fresh roots of C. abyssinica were collected from Kaptebee village, Turbo sub-county in Uasin Gishu county Kenya with the help of local herbalist under accepted bio-conservation methods from its natural habitat. This process was conducted during the months of January- March, a season in which the local herbalist believed that the medicinal plant had its maximum medicinal activity. A sample of fresh twigs with leaves was presented to a taxonomist for botanical authentication and a sample voucher was deposited at the National Museum of Kenya herbarium with the voucher number (NMK/BOT/CTX/1/2). Sample roots were then cleaned with tap water to remove any dirt and chopped into smaller pieces. The root samples of C. abyssinica were completely air dried under shade for two weeks. The samples were then packed into burlap sacks and transported to Kenyatta University Biochemistry laboratory. The samples were then pulverized into fine powder using laboratory electrical mill.
Extraction
A 400 g weight of powdered root sample was soaked in 1.3 litres of DCM with regular agitation at an interval of 1 hour to uniformly mix the sample within the first 10 hours and left to stand for 48 hours. Filtration was performed using Whatman’s filter paper No. 1. Concentration of the filtrate was performed using rotary evaporator at a temperature of 41°C under reduced pressure.
Laboratory animals
Only male Wistar albino rats aged between 8-9 weeks, weighing between 120-140 g were utilized for screening antipyretic activity of the plant extract because there are not affected by hormonal fluctuations during experiment as compared to female sex which are believed to be affected hormonal fluctuations and therefore may have an effect on the results. All the experimental animals were housed in animal house at Kenyatta University in standard laboratory cages. The experimental animals were kept at room temperature for 12 hours darkness followed by 12 hours light cycles throughout the entire experimental period. The animals were fed on rodent pellet diet and water ad libitum. In this study ethical guidelines and procedures were followed while handling the laboratory animals [18].
Evaluation of antipyretic effect
Male Wistar albino rats were grouped into 6 groups of five rats each. Prior to fever induction, rectal temperature of all the experimental animals were measured using digital thermometer and recorded. All the experimental animals were fasted for 12 hours during the entire experimental period, but were allowed access to water ad libitium. Steam distilled turpentine solution was used to induce fever and it was injected intraperitoneally at a dose of 20 ml/kg according to body weight and the animal left for one hour [19]. A raise in rectal temperature of Wistar albino rats by 0.8°C after one hour was termed pyretic and proceeded to be used in the assay.
One hour after fever induction, pyretic animals in groups IV-VI were administered intraperitoneally with the three experimental doses of 50, 100 and 150 mg/kg body weight respectively. Pyretic animals in group III (Positive control) were administered with aspirin (100 mg/ kg body weight). Pyretic animals in group II (negative control) were administered with 10% DMSO only. Experimental animals in group I (normal control) were not induced with fever but administered 10% DMSO only [20]. This design is summarized in Table 1.
| Group | Treatment |
|---|---|
| I(Normal control) | DMSO (10%) |
| II(Negative control) | Turpentine (20%) |
| III(Positive control) | Turpentine(20%)+Aspirin (100 mg/kg bw) |
| IV(Experimentalgroup A) | Turpentine (20%)+C.abyssinica (50 mg/kg bw) |
| V(Experimentalgroup B) | Turpentine (20%)+C.abyssinica (100 mg/kg bw) |
| VI(Experimentalgroup C) | Turpentine(20%)+C.abyssinica (150 mg/kg bw) |
Table 1: Treatment protocol for antipyretic activity of DCM root extract of C. abyssinica in wistar albino rats.
Plant extract doses were prepared on the same day of experiment. All the treatments were administered intraperitoneally. The rectal temperatures of rats were measured by inserting a well lubricated digital thermometer about 3 cm into the anus of the rats. The mean body temperature of Wistar albino rats was recorded at 20 minutes intervals over the 1 hour before turpentine injection and was recorded as the baseline/initial temperature. Rectal temperature was measured and recorded at an interval of one hour continuously for four hours after treatments. Percentage inhibition anal temperature was calculated by formula described by Ref. [20].
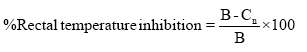
Where, B: Rectal temperature at 1 hour after turpentine injection; Cn: Rectal temperature after dose administration.
Qualitative phytochemical screening
The DCM root extract of C. abyssinica was subjected to qualitative phytochemical screening to determine the presence/absence of different active secondary metabolites constituents following the methodology described by Ref. [21]. The phytochemicals tested included flavonoids, phenolics, saponins, alkaloids, cardiac glycosides, steroids and terpenoids.
Data management and statistical analysis
Raw data on anal temperature was measured and tabulated on a broad sheet using Microsoft excel program. Data on rectal temperature was then exported to statistical software (Minitab version 17.0) for analysis. Data was subjected to descriptive statistics and results were expressed as Mean ± Standard Error of the Mean (SEM). Results among the groups was analysed for statistical significance using one way ANOVA followed Tukey’s post hoc test for pairwise comparison and separation of means. Results were presented in tables and graphs. A value of P ≤ 0.05 was considered significant.
Antipyretic activity of DCM root extract of C. abyssinica
The DCM root extract of C. abyssinica showed antipyretic effects against turpentine-Induced pyrexia in rats, which was indicated by reduction in rectal temperature (Table 2; Figure 1). In the first hour after treatment with the experimental doses, all the three doses (50, 100 and 150 mg/kg body weight), lowered the elevated anal temperature by 0.81%, 0.68% and 1.14% respectively (Table 2; Figure 1). On the other hand, aspirin lowered the anal temperature significantly by 3.32%, indicating a stronger antipyretic activity than the all the three experimental doses (P˂0.05; Table 2; Figure 1). However, the antipyretic effects of all the three doses of the DCM root extract of C. abyssinica were not significantly different (P>0.05; Table 2).
| Group | Treatment | % change in anal temperature in (°C) after dose administration | ||||
|---|---|---|---|---|---|---|
| 0hr | 1hr | 2hr | 3hr | 4hr | ||
| Normal Control | 10% DMSO | 100.00± 0.00 | 99.79± 0.16bc | 99.79± 0.16bc | 99.94± 0.12c | 99.95± 0.31c |
| (0.00) | (0.21) | (0.21) | (0.06) | (0.05) | ||
| Negative Control | Turpentine | 100.00 ± 0.00 | 101.08 ± 0.51c | 101.39 ± 0.5c | 100.82 ± 0.2c | 100.61 ± 0.4c |
| (0.00) | (-1.08) | (-1.39) | (-0.82) | (-0.61) | ||
| Positive Control | Turpentine+Aspirin (100 mg/kg bw) | 100.00 ± 0.00 | 96.68 ± 0.19a | 96.47 ± 0.12a | 95.44 ± 0.29a | 95.04 ± 0.23a |
| (0.00) | (3.32) | (3.53) | (4.56) | (4.96) | ||
| Experimental group A | Turpentine+C. abyssinica(50 mg/kgbw) | 100.00 ± 0.00 | 99.19 ± 0.56b | 97.46 ± 0.48a | 97.62 ± 0.53b | 97.56 ± 0.46b |
| (0.00) | (0.81) | (2.54) | (2.38) | (2.44) | ||
| Experimental group B | Turpentine+C. abyssinica(100 mg/kgbw) | 100.00 ± 0.00 | 99.32 ± 0.54b | 98.17 ± 0.68ab | 97.12 ± 0.80ab | 97.12 ± 0.79ab |
| (0.00) | (0.68) | (1.83) | (2.88) | (2.88) | ||
| Experimental group C | Turpentine+C. abyssinica(150 mg/kgbw) | 100.00 ± 0.00 | 98.86 ± 0.16b | 97.52 ± 0.34a | 96.64 ± 0.33ab | 96.79 ± 0.45ab |
| (0.00) | (1.14) | (2.48) | (3.34) | (3.21) | ||
Values are expressed as Mean ± SEM for five animals per group. Values with the same superscript letter are not significant different (one way ANOVA followed by Tukey’s test) (p>0.05). Percentage reduction in rectal temperature is within parenthesis.
Table 2: Antipyretic activities of DCM root extracts of C. abyssinica on Turpentine-Induced pyrexia in Wistar albino rats.
In the 2nd hour, experimental animals administered with DCM root extract of C. abyssinica at 50, 100 and 150 mg/kg body weight, reduced the anal temperature by 2.54%, 1.84% and 2.48% respectively. The reference drug, at this hour, reduced rectal temperature by 3.53% (Table 2; Figure 1). At this hour, a dose of 50 mg/kg body weight demonstrated the highest fever reducing activity. Even though the three experimental doses of C. abyssinica and the reference drug reduced the rectal temperature at different percentages, their antipyretic effects were not significantly different (P>0.05; Table 2; Figure 1).
In the third hour, the DCM root extract of C. abyssinica, at the three doses exhibited antipyretic effects by reducing the elevated anal temperature by 2.38%, 2.88% and 3.34% respectively in a dose dependent manner. Reference drug (aspirin) reduced elevated rectal temperature by 4.56% (Table 2; Figure 1). The antipyretic effects of 150 mg/kg body weight and 100 mg/kg body weight were comparable to the reference drug (aspirin) (P>0.5; Table 2).
At the end of four hours of the test period, the DCM root extract of C. abyssinica at all three dose levels, reduced the rectal temperature by 2.44%, 2.88% and 3.21% respectively in a dose dependent manner. The reference drug reduced rectal temperature by 4.96% (Table 2; Figure 1). Administration of extract to the experimental groups at all the three dose levels (50, 100 and 150 mg/kg body weight), exhibited strong antipyretic activity but showed no significant difference (P>0.5; Table 2; Figure 1). The antipyretic effects of 100 mg/kg body weight and 150 mg/kg body weight were comparable to the reference drug (aspirin) (P>0.5); Table 2; Figure 1).
Qualitative phytochemical screening
The DCM root extract of C. abyssinica upon phytochemicals test revealed the presence of the following compounds; terpenoids, alkaloids, flavonoids, steroids, cardiac glycosides and saponins. This phytochemical compounds have been associated with antipyretic activities (Table 3).
| Phytochemicals | DCM root extract of C. abyssinica |
|---|---|
| Alkaloids | + |
| Flavonoids | + |
| Steroids | + |
| Saponins | + |
| Cardiac glycosides | + |
| Phenolics | - |
| Terpenoids | + |
Table 3: Phytochemical composition of DCM root extract of C. abyssinica. Present phytochemicals denoted by (+), absent phytochemicals denoted by (-)
The study was designed to evaluate the antipyretic activity of DCM root extract on turpentine-Induced pyrexia in male Wistar albino rats. Fever can be induced in laboratory animals using several agents collectively termed as pyrogens such as lipopolysacharides (LPS), E-coli, amphetamines, sulphur, brewer’s yeast and turpentine. These agents are considered exogenous pyrogens [16,22]. Turpentine is a clear flammable liquid with pungent odour and bitter taste, refined from resin pine. It is a mixture of organic compounds especially terpenes. Subcutaneous administration of turpentine is a well established model for sterile inflammation. Turpentine causes tissue damage and induces acute phase response as well as fever [23]. According to Soszynski [24] rats respond with acute fever to intramuscular injection of turpentine reaching its peak value in 9th hour and 11th hour. Turpentine injection into experimental animals induces a persistently high fever pattern (Kuochung et al.). According to Ref. [25] turpentine causes local inflammation and robust fever when administered into laboratory animals.
The DCM root extract of C. abyssinica, in this study demonstrated antipyretic activities against turpentine-induced pyrexia in Wistar albino rats (Table 2; Figure 1). Findings from this study were relatable to other earlier research studies on evaluation of antipyretic effects of herbal extracts of herbal plants using animal models. Similar study carried out by Ref [26] showed that the methanolic stem extracts of Harrisonia abyssinica and Ladolphia buchananiii staphf possess antipyretic activities against turpentine-induced pyrexia in Wistar albino rats. Likewise, a study carried out by Ref [27] on antipyretic properties methanolic leaf extracts of Kigelia africana (Lam) and Acacia hockii de wild showed significant antipyretic activities in Wistar albino rats.
To relieve fever nonsteroidal anti-inflammatory drugs (NSAIDS) such as aspirin are prescribed [28]. Antipyretic drugs such as aspirin, exhibit their antipyretic activity by inhibiting cyclooxygenase enzyme (COX). Inhibition of cyclooxygenase enzyme results in blockage of synthesis of prostaglandins consequently the levels of PGE2 in the hypothalamic region is also reduced [11]. The extract lowered elevated temperature by inhibiting cyclooxygenase enzyme, a key enzyme necessary for synthesis of prostaglandins, thereby reducing fever. Moreover, the extract may have reduced the concentration of PGE2 in the hypothalamus through its action on cyclooxygenase enzyme or by enhancing of body’s own antipyretic substances like vasopressin, IL 10 and arginine [28]. However, other mechanism for blocking fever cannot be ignored.
The dose range of (50, 100 and 150 mg/kg body weight) applied in bioscreening of antipyretic activities of DCM root extract in the study were comparable to dose ranges used by Ref. [29] while evaluating the antipyretic, anti-inflammatory and analgesic activity of methanolic leaf extract of Acacia hydaspica (R) parker in laboratory animals. Similarly, Ref. [30] used these doses while evaluating antipyretic activity of methanolic leaf extract of Kigelia africana (lam) benth and stem bark extract of Acacia hockii de wild in animal models.
The DCM root extract of C. abyssinica, lowered the elevated rectal temperature more in the fourth hour than (in the third hour, the second and the first hour) (Table 2; Figure 1). This might be attributed to the fact that most of the bioactive compounds may have not been completely absorbed across the peritoneum cavity while in the third and fourth hours most of the bioactive compounds may have been absorbed across the peritoneum cavity thereby causing higher antipyretic activities. Lower percentage inhibition in first and the second hours could also be attributed to the fact that the drug needed time to be biotransformed into an antipyretic agent. Another factor is that at certain dose ranges, the active antipyretic phytochemicals compounds in the extract were not sufficient enough to lower the rectal temperature. However, the plant extract at 50 mg/kg body weight lowered the rectal temperature in the second hour, then reducing antipyretic activity in the third and fourth hours respectively (Table 2; Figure 1). This scenario could be ascribed to the fact that most of the bioactive compounds might have been quickly metabolized and excreted because of the small concentrations of phytochemicals compounds in the extract.
In the fourth and the third hours, the DCM root extract of C. abyssinica, showed a dose dependent response in reducing the elevated rectal temperature in turpentine-induced pyrexia in Wistar albino rats (Table 2; Figure 1). The dose dependent response observed in this study are in agreement with studies by Ref [31], who observed a similar trend while examining antipyretic activities of methanolic bark extract of T. brownii in rats. Likewise, [26] Ref observed similar dose dependent response while evaluating the antipyretic properties of methanolic stem bark extracts of H. abyssinica oliv and L. buchananiii staphf against turpentine-induced pyrexia in Wistar albino rats. Within the first and second hours, the extract demonstrated a non-dose dependence antipyretic activity. This phenomenon can be explained in terms of certain dose limits when excited the activity of that particular drug is reduced or is rendered inactive.
The presences of active phytochemical compounds in the DCM root extract of C. abyssinica might have contributed to the antipyretic activity. Flavonoids have been found to inhibit prostaglandin E synthase production and its transcription, resulting in blockage of prostaglandin synthesis [32]. Flavonoids such as baicalin have been shown to exert antipyretic activity by inhibiting TNF-α [33].
The DCM root extract reduced the elevated temperature when compared to the aspirin (reference drug). This signifies that the plant is endowed with potent antipyretic properties and it might be useful in obtaining therapeutic agents capable of relieving fever. The study therefore scientifically confirms the traditional use of the medicinal plant in management of pyrexia.
The authors acknowledge the technical support provided by James Adino, Daniel Gitomga and James Ngujiri.