Chemotherapy: Open Access
Open Access
ISSN: 2167-7700
ISSN: 2167-7700
Research Article - (2023)Volume 11, Issue 1
IL-2 and TRAIL are therapeutic agents known for their antitumor role, but they can be cleared quickly by the body or be cytotoxic, compromising their function. To reverse this impasse, the synthesis of these agents by live bacteria directly in the tumor is a viable approach, due to bacteria’s preference to colonize tumor tissue. It happens because of the high nutrient grid in this microenvironment and possible escape from the immune system since solid tumors are ischemic and have regions of hypoxia. In this context, attenuated Salmonella represents a promising live vector for delivery of antitumor molecules. The present study investigated the effects of IL-2, TRAIL and mixture of proteins expressed by recombinant Salmonella, strain SL3261, with unique and innovative construction with IL-2 and TRAIL proteins, on bladder cancer cells and in mice od C57BL/6. Human bladder cancer cell line RT4 was exposed for 24 and 48 hours to these proteins and were analyzed with flow cytometry, ELISA, dyes and fluorescent antibodies. In the animal model, tumor induction was performed with MB49 cells, followed by treatment with bacterial strains and collection of tissues and organs for analysis. The results demonstrated that both agents are cytotoxic to the tumor cells, as they cause a decrease in cell viability, modification of its proper morphology and induction of apoptosis. This effect is caused by the activation of the iNOS enzyme by IL-2, which leads to the production of Nitric Oxide (NO) with consequent activation of genes that lead to DNA degradation and by the activation of the Caspase family by TRAIL, causing apoptosis. In mice, there was an important regression of tumor progression and modulation of the immune system. Therefore, IL-2, TRAIL and MIX synthesized by recombinant Salmonella show promising potential in bladder cancer therapy.
Apoptosis; Cancer; Drug delivery; Live vector; SL3261
IL-2: Interleukin 2; TRAIL: Tumor necrosis factor-Related Apoptosis-Inducing Ligand; NK: Natural Killers; YFP: Yellow Fluorescent Protein; FBS: Fetal Bovine Serum; DMSO: Dimethyl Sulfoxide; AAU: Arbitrary Autophagy Units; NO: Nitric Oxide; PS: Phosphatidylserine; EDTA: Ethylenediamine Tetraacetic Acid - Tetraacetic Ethylenediamino Acid; OD: Optical Density
IL-2 (Interleukin 2) and TRAIL (Tumor necrosis factor-Related Apoptosis-Inducing Ligand) proteins are known to have antitumor effects against several types of cancer [1-3]. IL-2 may lead to complete and permanent cancer regression in patients with metastatic melanoma and kidney cancer [3], because this protein is responsible for TCD4 lymphocytes proliferation and, consequently, activation of TCD8 lymphocytes, Natural Killers (NK) lymphocytes and dendritic cells [3,4]. TRAIL, on the other hand, shows the ability to induce cell death by apoptosis by interacting with its membrane receptors. Studies have shown that it acts specifically in multiple tumor cells rather than healthy cells [5,6]. However, TRAIL has been cleared by the kidneys very quickly, in addition to being able to cause liver cells death and patients who received high doses of IL-2 developed severe cytotoxicity [7,8].
The cancer therapy by application of genetically modified bacteria expressing antitumor molecules has been shown to be effective, as bacteria of various strains act as living vectors that synthesize proteins of interest directly in the tumor [9]. This specific targeting of bacterium, which is often inoculated at a distant location from tumor, occurs due to the tumor´s microenvironment that is rich in nutrients and protected from the immune system because of ischemia and hypoxia [10].
The use of live bacteria in cancer treatment was first reported almost 150 years ago, in a study about immunomodulation against this disease, in which patients in an advanced stage of cancer could be recovered [11]. With the help of genomic sequencing and genetic engineering, bacterial strains that act as vectors and have the ability to control cancer, have been sought [12,13]. The bacteria of Salmonella genus showed its preferential replication in solid tumors and its ability to transport and express genes coding for therapeutic molecules [10].
Bladder cancer is among the most prevalent cancers worldwide, with 549,393 new cases reported in 2018 [14]. Non-muscle-invasive, muscle-invasive and metastatic are different forms of the disease, each one with different molecular conductors [15]. It is the 9th most common type of cancer and is more prevalent in men, with high recurrence, which is an important health problem [16,17].
The current cancer treatments use radio and chemotherapy, however these approaches do not result in the necessary effectiveness, since metastases are still recurrent and are the main cause of death resulting from this disease. The low selectivity of this type of treatment for tumor cells reduces the benefit of these therapies because the mechanism involved in this process cannot provide limited cytotoxicity to tumor tissue, resulting in devastating side effects as healthy cells are equally exposed. Thus, a proposal for selective cancer therapy should overcome these obstacles and the inhibition of metastasis appears as the main target for new therapies [13]. Therefore, this study aimed to evaluate the antitumor effect of the therapeutic agents IL-2 and TRAIL expressed by recombinant Salmonella against bladder tumor in vitro.
Recombinant lineages of S. typhimurium
Three recombinant strains of S. typhimurium, SL3261 (with the plasmid insertion but without the genes), SL3261_IL-2 (with insertion of plasmid for IL_2 and fused with Yellow Fluorescent Protein (YFP)) and SL3261_TRAIL (with insertion of plasmid for TRAIL fused with Emerald Green Fluorescent Protein-EmGFP ) were provided by Dr. Luciana Camillo from Laboratory of Inflammation and Infectious Diseases and Dr. Adilson José da Silva from Laboratory of Cellular Factory, both in Federal University of São Carlos, UFSCar, São Carlos-SP, Brazil. For the insertion of these genes the plasmid chosen was pAE containing the nirB promoter, the expression of which is induced in anaerobiosis and already used for this type of approach [18,19]. The cultivation and maintenance of strains and the expression of IL-2 and TRAIL proteins have been previously described in murine bladder cancer cells [20].
Human bladder cancer cell culture (RT4)
The non-invasive superficial human bladder cancer cell line RT4 was used [21]. Cells were grown in culture bottles in DMEM F12 medium (Dulbecco's Modified Eagle's Medium: Nutrient Mixture F-12, Sigma-ALDRICH, USA) in the presence of 100 µL/mL penicillin/streptomycin antibiotics (LGC Biotechnology) and addition of 10% heat-inactivated Fetal Bovine Serum (FBS) (LGC Biotechnology). The bottles were incubated in a humid environment at 37°C and 5% CO2 until reaching 90% confluence, under passages.
In vitro assays
The six proposed groups and the established treatment are shown in Table 1. All tests were performed with a period of exposure to treatments of 24 and 48 hours. For each assay, fresh culture of all bacterial strains was performed.
| Groups | DMEM medium | LB medium | SL3261 | SL3261_IL-2 | SL3261_TRAIL |
|---|---|---|---|---|---|
| CTRL- | 200 µl | - | - | - | - |
| LB | 100 µl | 100 µl | - | - | - |
| SL3261 | 100 µl | - | 100 µl | - | - |
| IL-2 | 100 µl | - | - | 100 µl | - |
| TRAIL | 100 µl | - | - | - | 100 µl |
| MIX | 100 µl | - | - | 50 µl | 50 µl |
Table 1: Experimental groups and treatment. The RT4 cells were used. DMEM F12: Dulbecco's Modified Eagle's Medium: Nutrient Mixture F-12. LB medium: Bacterial culture medium. The CTRL- group did not receive treatment. The LB group did not receive treatment, but it was added LB medium. The groups SL3261, IL-2, TRAIL and MIX received treatment with bacterial culture supernatant.
Cell viability with MTT salt and neutral red dye: For cell viability tests, two methods were used independently. Tetrazolium 3-(4.5-dimethyliazole-2-il) 2.5-diphenylbromide (MTT) (SIGMA-ALDRICH, USA) was performed to evaluate the integrity of mitochondrial function by measuring the formation of formazan crystals by mitochondrial enzymes. The higher the crystals production, the greater the cell viability [22]. The neutral red dye assay is based on dye accumulation in liposome membranes. The more viable cells, the greater dye diffusion through the membrane [21]. In a 96-well plate, 1 × 104 cells/well were inoculated. After the adhesion and treatment period, the wells were washed twice with 1X PBS (phosphate saline buffer, pH 7.4) and 100 μL of MTT solution at 0.5 mg/mL and 100 μL dye at 30 μg/mL were added. The reaction occurred for 3 hours in MTT assay and for 2 hours in neutral red dye assay at 37°C and 5% of CO2. Blank controls were made containing only MTT and red dye solution in addition to cell death control (C+) with 5% extran enzymatic detergent (only for MTT). Then, the reagent solutions were removed and 100 µL of Dimethyl Sulfoxide (DMSO) diluent for MTT and 200 µL of 1% Acetic Acid and 50% Ethanol (1:1) diluent were added per well, followed by reading the absorbance at 570 nm (MTT) and 540 nm (red dye) in spectrophotometer (Thermo Scientific™ Multiskan™ GO Microplate Spectrophotometer). The percentage of cytotoxicity was shown by comparing the data obtained with C-group according to equation 1.
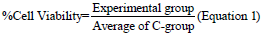
Cell death by autophagy: The index of Arbitrary Autophagy Units (AAU) indirectly measures autophagic cell death by calculating the correlation between the proportion of autophagic vacuoles stained in the neutral red dye assay and the cell survival rate obtained by the average of the values from MTT assay according to equation 2.

There is a greater incorporation and retention of red dye in late autophagic vacuoles which leads to an overestimated value of cell viability in red dye assay, so it must be normalized by the mean of cellular viability obtained in MTT. The positive correlation with cell death by autophagy is described when the AAU is more than 1. Thus, the data obtained in MTT and neutral red dye assays in different groups and periods were used to calculate the AAU [23].
Cellular morphology by optical microscopy: Cell morphology of RT4 strain after exposure to treatments was observed by optical microscopy. In a 96-well plate, 1 × 104 cells/well were inoculated. After the period of adhesion and treatment, the wells were observed under an optical microscope Axiovert 40 CFL (Zeiss), with 10X objective lens, whose images were captured with camera coupled model LOD-3000 (Bio Focus) and analyzed by Future WinJoeTM 2.0 version software in final resolution of 100 X.
Nitric Oxide (NO) production by griess reaction: The indirect production of NO from nitrite ion (NO2-) was performed by Griess reaction [24,25]. In a 96-well plate, 1 × 104 cells/well were inoculated. After the period of adhesion and treatment, 50 μL of the supernatant was collected and added to a new plate followed by the addition of 50 μL of Griess solution (mixture 1:1 of solution A [sulfanilamide 1% in phosphoric acid 5%] and solution B [0.1% N-(1-Naphthyl) ethylenediamine dihydrochloride]) for 15 minutes at room temperature. The absorbance reading was performed at 554 nm in spectrophotometer (Thermo Scientific™ Multiskan™ GO Microplate Spectrophotometer). The nitrite concentration in supernatant was quantified using a standard curve with known concentrations of nitrite in mM as described in the kit.
Caspase 3 levels dosage: For the detection of apoptosis via increased activity of Caspase-3 enzyme, the Kit EnzChek® Caspase-3 Assay Kit #1 was used. It continuously monitors the activity of Caspase-3 and closely related proteases in cell extracts by substrate derived from 7-amino-4-methylcoumarin (Z-DEVD-AMC) which produces a bright blue fluorescent product after proteolytic cleavage. In a 24-well plate, 1 × 105 cells/well were inoculated. After the adhesion and treatment period, the plate was centrifuged and washed with 1X PBS and the cells were removed from the wells with a scraper. The cell extract was lysed by a lysis buffer for 30 minutes at -20°C and centrifuged at 5000 g for 5 minutes. Then, 50 μL of the supernatant was added in a new 96-well black microplate with transparent background and 50 μL of the reagent solution (Z-DEVD-AMC in reaction buffer) was added. The reaction occurred for 30 minutes at room temperature. Fluorescence emission was read at 342-441 nm in Spectra MAX i3TM (Molecular Devices) equipment.
Apoptosis with Annexin V marker: Cell death by apoptosis was also analyzed using the Annexin V marker (BD Biosciences detection kit) with fluorescent markers PE and 7AAD. In apoptotic cells, the phospholipid membrane Phosphatidylserine (PS) is translocated from the inner to the outer leaflet of the plasma membrane, exposing the PS to the external cellular environment. Annexin V is a binding protein with high affinity for PS and binds to cells with exposed PS. In a 24-well plate, 1 × 105 cells/well were inoculated. After the adhesion and treatment period, the plates were centrifuged, washed with PBS 1X and the antibodies Annexin V PE and 7AAD [1:1] were added. The reaction occurred for 15 minutes at room temperature protected from light. Then, the cells were removed with a scraper and resuspended in microtubes with 300 μL of binding buffer (1:10). The reading was performed in Flow Cytometer Accuri™ C6 BD Biosciences, selecting gates with 10,000 events. The analysis was performed using FlowJo™ version XV (BD Biosciences). Live cells and double labeled controls were performed, along with two death controls (10 minutes in a dry bath at 80°C) for equipment calibration: One marked only with PE and other marked only with 7AAD.
Il-6, IL-8 and TNF cytokine dosage: For dosage of IL-6, IL-8 and TNF cytokines in cell culture supernatant, direct ELISA (OptEIATM, BDBiosciences kit) was performed. Between each protocol step described below, the plates were washed with 300 μL/well of washing solution (PBS 1X+Tween 20 to 0.05%) and incubated at room temperature. The assay occurred in 96-well high-affinity microtiter plates. After sensitizing the plates with 100 μL/well of specific capture antibody in carbonate buffer 1:10000 (7.13 g of NaHCO3 and 1.59 g of Na2CO3:1 L of distilled water) there was incubation for 16 hours at 20°C. Then, the blocking reaction was performed with 200 µL/well with 10% milk (0% fat), followed by incubation for 1 hour. After, 50 µL of the samples and standards for cytokine titration curve were added with 2-hour incubation. The capture antibody conjugated to peroxidase enzyme was added at 100 µL/well protected from light for 1 hour and 30 minutes. Then, 100 μL/well of TMB substrate was added and the plates were incubated, still protected from light, for approximately 15 to 30 minutes. The reaction was blocked with 50 μL/well of 2 M sulfuric acid, and the plates were read at a wavelength of 450 nm, using a plate spectrophotometer (Thermo Scientific™ Multiskan™ GO Microplate Spectrophotometer). The concentrations were calculated based on cytokines titration curves patterns and expressed in pg/mL.
Cell recovery via colony formation: The recovery capacity of RT4 cells after the proposed treatment was evaluated by the cells ability to recover through the formation of colonies via clonogenic assay [26]. In a 24-well plate, 2 × 103 cells/well were inoculated. After the adhesion and treatment period, supernatant was discarded, and a new culture medium was added. After 7 days of recovery, and change of medium twice, the cells were fixed with methyl alcohol P.A. (absolute methanol) and stained with 0.1% crystal violet. The wells were photographed and colonies were counted using ImageJ software version 1.53ª [27]. The wells absorbance reading was also performed with colony dilution in 1% SDS (sodium dodecyl sulfate), at 540 nm in spectrophotometer (Thermo Scientific™ Multiskan™ GO Microplate Spectrophotometer).
In vivo assays
The experimental design of this study was based on the recommendations of the Ethical Principles of Animal Experimentation adopted by the Brazilian Society of Laboratory Animal Science (SBCAL) and approved by the Ethics Committee on Animal Use (CEUA) of the Federal University of São Carlos (UFSCar), under the license number 8915061118 (Supplementary Material 1).
Animals: Female mice of the C57BL/6 lineage, weighing between 18 and 20 grams, were obtained from the company ANILAB Animal Farm and Commerce Ltda. These animals have the Specified Pathogen Free-SPF certificate, which ensures that they are free of pathogens. All animals were kept in the Bioterium of the Department of Morphology and Pathology of the Federal University of São Carlos (DMP-UFSCar), with free access to water and food for rodents in individual cages with air control (ALESCO).
Experimental groups, cancer induction and treatment: Six experimental groups were defined with 10 animals per group (n=10) analyzed individually. The sample groups are shown below (CAbxg: bladder cancer):
• CTRL-: Healthy animals
• CTRL+: CAbxg
• SL3261: CAbxg+Treated with SL326
• SL3261_IL-2: CAbxg+Treated with SL3261_ IL-2
• SL3261_TRAIL: CAbxg+Treated with SL3261_TRAIL
• SL3161_MIX: CAbxg+Treated with SL3261_ MIX
For tumor induction, under the effect of anesthetics, the animals were submitted to transurethral catheterization with the aid of a 24-gauge polyethylene catheter without needle and the bladder was completely emptied by manual compression for the removal of urine residues. Then, the bladder epithelium was injured with 70 μl with 22% ethanol, followed by washes with 0.9% saline solution. The tumor was induced by a lesion in the internal tissue of the bladder followed by local inoculation of tumor cells MB49. Then, intravesical instillation of 70 μl of MB49 to 1 × 104 cells/ mouse suspension was performed. After instillation procedures, the catheters were removed and the animals were followed daily, analyzing tumor implantation through observation of hematuria and abdominal augmentation.
Treatment was intratumoral with 5 × 107 CFU/ml of the bacterium after 3 days of tumor induction. The time of euthanasia was determined by the weakened state of the animals, occurring 11 days after tumor implantation.
Euthanasia, obtaining and counting blood cells: The animals were anesthetized with xelasin and ketain at 20 mg/kg intraperitoneal (i.p.). The blood of the animals was obtained by puncture of the left brachial vein, using EDTA (Ethylenediamine Tetraacetic Acid- Tetraacetic Ethylenediamino Acid) as anticoagulant at the final concentration of 0.3M. The overall blood cell count was performed individually in the Neubauer chamber, and the samples were added in Turk solution (3% acetic acid and methylene blue at 1%) at 1:20 dilution. For the differential count and % (Eosinophils, Neutrophils and Mononucleated Leukocytes) smear slides were made with Fast Pannotic Dye. In each slide, 100 cells were counted, with the aid of light microscopy with a final magnitude of 1000 X. The plasma of the animals was obtained after centrifugation of the whole blood, at a speed of 1500 g, for a period of 15 minutes. Subsequently, the plasma was stored at a temperature of -20°C to perform cytokine dosage.
Plating of spleen, liver and tumor cultures for CFU count- Biodistribution: The spleens, livers and tumors of the animals were macerated with 1.0 ml of 0.9 % NaCl saline solution. Then, serial dilution of the samples was performed with saline solution (up to 1:10 or 1:100) and aliquots of 100 l of them were plated in AGAR LB. The plates were incubated at 37°C for 48 hours for later counting of colony-forming units.
Bladder weight analysis: The bladders were removed and weighed on a precision analytical scale and photographed.
Quantification of IL-12, TNF-α and IFN-γ: For the measurement of cytokines IL-12, TNF and IFN-γ the plasma of the animals was used individually by the direct ELISA assay (Kit OptEIATM, BDBiosciences) following the protocol described in item 2.3.7, being used carbonate buffer for IFN-γ and phosphate for TNF and IL-12 all with dilutions of detection antibodies, capture and enzyme in the ratio of 1:250.
Statistical analysis
The data obtained were analyzed using GraphPad Prism 7.0 (San Diego, California, USA). The entire study was conducted in at least three biological replicates and three independent experiments. Discrepant data were identified using Grubbs analysis, followed by the Shapiro-Wilk test to verify the parametric or non-parametric data characteristic. For that, the ANOVA test (analysis of variance) was applied to the parametric data and the post-test of Tukey's multiple comparisons (results were presented in mean and standard deviation). For nonparametric data, the Kruskal-Wallis test and Dunn's multiple comparison post-test were used (results were presented as the median with the upper and lower quartiles: Me [Q1; Q3]). Statistical significance was established at p<0.05.
Figure 1 represents cell viability in % obtained with MTT assay (A, B), neutral red dye (C, D) and the Arbitrary Autophagic Units (D, E) for 24 and 48 hours of exposure to treatments. Cell viability decreases significantly in IL-2, TRAIL and MIX groups, both in MTT and neutral red dye assays, when compared to the CTRL- group in 24 and 48 hours, being higher in 48 hours of exposure. In 24 hours, there was autophagy only in the IL-2 group and in 48 hours autophagy is positive in the groups SL3261, IL-2, TRAIL and MIX. The dosage of pro-inflammatory cytokines produced after 24 and 48 hours of exposure to treatments is also shown in Figure 1. At both times, levels of cytokines IL-6 (G) and IL-8 (H) were slightly higher in IL-2, TRAIL and MIX groups when compared to the others. The TNF (I) levels were significantly higher in IL-2, TRAIL and MIX groups when compared to the others.
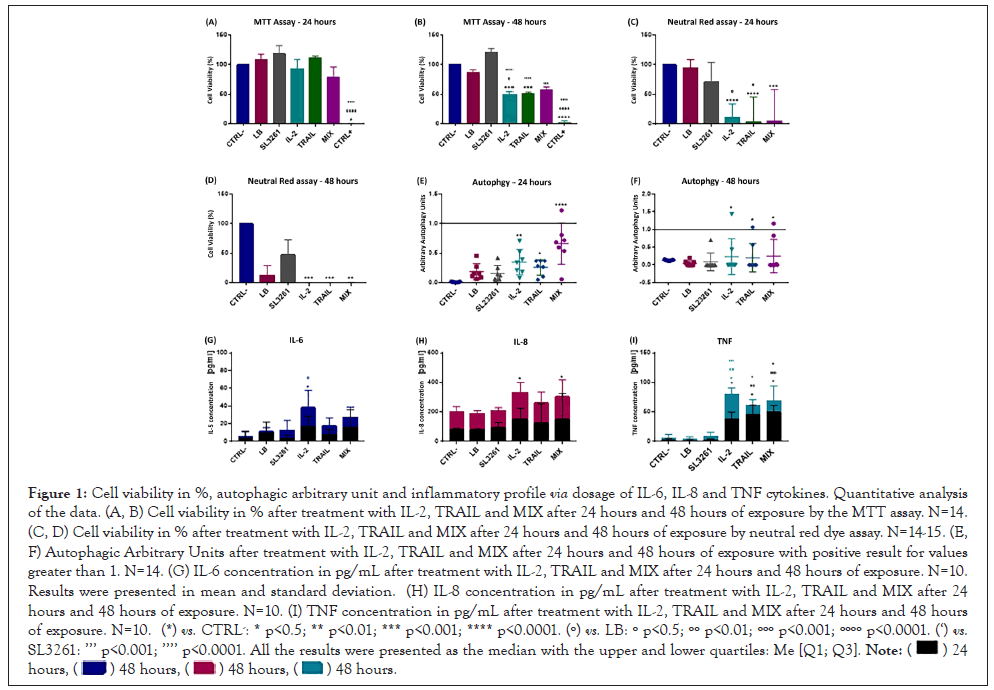
Figure 1: Cell viability in %, autophagic arbitrary unit and inflammatory profile via dosage of IL-6, IL-8 and TNF cytokines. Quantitative analysis of the data. (A, B) Cell viability in % after treatment with IL-2, TRAIL and MIX after 24 hours and 48 hours of exposure by the MTT assay. N=14. (C, D) Cell viability in % after treatment with IL-2, TRAIL and MIX after 24 hours and 48 hours of exposure by neutral red dye assay. N=14-15. (E, F) Autophagic Arbitrary Units after treatment with IL-2, TRAIL and MIX after 24 hours and 48 hours of exposure with positive result for values greater than 1. N=14. (G) IL-6 concentration in pg/mL after treatment with IL-2, TRAIL and MIX after 24 hours and 48 hours of exposure. N=10. Results were presented in mean and standard deviation. (H) IL-8 concentration in pg/mL after treatment with IL-2, TRAIL and MIX after 24 hours and 48 hours of exposure. N=10. (I) TNF concentration in pg/mL after treatment with IL-2, TRAIL and MIX after 24 hours and 48 hours of exposure. N=10. (*) vs. CTRL-: * p<0.5; ** p<0.01; *** p<0.001; **** p<0.0001. (º) vs. LB: º p<0.5; ºº p<0.01; ººº p<0.001; ºººº p<0.0001. (‘) vs. SL3261: ’’’ p<0.001; ’’’’ p<0.0001. All the results were presented as the median with the upper and lower quartiles: Me [Q1; Q3].
 .
.
Cell morphology is shown in Figure 2 in the 24 and 48-hour periods. In both time intervals, cell morphology in CTRL-, LB and SL3261 groups are preserved. On the other hand, cells treated with IL-2, TRAIL and MIX present alterations in morphology with reduced cell size, lower adhesion, extravasation of cytoplasmic content and cell membrane with lower definition.
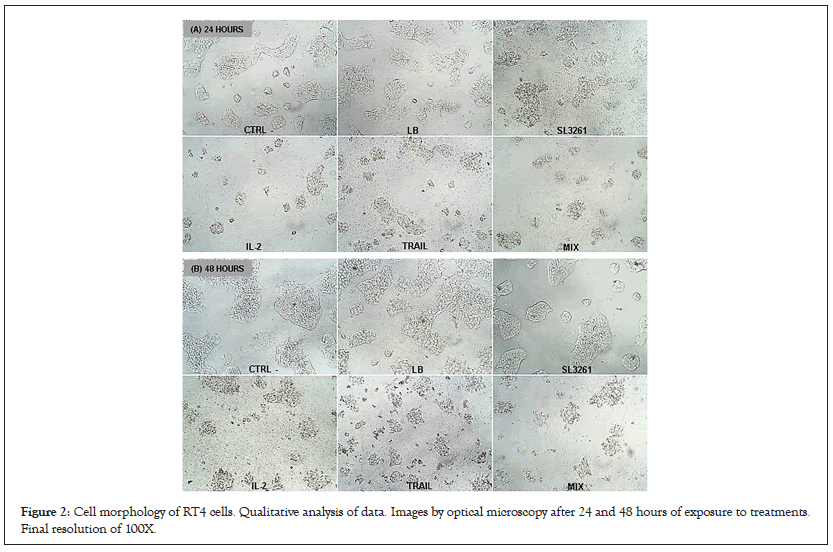
Figure 2: Cell morphology of RT4 cells. Qualitative analysis of data. Images by optical microscopy after 24 and 48 hours of exposure to treatments. Final resolution of 100X.
Figure 3 shows the dosage of NO produced (A) and the activity of Caspase-3 (B) in Optical Density (OD). The concentration of NO at 24 hours was slightly higher only in the IL-2 group. In 48 hours, there was a significant increase in NO´s concentration in IL-2, TRAIL and MIX groups. The activity of Caspase-3 enzyme was higher in IL-2, TRAIL and MIX groups, but a significant difference was observed only in TRAIL and MIX groups. The values obtained were higher in 24 hours than in 48 hours, when there was also a slight increase in Optical Density, without significant difference in IL-2, TRAIL and MIX groups.
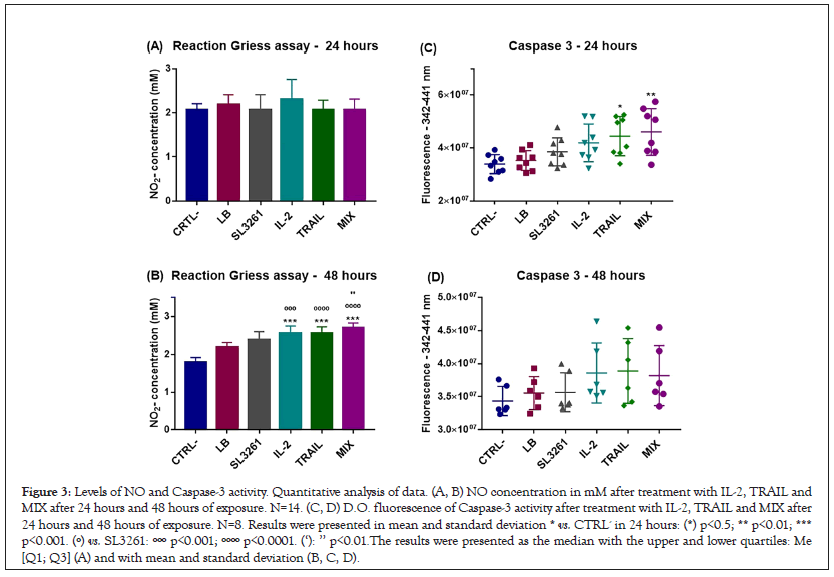
Figure 3: Levels of NO and Caspase-3 activity. Quantitative analysis of data. (A, B) NO concentration in mM after treatment with IL-2, TRAIL and MIX after 24 hours and 48 hours of exposure. N=14. (C, D) D.O. fluorescence of Caspase-3 activity after treatment with IL-2, TRAIL and MIX after 24 hours and 48 hours of exposure. N=8. Results were presented in mean and standard deviation * vs. CTRL- in 24 hours: (*) p<0.5; ** p<0.01; *** p<0.001. (º) vs. SL3261: ººº p<0.001; ºººº p<0.0001. (‘): ’’ p<0.01.The results were presented as the median with the upper and lower quartiles: Me [Q1; Q3] (A) and with mean and standard deviation (B, C, D).
Cell death by apoptosis was analyzed by flow cytometry and the data are shown in Figure 4. The initial apoptosis (positive for PE and negative for 7AAD) and late apoptosis (positive for PE and 7AAD) were differentiated. It is notable that in treated groups with IL-2, TRAIL and MIX, there was a higher total apoptosis in both exposure intervals. However, late apoptosis was higher than initial apoptosis at both times, mainly at 48 hours. Dot plot graphs obtained through flow cytometry of the groups CTRL-, LB, SL3261, IL-2, TRAIL and MIX in RT4 cell culture in 24 and 48 hours are showed in the Figures 4A and 4B with 7-AAD on the y-axis and PE Annexin V on the X-axis. The Figures 4C and 4D are representative of histograms showing the fluorescence peak emission of the respective markers, according to each group analyzed in both 24 hours and 48 hours. At both times the emission peaks of the IL-2, TRAIL and MIX groups are significantly higher than the peaks of the CTRL-, LB and SL3261 groups. The data presented are representative (Figures 4A-D), showing a single reading from each group, since the sample number is n=11. The graphs in Figures 4E, 4F, 4I and 4J show the fluorescence in % of each marker. The cells that are considered viable are PE Annexin V and 7-AAD negative. The cells that are in early apoptosis are PE Annexin V positive and 7-AAD negative, while cells that are in late apoptosis or already dead are both PE Annexin V and 7-AAD positive. In Figure 4G and 4H are the heatmap graphs where gray represents the lowest values and blue and pink the highest values at 24 and 48 hours, respectively.
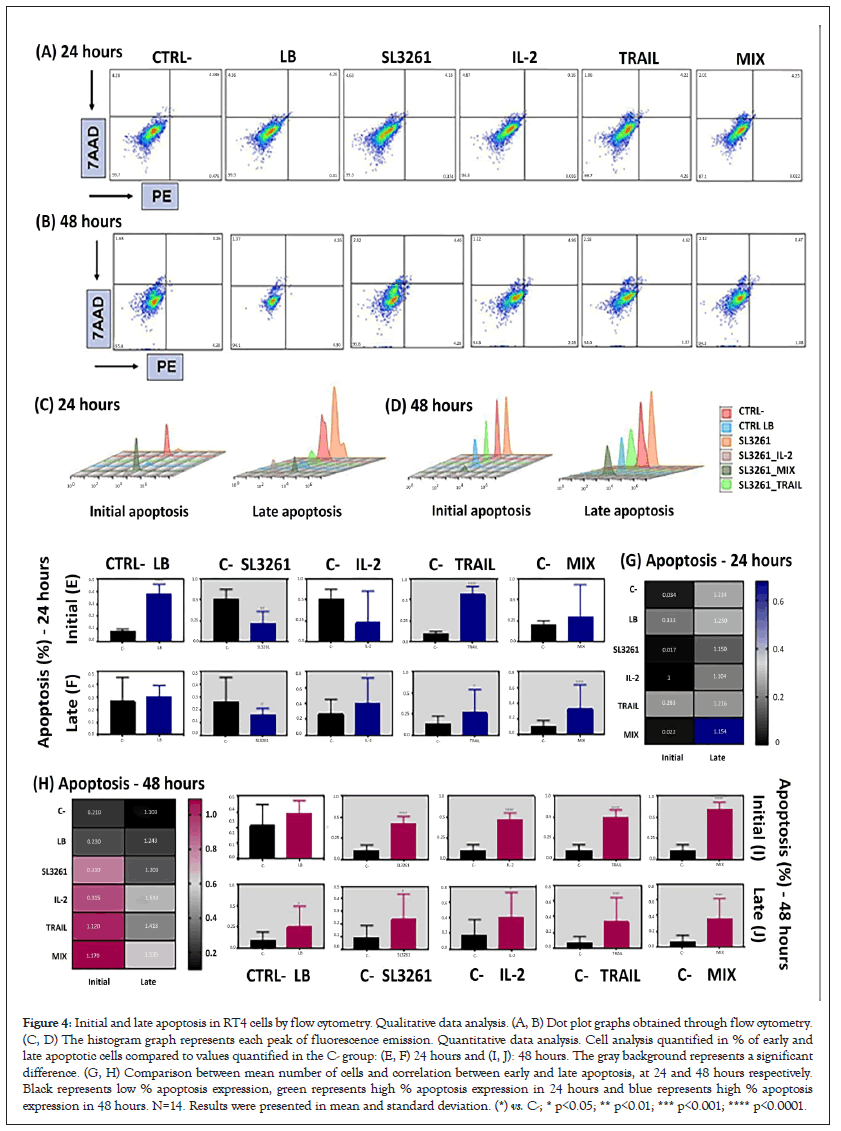
Figure 4: Initial and late apoptosis in RT4 cells by flow cytometry. Qualitative data analysis. (A, B) Dot plot graphs obtained through flow cytometry. (C, D) The histogram graph represents each peak of fluorescence emission. Quantitative data analysis. Cell analysis quantified in % of early and late apoptotic cells compared to values quantified in the C- group: (E, F) 24 hours and (I, J): 48 hours. The gray background represents a significant difference. (G, H) Comparison between mean number of cells and correlation between early and late apoptosis, at 24 and 48 hours respectively. Black represents low % apoptosis expression, green represents high % apoptosis expression in 24 hours and blue represents high % apoptosis expression in 48 hours. N=14. Results were presented in mean and standard deviation. (*) vs. C-; * p<0.05; ** p<0.01; *** p<0.001; **** p<0.0001.
Figure 5 represents the ability of cell recovery through colony formation after 24 and 48 hours of exposure. A and B shows the colony numbers and C and D shows Optical Density. The recovery capacity of RT4 cells is compromised after treatments with IL-2, TRAIL and MIX because both the number of colonies and O.D. are significantly lower when compared to the other groups.
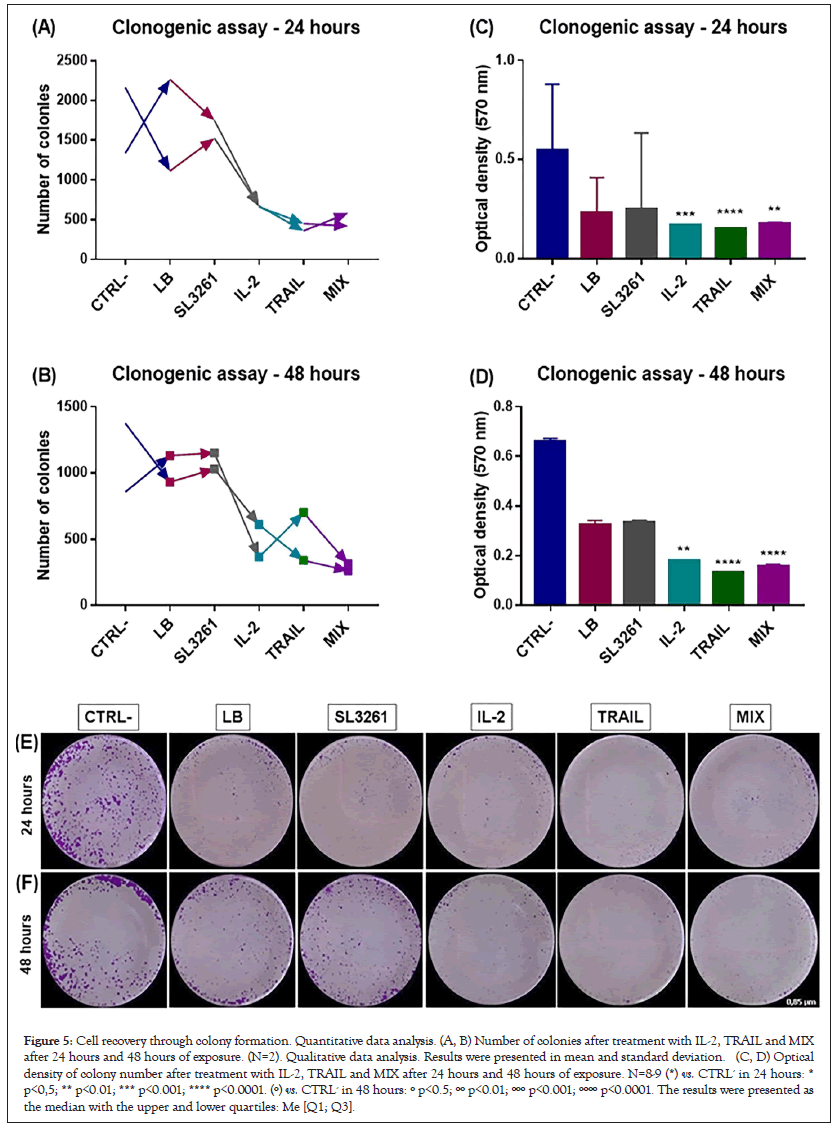
Figure 5: Cell recovery through colony formation. Quantitative data analysis. (A, B) Number of colonies after treatment with IL-2, TRAIL and MIX after 24 hours and 48 hours of exposure. (N=2). Qualitative data analysis. Results were presented in mean and standard deviation. (C, D) Optical density of colony number after treatment with IL-2, TRAIL and MIX after 24 hours and 48 hours of exposure. N=8-9 (*) vs. CTRL- in 24 hours: * p<0,5; ** p<0.01; *** p<0.001; **** p<0.0001. (º) vs. CTRL- in 48 hours: º p<0.5; ºº p<0.01; ººº p<0.001; ºººº p<0.0001. The results were presented as the median with the upper and lower quartiles: Me [Q1; Q3].
Figure 6 shows the weight of the bladders of c57BL/6 animals. In Figure 6A are the data of all animals showing that there was tumor implantation when compared with the CTRL- group and that treatments with the 3 bacterial lines are effective in tumor reduction. Figure 6B is an image as photographs of bladders of all groups. The bladders of the animals treated with SL3261_IL-2, SL3261_TRAIL and SL3261_MIX presented in addition to lower weight aspect better, with reduced size and color similar to CTRL-. The MIX group presented the highest tumor regression, in which the bladders presented size equal to or less than the CTRL- group. Figure 6 also represents the accumulation of the 3 strains in the tumor microenvironment analyzed by counting colony-forming units in the tumor, spleen and liver of the animals (Figure 6C). Photograph of the culture plates from the macerated organs in 2 dilutions, 100 and 200 times (Figure 6D). Observa-se que a acumulação de S. typhimurium é preferencial no tumor (bexiga) do que nos órgãos naturalmente colonizados (fígado e baço).
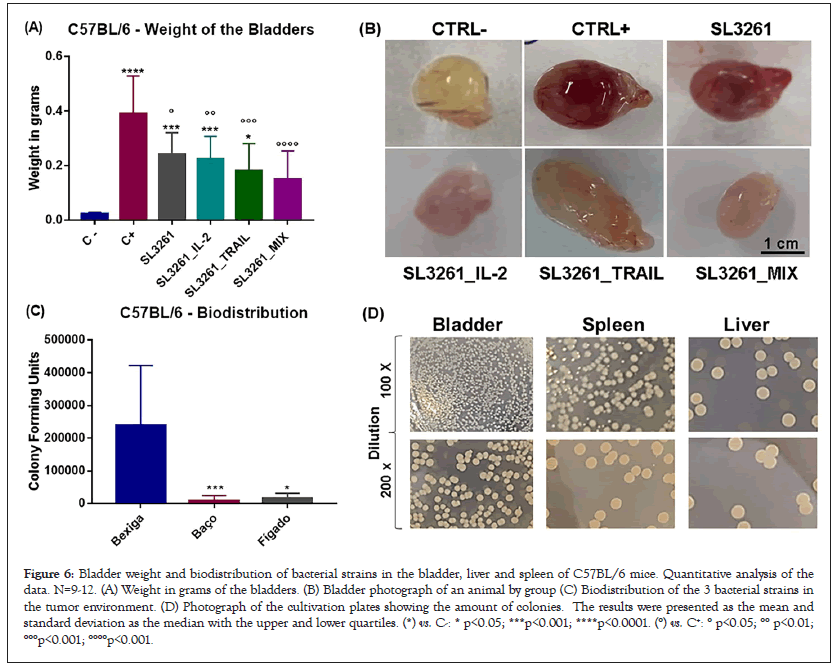
Figure 6: Bladder weight and biodistribution of bacterial strains in the bladder, liver and spleen of C57BL/6 mice. Quantitative analysis of the data. N=9-12. (A) Weight in grams of the bladders. (B) Bladder photograph of an animal by group (C) Biodistribution of the 3 bacterial strains in the tumor environment. (D) Photograph of the cultivation plates showing the amount of colonies. The results were presented as the mean and standard deviation as the median with the upper and lower quartiles. (*) vs. C-: * p<0.05; ***p<0.001; ****p<0.0001. (°) vs. C+: ° p<0.05; °° p<0.01; °°°p<0.001; °°°°p<0.001.
Figure 7 shows the cellular profile in the plasma and blood of the animals. In Figure 7A there is the overall cell count in plasma. It is noted that healthy animals, CTRL- group, have a higher number of cells. On the other hand, animals with cancer and without treatment, CTRL+ group, the number of cells is markedly lower when compared to the CTRL- group. The other treated groups also have a lower number of cells than CTRL-, but higher than CTRL+. Figure 7B shows the amount of mononucleated leukocytes (lymphocytes, mastocytes, monocytes, and basophils) in the blood. The cell number is higher in CTRL-, lower in CTRL+ and intermediate in the treated groups. In Figure 7C there is the number of neutrophils in the blood. In the groups treated with SL3261, SL3261_IL-2 and SL3261_MIX there is an increase in the number of neutrophils, which is not observed with a group treated with SL3261_TRAIL. In Figure 7D there is the number of eosinophils in the blood and is significantly higher in CTRL+ and slightly present in SL3261_IL-2 and SL3261_TRAIL. Figure 7 also shows the dosage of TNF, IL-12 and IFN-γ in the plasma of c57BL/6 animals. From the levels of TNF in plasma, it is observed that there is a slight increase in the group treated with SL3261_TRAIL in plasma. Figure 7F shows il-12 levels in plasma. There is a slight decrease in concentration in the CTRL+ group. In Figure 7G there is the dosage of IFN-γ in plasma, in which there is a significant increase in the groups treated with SL3261_TRAIL and MIX. Figure 7H shows the survival rate (%) of the animals. It is noted that animals died primarily in the groups treated with SL3261_TRAIL and SL3261_MIX, followed by death in SL3261_IL-2, CTRL+ and no deaths in CTRL- and SL3261, so much so that the curves are overlapping. Figure 7I is a representative scheme of experimental design in which immunotherapy began on the 4th day after tumor induction and euthanasia occurred on the 11th day.
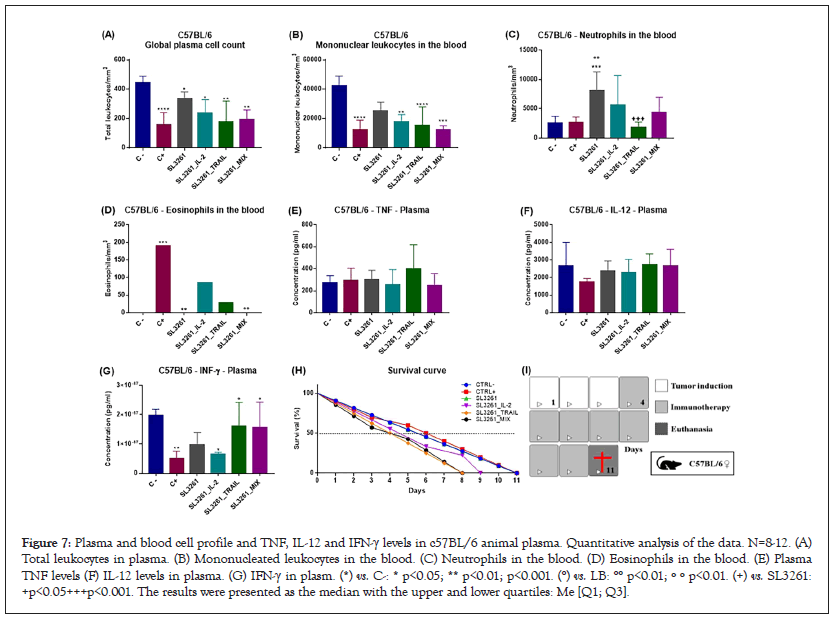
Figure 7: Plasma and blood cell profile and TNF, IL-12 and IFN-γ levels in c57BL/6 animal plasma. Quantitative analysis of the data. N=8-12. (A) Total leukocytes in plasma. (B) Mononucleated leukocytes in the blood. (C) Neutrophils in the blood. (D) Eosinophils in the blood. (E) Plasma TNF levels (F) IL-12 levels in plasma. (G) IFN-γ in plasm. (*) vs. C-: * p<0.05; ** p<0.01; p<0.001. (°) vs. LB: °° p<0.01; º º p<0.01. (+) vs. SL3261: +p<0.05+++p<0.001. The results were presented as the median with the upper and lower quartiles: Me [Q1; Q3].
A reduced and representative scheme of the action of IL-2 and TRAIL proteins on T4 cells is shown in Figure 8. The TRAIL protein finds its TRAIL-R receptor on the surface of cell MB49 and RT4, which generates a signal for the cell to synthesize more TRAIL molecules. Because of this signal amplification, caspase proteins are activated. IL-2 also finds its receptor, IL-2R, on the surface of cells. Once this happens, the activation of the enzyme iNOS occurs, which leads to the production of ON. Consequently, there is a decrease in cellular respiration, DNA fragmentation, increased transcription of the p53 gene. Both processes lead cells to the apoptosis process, also causing damage to the cell membrane, mitochondria and lysosomes and synthesis of inflammatory proteins. In animals there is reduction of tumor size and stimulation of the immune system to try to fight the progression of the disease.
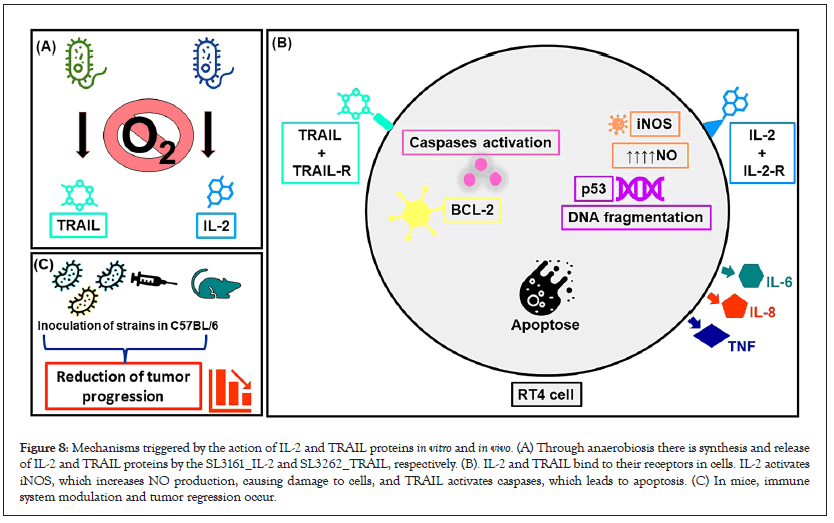
Figure 8: Mechanisms triggered by the action of IL-2 and TRAIL proteins in vitro and in vivo. (A) Through anaerobiosis there is synthesis and release of IL-2 and TRAIL proteins by the SL3161_IL-2 and SL3262_TRAIL, respectively. (B). IL-2 and TRAIL bind to their receptors in cells. IL-2 activates iNOS, which increases NO production, causing damage to cells, and TRAIL activates caspases, which leads to apoptosis. (C) In mice, immune system modulation and tumor regression occur.
The present study proposed to analyze the antitumor effect of IL-2, TRAIL and a mixture (MIX) of these proteins expressed by the recombinant strain of Salmonella SL3261 on an in vitro model of bladder cancer therapy using a human cell line, RT4 and C57BL/6 mice. The proteins were expressed extracellularly, making it possible to use the culture supernatant as a source of the recombinant proteins. Cell death by apoptosis through marked antibodies and Caspase-3 activity, inflammatory profile generated via inflammatory cytokine synthesis, cytotoxicity through cell viability assays and cell morphology by optical microscopy was analyzed. In the murine assays, the cellular and immunological profile and tumor progression were analyzed.
The tumor microenvironment consists of malignant and non-malignant cells in which cancer cells aggregate healthy cells to help them proliferate. It is increasingly clear that the interaction of cell compartments affects cancer progression and response to therapy. Therefore, current preclinical models such as tests on human cell lines incorporate these compartments and allow the investigation of dependent contributions of specific cell types to cancer progression [15].
Bladder cancer is very malignant with the main cause represented by smoking. The appearance of metastases and recurrence is high [16]. Therefore, the experimental design proposed in this study using human bladder tumor cell line can contribute to the development of more selective therapies for bladder cancer.
The S. typhimurium SL3261 strain used in this study presents attenuation of the aroA gene that leads to blockage of the synthesis pathway of aromatic molecules and impairs reproduction. These organisms rely, for example, on p-Aminobenzoic acid (pABa) and 2,3-Dihydrobenzoic acid (DHB), which is a precursor of compounds used to extract iron from bacteria. Salmonella also synthesizes pABa and DHB from chorismic acid, the final product of the aromatic biosynthetic pathway. Interfering in any step of this pathway makes the bacteria auxotrophic and less pathogenic to two components not available in vertebrate tissue which makes this attenuation safe [19,28,29].
A previous study that used this SL3261 strain that synthesizes IL-2+YFP and TRAIL+EmGFP, construction that is novel and that was published in a single article by our group using the murine bladder tumor cell line MB49 [18]. This work showed that the two strains showed stronger fluorescence when the bacterial culture supernatant analyzed in a fluorescence reader and also by ELISA, confirming the heterologous expression of IL-2 and TRAIL proteins [20]. This approach is common for both IL-2 and TRAIL for this type of construction in tumor model studies [2,30]. In addition, EmGFP protein and its derivatives do not cause obvious biological damage and do not require substrates or cofactors [31]. Another advantage of this type of structure is that the acquisition of fluorescent images, widely used in oncogenic therapy and in vitro and in vivo studies, is totally noninvasive.
The mechanism of IL-2 in vitro starts when the protein finds its receptor in the membrane (IL-2R) of the RT4 cell [32]. IL-2R is present in tumor cells [33]. This IL-2 and receptor binding generates an activation signal of the enzyme nitric oxide synthase (iNOS-inducible isoform) that catalyzes the synthesis of NO from the amino acid L-arginine [1]. The NO is a highly reactive molecule because it is a free radical with an unpaired electron [34]. As a consequence of NO´s action, there is suppression of cellular respiration and DNA synthesis, DNA fragmentation, pro-apoptotic modulation via activation of proteases of the caspase family, increased expression of p53 (protein related to the cell cycle and tumor suppression) [35], and change in the expression of proteins associated with apoptosis [36].
TRAIL induces cell death by apoptosis when it binds to its receptors (TRAIL-R1 or TRAIL-R2) in RT4 cell membrane that activates apoptotic signal transduction cascades, mediated by members of the tumor necrosis factor receptor superfamily, initiating the apoptotic process via caspase activation. This is the extrinsic activation of apoptosis [37,38]. The intrinsic pathway of apoptosis is activated by intracellular signaling of mitochondria. Mitochondria are regulated by pre-apoptotic and antiapoptotic BCL-2 proteins and play important roles in chemotherapy and radiation-induced cell death [38].
The data obtained in this study show that there was an increase in NO production after 48 hours in the three treated groups and there was an increase only in the IL-2 group after 24 hours. These results were also found in a study that used the same bacterial strain (SL3261) synthesizing these two proteins in murine bladder cancer cells MB49 [20]. Another study showed that NO improved TRAIL action in pancreatic cancer via regulation of BCL-2, a protein that regulates the permeability of mitochondrial membrane and has apoptotic or anti-apoptotic role [39,40]. NO has antiangiogenic and antitumor properties [41]. In IL-2 and MIX groups, the synthesis of NO was due to the iNOS action, while in the cells treated with TRAIL the production was due to cell death by apoptosis induced by this protein [37]. IL-2 has demonstrated apoptotic effects as a result of NO production. So, IL-2 can act as a pro-apoptotic modulator, since it activates proteases of the caspase family by releasing mitochondrial cytochrome C into the cytosol. It results in an increased expression of the p53 gene and alters the expression of proteins associated with apoptosis, such as the BCL-2 family that regulates programmed cell death (apoptosis) [42].
The data obtained show that RT4 cells suffer cell death by apoptosis when exposed to IL-2, TRAIL and MIX proteins. This was observed through Caspase-3 analysis and Annexin V PE and 7AAD marking, fluorescent dyes commonly used in assays for verification and quantification of apoptosis by flow cytometry. Caspases are cysteine-aspartate enzymes whose sequential activation leads to programmed cell death [43]. Similar results were found in other study with MB49 cells using the same proteins synthesized by SL3261 when analyzed by flow cytometry and in a prostate cancer cells study with TRAIL treatment [20,44].
TRAIL induces apoptosis in tumor cells, but when this induction fails or is actively inhibited, there is an ability of human and mouse TRAIL receptors to induce necrosis, regardless of their ability to undergo apoptosis [45]. Late apoptosis, found in the effects of both IL-2 and TRAIL in the present study, may indicate necrosis [43]. Thus, in addition to the strategies established for induction of apoptosis, the induction of programmed necrosis by TRAIL may represent innovative options to combat tumor cells. The cytotoxic effects of IL2, TRAIL and MIX on necrotic tumor cells may contribute to more effective cancer treatment. It is noteworthy that the antitumor response induced by Salmonella expressing IL-2 was correlated with decreased angiogenesis and increased necrosis in tumor tissue [2].
RT4 cells suffered loss of viability demonstrated by MTT and neutral red dye assays that was supported by cell morphology images. Studies have shown that IL-2 has the ability to decrease cell viability in SKOV3 (human ovarian cancer) and HepG2 (human liver cancer) cells by the MTT assay. TRAIL also showed loss of cell viability in different liver cancer cells (ep3b, PLC, HepG2, HCT116-Bax/ e HCT116-Bax/) also by the MTT assay [46-48]. This reduction in cell viability induced by IL-2, TRAIL and MIX has also been demonstrated in MB49 tumor cells [20]. In the neutral red dye assay, a study showed that TRAIL decreased cell viability in lung cancer model (L-132) [49].
Autophagy is a natural process in which the cytoplasmic content of cells is renewed and can be activated by environmental stress such as hunger and infections or by the cell itself such as toxins, chemotherapy and immune system stimulation [50]. Depending on the stimulus, it can kill the cell or lead to its survival, both in healthy and tumor cells [51]. In addition, there is a strong correlation between autophagy and apoptosis, since their activation mechanisms are associated [51]. The IL-2, TRAIL and MIX proteins tested in this study in RT4 cells generated autophagy as a response to the cytotoxic proteins action. TRAIL is an autophagy activator, as reported in several studies and has a cross-effect between autophagy and apoptosis [51], which corroborates the data obtained. Studies have shown that IL-2 promotes autophagy in mouse tumor cells, MC38 strain of colorectal carcinoma and Panc-2 of chloroquine-associated adenocarcinoma, leading to tumor cell death and survival of animals [52].
As all these processes mentioned so far depend on signaling, the synthesis of pro-inflammatory cytokines IL-6, IL-8 and TNF was investigated. IL-6 is an inflammatory protein synthesized by T lymphocytes and macrophages and acts to combat bacterial infections and traumas such as burns [53]. In cancer, this protein is present at high levels as in cases of breast and prostate cancers [53,54]. IL-8 is produced by T lymphocytes, macrophages and dendritic cells in response to intracellular infections and chemotaxis for neutrophil activation [55,56]. IL-8 is a promising biomarker for cancer and has been studied for the diagnosis of this disease [57]. In cancer, IL-8 is responsible for tumor angiogenesis and metastasis [58]. In the data obtained in this study, there was a slight difference between the treated groups when compared with the control group for both IL-6 and IL-8.
TNF is also an inflammatory cytokine responsible for fighting tumors via necrosis, synthesized mainly by macrophages [59]. Depending on the cell situation, it can induce various effects such as apoptosis, necrosis, angiogenesis, immune cell activation, cell differentiation and migration. These processes are very important in immunological monitoring of the tumor and play an important role in tumor development and progression. Therefore, it is not surprising that TNF has a context-dependent precancerous and anticancer effect [60]. Data shows that there was an increase in TNF levels in the groups treated with IL-2, TRAIL and MIX, evidencing that in this experimental model the TNF promotes cell death.
Finally, the ability of RT4 cells to recover after the proposed treatment was evaluated, which was impaired by the action of IL-2, TRAIL and MIX. Similar pattern was observed in MB49 tumor cells treated with the same proteins synthesized by SL3261 [20]. Another study showed that three types of bladder cancer, murine (MB49) and human (T24 and UMUC3), had the ability to recover and form colonies [61], showing that tumor cells maintain this ability even after treatment. In view of this, the data obtained in the present study are promising, since cell recovery was affected by the proposed treatment.
A study previously carried out in our research group verified the safety of the use of SL3261 in balb/c animals. In the study, 5 × 105 CFU/ml of SL3261_IL-2 and SL3261_TRAIL. Il-12 and TNF levels were dosed in the plasma of the animals and the number of total leukocytes. One day after inoculation, Il-2 inflammatory cytokine levels increased due to the host's natural immune response to LPS present in SL3261. However, on the 7th day, these values decreased. Moreover, TNF levels did not increase at any time. Based on these findings, it was proven that the animals did not suffer sepsis. This is explained by the fact that there is a lack of accumulation of genetically modified S. typhimurium in normal tissues, including blood showing that the therapeutic use of SL3261 is safe. The data in the present study corroborate these findings since the levels of TNF and IL-12 in the plasma of C57BL/6 animals treated with SL3261, SL3261_IL-2, SL3261_TRAIL and SL3261_MIX were not high.
There was a significant decrease in bladder size of animals treated with SL3261, SL3261_IL-2, SL3261_TRAIL and SL3261_MIX. In the work of Camillo et al., the same bacterial strains were used in Balb/c Nude mice with human colorectal cancer, HCT8 strain, which showed the same marked decrease in the tumor located on the flanks of the animals treated with SL3261_TRAIL and SL3261_MIX [20]. Similar results were found in tests using the same line of S. typhimurium A1-R in the treatment of breast cancer also in Balb/c Nude. A study with immunodeficient mice with several types of cancer, such as breast carcinoma, colon carcinoma, pancreatic adenocarcinoma, glioma and myeloma showed that trail treatment was effective in tumor regression [38]. Trail's recombinant molecule was developed to reach various types of tumor cells, so that they have to present their receptor, TRAIL-R. It has an important advantage that is ability to eliminate tumor cells without causing lethal adverse effects, such as those found with TNF. As previously discussed, TRAIL induces apoptosis through interaction with its receptors and consequent activation of Caspases, but it also plays a role in p53 protein-mediated apoptosis, which is a tumor suppressor gene. Genetic lesions from trail activation pathway components have been identified in human tumors, suggesting trail pathway inactivation and/or trail-mediated immunovigilance escape and may function at tumor onset and progression [38]. So, TRAIL proves to be a very promising therapeutic agent for cancer therapy.
In this same work by Camillo et al., some of the Balb/c Nude animals died when treated with SL3261_TRAIL and SL3261_MIX, the same observed in the present study besides death with SL3261_IL-2. This can be explained by the fact that Il-2 and TRAIL proteins generated high inflammatory power at the dose of 5 × 107 CFU/ml. However, there were no deaths with treatment with SL3261. Therefore, IL-2 and TRAIL generated a strong tissue inflammatory reaction that evolved to a systemic response and that in this dose is not safe, even if there is a marked decrease in the tumor. As studies have been done in Balb/c with a dose 5 times greater than this the preferential accumulation of SL3261 in tumor tissue and not in healthy tissues is not able to activate this systemic inflammation, since there was no death of animals or increase of inflammatory cytokines as discussed in the previous paragraph. This tissue inflammatory reaction can be explained due to the synthesis of ON by the activation of iNOS by IL-2 which contributed to the inflammatory and antitumor responses as mentioned earlier.
S. typhimurium replicates preferentially in tumors, but a small number of them are capable to replicate in healthy tissues, which can be toxic which causes harmful effects on normal tissues. The proposed approach with the use of attenuated SL3261 and with the nirB promoter, which allows the specific expression of genes of localized interest in the hypoxia environment of the tumor, tends to be safe and avoid undesirable toxicities to normal tissues. One study compared the effects of intratumoral and intravenous Salmonella inoculation, analyzing safety, ability to limit bacterial dissemination, and efficacy in the propagation of therapeutic effects in primary and secondary tumors. They concluded that the intratumoral route is safer. Therefore, the intratumoral route was used in this study and there was the expected biodistribution with the use of SL3261.
IL-2 is produced by TCD4+ lymphocytes that stimulate The TCD4+ itself and associated with IL-15, produced by cells in the body, stimulate the TCD8+ lymphocyte. Lymphocytes identify specific cancer antigens and trigger a specific immune response by various mechanisms. The TCD8+ lymphocyte, for example, eliminates the tumor cell via perforin secretion (direct form) and by the synthesis of IFN-γ (indirect form) and, generally, both processes occur concomitantly. Data found in the present study show an increase in ifn-γ plasma levels of animals treated with TRAIL and MIX. IL-2 in addition to stimulating T cells stimulate NK lymphocytes, increase serum concentrations of other pro-inflammatory cytokines such as TNF, IFN-γ and IL-1. The U.S health surveillance agency, the FDA (Food and Drug Administration) has approved the application of high doses of IL-2 in the treatment of metastatic melanoma and renal carcinoma based on a study of 270 patients in 8 studies by the U.S. Therefore, IL-2 plays an important role in tumor regression by activating several antitumor mechanisms. Treatment with IL-2 against hepatocellular carcinoma was able to inhibit metastases in a study with Nude mice because NK cells were alive and active in the liver. The same study also demonstrated in vitro that NKs inhibited the proliferation, migration and invasion of hepatocarcinoma cells, preventing lung metastasis. The tumor decrease observed in the SL3261_MIX group may have been exerted by the antiangiogenic and cytotoxic action of IL-2 in addition to the cytotoxicity exerted by TRAIL directly in tumor cells. The administration of therapies based on a combination of TRAIL and IL-2 can be highly effective.
In addition to the action of IL-2 and TRAIL Salmonella itself may play a role in tumor regression. A work with recombinant S. typhimurium that secretes flagellin showed that it was able to generate tumor regression even though a control group treated with flagellin, in which there was no tumor regression. These findings verify the efficiency of Salmonella in cancer treatment. The explanation for this is that the tumor microenvironment incorporates the bacterium and there is competition between bacteria and tumor cells by nutrients, release of components derived from bacteria that are antitumor released by bacterial lysis, decreased angiogenesis, autophagy and increase of apoptotic molecules. Salmonella is also capable of synthesizing IFN-γ and ON, as already discussed and reducing the action of suppressor cells from myeloid cells of the tumor microenvironment, resulting in the recruitment of NK, macrophages, neutrophils, T and B lymphocytes.
The high levels of IFN-γ dosed in plasma of the groups treated with SL3261_TRAIL and SL3261_MIX are explained by the fact that IL-2 induces proliferation and activation of NK cells, responsible for the activation, proliferation and recruitment of other inflammatory and cytotoxic cells to the tumor region, via the release of cytokines such as IFN-γ. IFN-γ directly induces TRAIL in Jurkat human leukemia cells and HT29 colon cancer cells. Such TRAIL induction occurs by binding IFN-γ to a response element stimulated by IFN-γ, leading to TRAIL induction and consequent apoptosis, so the levels of this cytokine are increased in the groups treated with SL3261_TRAIL and SL3261_MIX. Tumor regression can also be explained by the action of IFN-γ it is able to inhibit tumor growth, activate macrophages and increase cellular immunity (TORRES, 2018).
The overall count of cells, mononucleated leukocytes and neutrophils in the blood can be explained by the fact that Salmonella is responsible for the recruitment of neutrophils and macrophages, resulting in the elimination of tumor cells. After infiltrating the tumor, the bacteria generate infection with high infiltration of immune cells (mainly neutrophils and dendritic cells), which are located between the viable and necrotic areas of the tumor. In the blood there was a higher number of mononucleated leukocytes, mainly lymphocytes in the groups treated with SL3261, SL3261_IL-2 and SL3261_TRAIL when compared to the C+ group. A recent study using the CRISPR-Cas9 technique has identified a subgroup of T lymphocytes capable of recognizing tumor cells and combating them. The mechanism occurs by the specific identification of unique metabolites related to the MR1 molecule, tumor cells and specific CRT. Treatment with these lymphocytes was effective against lung, kidney, uterus, melanoma and leukemia cancer.
Neutrophils infiltrate the tumor microenvironment and neutrophilia is one of the worst prognoses of the disease. A study that sought to elucidate the development of glioblastoma, one of the types of brain cancer, using animal models showed that neutrophils are located very close to the tumor and that throughout the development of the disease are constantly recruited from the bone marrow. In the early stages of the disease, recruited neutrophils caused the growth of the tumor to decrease. However, with the tumor advancement of the neutrophil bone marrow with a pro-tumorigenic phenotype, they were recruited. It is believed that there is a signaling pathway between the bone marrow and the tumor that can influence the synthesis of neutrophils with a phenotype that promotes tumor growth. In the present study, the established cancer was acute with only 11 days of disease development, so it was in the early stages. In the blood, neutrophils were more in animals treated with SL326, showing that neutrophils played an important role in tumor regression.
Eosinophils may play a role in the beginning of the antitumor response. One study evaluated eosinophilia in cancer and identified a lower incidence of colorectal cancer with an increase in the eosinophil count. In the data of the present study, the number of eosinophils was much higher in the blood in the C+ group and in the groups treated with SL3261_IL-2 and SL3261_TRAIL.
IL-2 and TRAIL, administered separately or together, contributed to generate a marked decrease in cell viability via NO production and Caspase activation, resulting in deleterious autophagy and cell death by apoptosis in human bladder cancer cells. In the mice tests both proteins generated significant tumor regression and synthesis of important cytokines in the tumor control process. The use of these therapeutic agents is relevant, as there is already a vast knowledge about their action in cancer therapy, not only from the positive aspects but also from the negative ones, such as cytotoxicity in high concentrations and rapid clearance by the body. Therefore, the proposed approach, that is the localized synthesis of these proteins by attenuated recombinant Salmonella, which has a proven preference for the tumor microenvironment and acts as a live vector, is promising and deserves attention and investment in new models.
The authors thank Dr. Heloisa Sobreiro Selistre de Araújo (Department of Physiological Sciences, Federal University of São Carlos, São Carlos, SP, Brazil) and Dr. Márcia Regina Cominetti (Department of Gerontology, Federal University of São Carlos, São Carlos, SP, Brazil) for providing the necessary equipment to carry out some analyses.
Conceptualization: Bruna, Luciana, Fernanda, André and Maria Teresa; Data curation: Bruna li and Joice; Formal analysis: Bruna, Joice and Krissia; Funding acquisition: Adilson and Fernanda; Investigation: Bruna; Methodology: Bruna, Joice and Krissia; Project administration: Bruna, Adilson and Fernanda; Resources: Ricardo, Adilson and Fernanda; Software: Bruna, Krissia and Joice; Supervision: Bruna; Validation: Bruna; Visualization: Bruna; Roles/Writing-original draft: Bruna; Writing-review & editing: Camila, Cynthia, Patricia, André, Maria Teresa.
This study was financed in part by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior - Brazil (CAPES) - Finance Code 001.
None to report. The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Google Scholar] [PubMed]
[Google Scholar] [PubMed]
[Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
Citation: Fragelli BDL, Rodolpho JMA, Franco de Godoy K, Camillo L, Aparecida de Castro C, Brassolatti P, et al (2023) Antitumor Effect of IL-2 and TRAIL Expressed by Salmonella: An Immunotherapeutic Proposal against Bladder Cancer In Vitro and In Vivo by iNOS, Caspase Activation, Immune System Modulation and Tumor Regression. Chemo Open Access. 11:171.
Received: 31-Jan-2023, Manuscript No. CMT-23-21648; Editor assigned: 03-Feb-2023, Pre QC No. CMT-23-21648 (PQ); Reviewed: 22-Feb-2023, QC No. CMT-23-21648; Revised: 01-Mar-2023, Manuscript No. CMT-23-21648 (R); Published: 08-Mar-2023 , DOI: 10.35248/2167-7700.23.11.171
Copyright: © 2023 Fragelli BDL, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.