
Journal of Clinical Chemistry and Laboratory Medicine
Open Access
ISSN: 2736-6588

ISSN: 2736-6588
Research Article - (2024)Volume 7, Issue 1
Use of dietary components as antineoplastic agents has been highlighted due to their high biological activity against tumor cells, chemo preventive effects and low toxicity. The present study evaluated antitumor effects and chemo protective potential of Bromelain (BL) alone and in combination with Doxorubicin (DOX) using in vitro tests: Alamar blue in the lines: AGP01, SKMEL103 and CAL27; MTT assay (3-(4, 5-dimethylthiazolyl-2)-2, 5-diphenyltetrazolium bromide), fluorescent labelling in murine Sarcoma 180 (S180) and comet assay in human lymphocytes. Cytotoxic effects of BL were noted with IC50 (μg/mL) of 124.80 (AGP01), 91.81 (SKMEL103), 95.75 (CAL27) and 25.27 (S180). When incubated in a BL+DOX combination, they demonstrated combination indices ranging from synergistic to antagonistic. In the cell death mechanism at S180, an increase in the number of cells undergoing early apoptosis was observed after incubation with BL (100 μg/mL). In genotoxicity assays, isolated BL was not genotoxic in human lymphocytes, unlike DOX. When combined (BL+DOX), BL modulated the DNA damage caused by the antineoplastic agent when compared to DOX alone, with Inhibition Degree (ID) values of 55.91% and Fractional Difference (FD) of 33.65%, presenting chemo protective potential. Thus, isolated BL exhibited ant proliferative effects on tumour lineages and was not genotoxic to human blood cells, with positive prospects for use in combination with DOX chemotherapy.
Antineoplastic agents; Bromelain; Cancer; Cytotoxicity; Genotoxicity; Pharmacological combination
The search for natural compounds that can be used in cancer prevention and in association with oncological treatment is increasing. These compounds are sought after due to their antioxidant properties and their ability to reduce side effects. Many natural compounds found in food have shown chemo preventive effects on Deoxyribonucleic Acid (DNA), demonstrating the ability to prevent or interact with processes leading to the development of neoplasia during its initiation, promotion and progression stages or the ability to interrupt its development [1]. Among these dietary compounds, bromelainstands out, which is a complex natural mixture of proteolytic enzymes derived from pineapple (Ananas cosmosus) that possesses therapeutic properties [2].
Bromelainhas been studied and employed in various therapeutic applications due to its biochemical and pharmacological properties, with a glycoprotein as the main key factor [3]. It also contains insoluble materials such as minerals, pigments, protease inhibitors, acids and organic solvents [2]. Among the therapeutic applications of BL, its effects stand out in the treatment of rheumatic diseases such as arthritis, thrombophlebitis, hematomas, oral inflammation, diabetic ulcers, rectal and perirectal inflammation, wounds, platelet aggregation inhibition, bronchitis, sinusitis, surgical trauma and pyelonephritis. Additionally, it has been used to enhance drug absorption, especially antibiotic. An important aspect of BL’s potential as an anticancer agent is its ant metastatic effect, acting as a tumor protein p53 activator and a pro-apoptotic agent for tumor cells [2,4].
The lack of selectivity and side effects caused by chemotherapy drugs, such as doxorubicin, has encouraged researches to find out alternative substances and the application of natural compounds and phytotherapy [5]. These compounds have received significant attention due to their antitumor potential and relatively low toxicity compared to traditional antineoplastic agents [6-8]. Furthermore, many of these bioactive compounds, such as BL, have the potential to inhibit the carcinogenesis process by interfering with one or several cellular pathways, playing a crucial role in both chemoprevention and cancer treatment [9].
Recent studies have highlighted the anti-tumoral activity of BL in skin, ovarian, glioma and breast cancers [4,10,11]. Several mechanisms have been proposed to explain its action, however, these mechanisms still need to be verified and confirmed, especially when combined with chemotherapy, where the researches on such combinations are still in the early stages [12]. Therefore, this study aimed to evaluate the ant proliferative activity of the bromelain enzyme complex both in isolation and in combination with the chemotherapy drug doxorubicin in tumor cell lines and normal human lymphocytes. The study also aimed to assess chemo protection and investigate the type of cell death caused by this phytochemical compound.
Salts and reagents
Purified BL (P.A. ≥ 98%), Roswell Park Memorial Institute (RPMI) 1640 medium, Fetal Bovine Serum (FBS), trypsin, streptomycin and phytohemagglutinin were acquired from Sigma-Aldrich (Brazil). Doxorubicin (DOX) was obtained from Eurofarma Laboratórios S.A. (Sao Paulo, Brazil).
BL was initially diluted in Dimethyl Sulfoxide (DMSO) from Sigma-Aldrich (Brazil) at a concentration of 1 mg/mL and stored at 4°C. Prior to the experiments, BL was further diluted to concentrations ranging from 12.5 μg/mL to 400 μg/mL. The final concentration of DMSO did not exceed 0.1%.
Cell culture
The tumor cell lines AGP01 (Human gastric adenocarcinoma), CAL-27 (Oral carcinoma) and SKMEL-103 (Human melanoma) were obtained from the Rio de Janeiro Cell Bank (BCRJ, Brazil). Normal human lymphocytes were used as a control and were isolated from peripheral blood. Cells in this study were grown in RPMI 1640 medium supplemented with FBS 10%, 2 mM of L-glutamine and 50 μg/mL of gentamicin. Cultures were maintained in a humidified incubator with 5% CO2 at 37°C. Cells were sub cultured every 3-4 days and allowed to reach high confluence (80%-90%) before conducting experiments.
Primary culture of sarcoma 180
The S180 cell line was obtained from the peritoneal cavity of mice previously inoculated (5 × 104, intraperitoneal) as described by Mororo, et al. [13]. Subsequently, 0.5 × 106 cells/mL were incubated in Roswell Park Memorial Institute (RPMI) 1640 supplemented with 1 mM L-glutamine, FBS 10% and 1% antibiotics (penicillin/streptomycin). 50 μM of isolated BL, 10 μM of DOX and the respective combinations were maintained in an incubator at 37°C for 72 hours. The animal experiments followed the guidelines established by the Institutional Animal Research Ethics Committee (UFPI #167/16).
In vitro determination of cytotoxicity
In vitro cytotoxicity assessment was performed using the Alamar Blue assay, as described by Ahmad, et al. [14]. The tumor cell lines AGP01, SKMEL-103 and CAL-27 were seeded in 96-well plates at a concentration of 5 × 106 cells/well and incubated in a humidified incubator for 24 hours at 37°C with 5% CO2. After this period, the cells were treated with BL at concentrations of 100 and 400 μg/mL in three independent experiments. DOX at 5 and 10 μM was used as a positive control.
Furthermore, a serial dilution was performed for BL (0 μg/mL-400 μg/mL) and DOX (0 μM-100 μM) as well as the combination of BL+DOX at the same concentrations. The negative control group received the same amount of DMSO (0.1%) as the highest substance concentration. After 72 hours of treatment, the Alamar Blue solution (diluted at 1:20 in Dulbecco’s Modified Eagle Medium (DMEM) culture medium without FBS) was added and the plate was re-incubated for 3 hours. The plates were read using a plate spectrophotometer at a wavelength of 465 nm/540 nm.
The half-maximal Inhibitory Concentration (IC50) and growth inhibition were calculated using GraphPad Prism software (version 5.01). The Combination Index (CI) was determined using CompuSyn software (version 1.0, CombSyn, Inc.). For the evaluation of the CI between BL+DOX, the following criteria were considered: CI<1 as a synergistic effect, CI=1 as an additive effect and CI>1 as an antagonistic effect [15].
Cell viability by MTT assay
The 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay was used to assess the cytotoxic potential of BL, either in isolation or in combination with DOX, as described by Mosmann, et al. [16]. The cell culture was distributed in a 96-multiwell plate at a density of 1 × 106 cells/mL. The triplicate experimental groups were subdivided into a negative control (culture medium) a positive control (doxorubicin 10 μM), isolated BL (50 μM) and combined with DOX (10 μM+50 μM). They were incubated for 68 hours along with the cells. After this period, 20 μL of the MTT solution (5 mg/mL) was added to the cultures and re-incubated for 4 hours in an incubator at 37°C with 5% CO2. The plates were allowed to dry, leaving the cells overnight and their precipitate was resuspended in 100 μL of isopropyl alcohol. To measure the salt reduced by viable cells, the absorbance was measured in a microplate reader at 550 nm. The absorbance data were normalized to the control (Treatment absorbance/Control absorbance × 100).
Evaluation of the cell death mechanism using double staining with propidium iodide and acridine orange
The quantification and differentiation of S180 cells into early apoptosis, late apoptosis or necrosis were performed using a double staining method with Propidium Iodide (PI) and Acridine Orange (AO) through a fluorescence microscope (Olympus DSU, Japan). In summary, the cells were plated at a density of 1 cells/mL × 106 cells/mL in 35 mm diameter microplates and treated with 100 μg/mL of BL. The cells were incubated in a 5% CO2 atmosphere at 37°C for 24 hours and then washed twice with Phosphate-Buffered Saline (PBS) to remove the remaining medium. A solution of AO (10 μg/mL) and PI (10 μg/mL) at a 1:1 ratio was added to the cell pellet and immediately observed by epifluorescence at 488 nm/550 nm (AO) and 550 nm/650 nm (PI). Approximately 200 cells per group were evaluated. The identification criteria were as follows: (a) Viable cells have a clear or non-stained light green nucleus with an intact structure; (b) Early apoptotic cells display a bright green nucleus with chromatin condensation; (c) Late apoptotic cells exhibit dense orange (red+green) areas of chromatin condensation and bleb formation on the membrane; (d) Secondary necrotic/dead cells have a red nucleus. This assay was performed in triplicate [17,18].
Comet assay on normal human lymphocytes
The alkaline version of the comet assay was conducted as described by Speit, et al. [19]. Aliquots of 10 μL of human lymphocyte cell suspension (0.5 cells/mL × 106 cells/mL) were mixed with a thin layer of low-melting-point agarose (0.75%, 90 μL) and placed on slides pre-coated with 1.5% normal agarose. Subsequently, the slides were immersed in a lysis solution (2.5 M Sodium chloride (NaCl), 100 mM Ethylenediaminetetraacetic Acid (EDTA) and 10 mM Tris, pH 10, with the addition of 1% Triton X-100 and 10% DMSO at the time of use) for up to 72 hours at 4°C. After this period, the slides were incubated in an alkaline buffer (300 mM Sodium Hydroxide (NaOH) and 1 mM EDTA, pH>13) for 20 minutes and then exposed to an electric current of 300 mA and 25 V (0.90 V/cm) for 15 minutes in an electrophoresis tank. Finally, the slides were neutralized with Tris buffer (0.4 M, pH 7.5) and stained with a silver solution. The slides were analyzed for the photo micrographic profile of the cells (at 400X magnification under an optical microscope) and the results were expressed in terms of the Damage Index (DI), Damage Frequency (DF) and % apoptosis out of 100 cells, in triplicate. The DI was calculated using the formula: DI = Σ (number of cells in a particular damage class X damage class), which ranged from 0 to 400X and DF was calculated using the following formula: DF=100-number of cells in damage class 0 [19].
Statistical analysis
The data were analyzed using one-way Analysis of Variance (ANOVA) followed by Dunnett's post-test for the Alamar Blue assays and Tukey’s post-test for the other assays. A significance level of p<0.05 was considered. The IC50 values were obtained using the Hill equation:
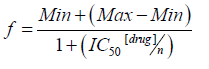
Where Max and Min represent the maximum and minimum viability values, respectively; IC50 is the concentration that inhibits 50% of viability and ‘n’ is the hill coefficient. The statistical program used was GraphPad Prism version 7.0 (GraphPad, USA).
Among the tested concentrations of BL (12.5; 25; 50; 100; 200 and 400 μg/mL), the ones that significantly interfered (p<0.05) were 100 and 400 μg/mL in the viability of AGP01, SKMEL-103 and CAL-27 cells when compared to the untreated group (NC). They showed a decrease in viability lower than 50% in all the evaluated tumor cell lines, indicating their cytotoxic effects. As expected, DOX at concentrations of 5 and 10 μM exhibited anti-proliferative effects when compared to the untreated group (NC).
When associated with BL at two concentrations (BL+DOX; 100 μg/mL+5 μM) and the other combination (BL+DOX; 400 μg/mL+10 μM), they also showed statistically significant anti-proliferative ability compared to NC, as evidenced by cellular viability lower than 25% in all three evaluated tumor cell lines (Figure 1).
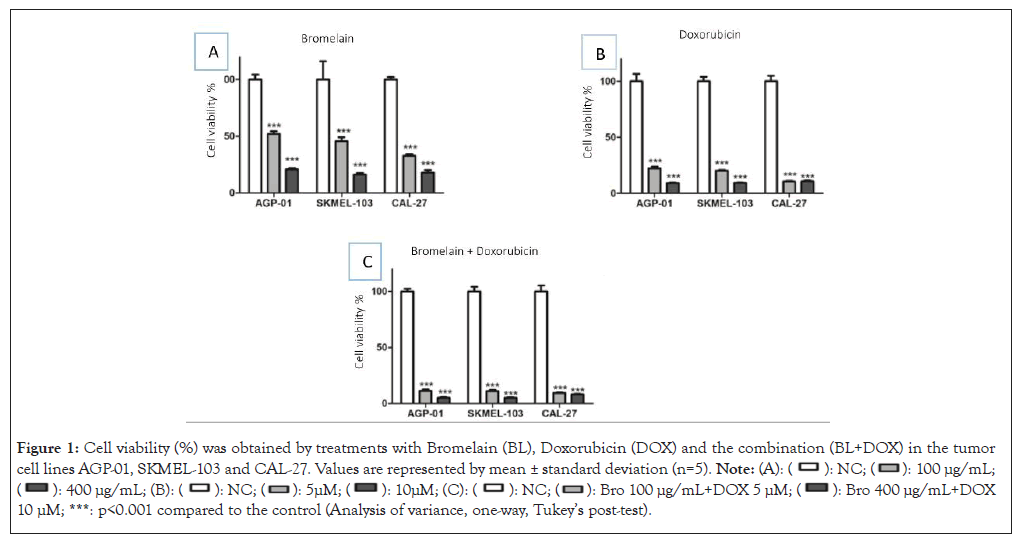
Figure 1: Cell viability (%) was obtained by treatments with Bromelain (BL), Doxorubicin (DOX) and the combination (BL+DOX) in the tumor
cell lines AGP-01, SKMEL-103 and CAL-27. Values are represented by mean ± standard deviation (n=5). 
 10 μM; ***: p<0.001 compared to the control (Analysis of variance, one-way, Tukey’s post-test).
10 μM; ***: p<0.001 compared to the control (Analysis of variance, one-way, Tukey’s post-test).
Table 1 shows the IC50 values obtained after incubation of AGP01, SKMEL-103 and CAL-27 with BL and DOX, both individually and in combination (BL+DOX). According to the results, it was observed that the BL+DOX combination induced an increase in the anti-proliferative effects in all the tested cell lines.
| IC50 (CI 95%) | |||
|---|---|---|---|
| Bromelain (μg/mL) | Doxorubicin (μM) | Bromelain+Doxorubicin (μg/mL) | |
| AGP-01 | 124.80 (109.8-141.8) | 0.75 (0.66-0.85) | 26.29 (23.99-28.82) |
| SKMEL-103 | 91.81 (78.02-108.0) | 0.70 (0.59-0.84) | 29.04 (26.98-31.26) |
| SCAL-27 | 95.75 (78.22-117.2) | 0.02 (0.012-0.033) | 2.68 (2.14-3.37) |
Note: Inhibitory Concentration (IC50); Confidence Interval (CI).
Table 1: Effects of Bromelain (BL), Doxorubicin (DOX) and the combination BL+DOX on different tumour cell lines. The values are expressed by IC50 and a confidence interval of 95%.
The IC values were used to characterize the interactions between BL and DOX as synergistic (<1), additive (=1) or antagonistic (>1). A synergistic effect of BL was observed at 50, 100 and 200 μg/mL concentrations when combined with DOX at 12.5, 25 and 50 μM in the SKMEL-103 and CAL-27 (Figure 2). For the AGP-01, the highest concentrations also exhibited a synergistic effect, indicating the cytotoxic potential of the combination. However, in the highest concentration and the two lowest concentrations of BL+DOX, an antagonistic effect was observed in the SKMEL-103 and CAL-27. In the AGP-01 cell line, this antagonistic effect was observed only at the lowest CI concentration (Figure 2).
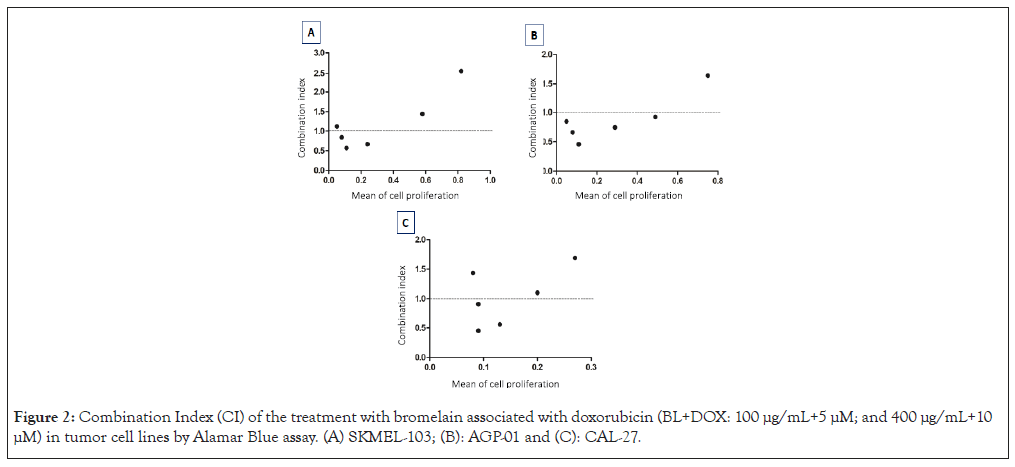
Figure 2: Combination Index (CI) of the treatment with bromelain associated with doxorubicin (BL+DOX: 100 μg/mL+5 μM; and 400 μg/mL+10 μM) in tumor cell lines by Alamar Blue assay. (A) SKMEL-103; (B): AGP-01 and (C): CAL-27.
Values of the Combination Index (CI) calculated by the COMPSYN software, relative viability (Alamar Blue assay), characterizing interactions as synergistic (<1) when points are below the line, additive (=1) when on the line or antagonistic (>1) when above the line.
The treatments with isolated bromelain (50 μg/mL), doxorubicin (10 μM) and the combination of these two chemical compounds showed significant cytotoxic effects (p<0.05) compared to the control in murine sarcoma tumor cells. For isolated BL, an IC50 of 25.27 μM was observed, while for DOX, an IC50 of 2.80 μM was obtained and in combination, an IC50 of 6.11 μg/mL was observed (Table 2).
| IC50 (CI 95%) | |||
|---|---|---|---|
| Bromelain (μM) | Doxorubicin (μM) | Bromelain+Doxorubicin (μg/mL) | |
| S-180 | 25.27 (10.79-59.18) | 2.80 (0.91-4.11) | 6.11 (3.90-9.18) |
Note: Inhibitory Concentration (IC50); Confidence Interval (CI).
Table 2: Inhibitory Concentration of 50% (IC50), Confidence Interval of 95% (CI 95%) and percentage of cell viability obtained from the treatments with Bromelain (BL), Doxorubicin (DOX) and the combination (BL+DOX) in the murine Sarcoma 180 tumor cell line.
The cytotoxic capacity in the assessment of cell death after a 24-hour exposure showed the expected effects of DOX when compared to the control (p<0.05). Treatment with BL at a concentration of 100 μg/mL also exhibited cytotoxic effects (reduced viable cells and increased early apoptosis in 66.8%) in S180 cells compared to NC. However, there was no significant difference in late apoptosis and necrosis between BL and the control (p>0.05).
Compared to DOX (35.5% viable cells, 42.4% in early apoptosis and 19.5% in late apoptosis), BL showed a statistically higher percentage of cells in early apoptosis (63.9%) (p<0.05). On the other hand, BL showed a lower percentage of viable cells (16.8%) and late apoptosis (4.8%) (p<0.05) (Figures 3 and 4).
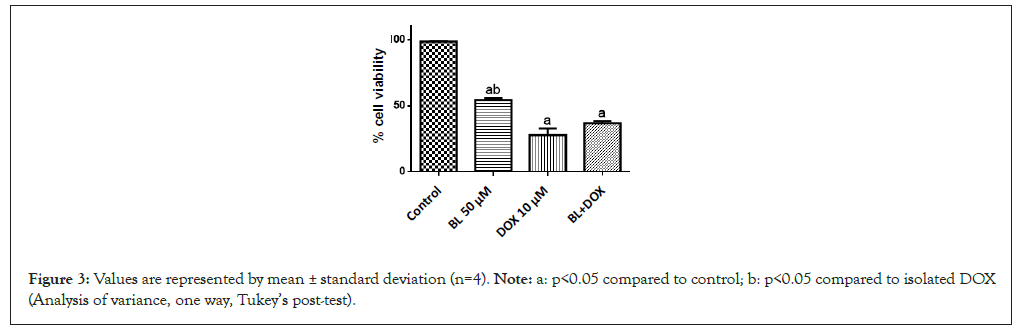
Figure 3: Values are represented by mean ± standard deviation (n=4). Note: a: p<0.05 compared to control; b: p<0.05 compared to isolated DOX (Analysis of variance, one way, Tukey’s post-test).
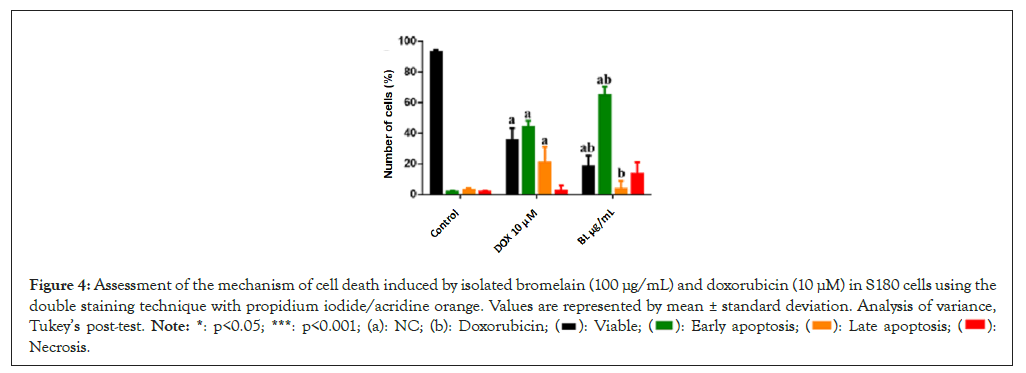
Figure 4: Assessment of the mechanism of cell death induced by isolated bromelain (100 μg/mL) and doxorubicin (10 μM) in S180 cells using the
double staining technique with propidium iodide/acridine orange. Values are represented by mean ± standard deviation. Analysis of variance,
Tukey’s post-test. Note: *: p<0.05; ***: p<0.001; (a): NC; (b): Doxorubicin;  Necrosis.
Necrosis.
To assess the genotoxicity of BL in normal cells (human lymphocytes) and its potential for protecting against DNA damage caused by the chemotherapy drug DOX, the comet assay was conducted. The evaluation of the Damage Index (DI) and Damage Frequency (DF) showed a significant increase (p<0.05) for DOX, confirming its genotoxic potential in normal human blood cells, with an average DI of 255.0 and DF of 94.25. In contrast, the isolated BL did not cause significant DNA damage in the same cells compared to the control, with DI of 28.75 and DF of 13.5. The combined treatment of these compounds showed genotoxic effects on lymphocytes compared to the control. However, BL caused a significant modulation with percentages of 55.91% for DI and 33.65% for DF when compared to the DI and DF values for isolated DOX, indicating a chemo-protective effect for these cells (Figures 5 and 6).
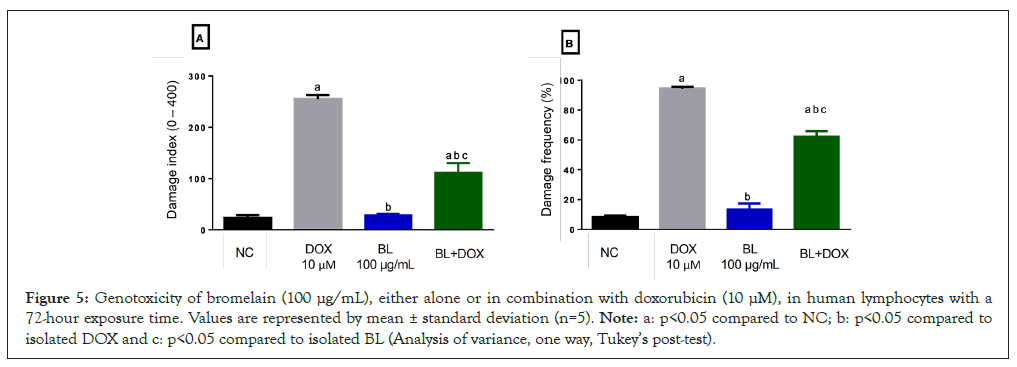
Figure 5: Genotoxicity of bromelain (100 μg/mL), either alone or in combination with doxorubicin (10 μM), in human lymphocytes with a 72-hour exposure time. Values are represented by mean ± standard deviation (n=5). Note: a: p<0.05 compared to NC; b: p<0.05 compared to isolated DOX and c: p<0.05 compared to isolated BL (Analysis of variance, one way, Tukey’s post-test).
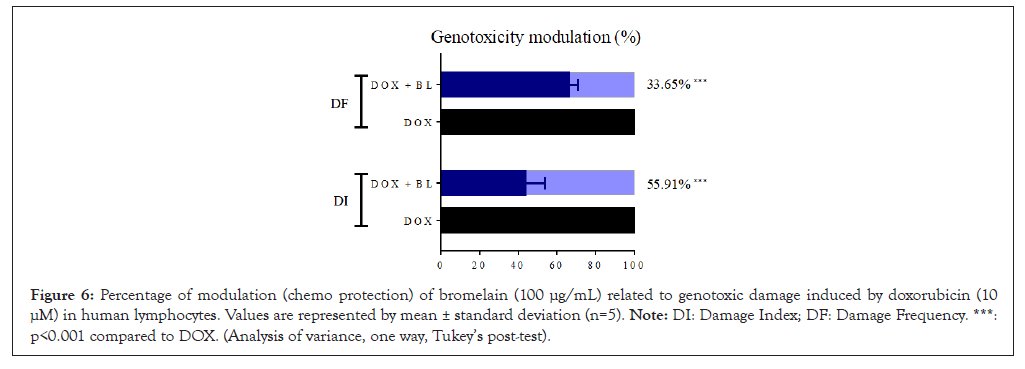
Figure 6: Percentage of modulation (chemo protection) of bromelain (100 μg/mL) related to genotoxic damage induced by doxorubicin (10 μM) in human lymphocytes. Values are represented by mean ± standard deviation (n=5). Note: DI: Damage Index; DF: Damage Frequency. ***: p<0.001 compared to DOX. (Analysis of variance, one way, Tukey’s post-test).
Bromelain, a mixture of proteolytic enzymes, has been reported to have beneficial effects as an anti-inflammatory agent, enhancing antibiotic absorption, fibrinolytic activity, cytokine and immune modulation, mucolytic action, wound healing improvement, cardiovascular and circulatory enhancement and in a variety of gastrointestinal diseases, including inflammatory bowel disease [20-22]. In the present study, it was demonstrated that bromelain exerted anti-proliferative/pro-apoptotic effects in human and murine tumour cell lines, as well as exhibited chemo preventive effects against genotoxic damage caused by Doxorubicin.
In this study, the anticancer effects of bromelain were investigated and confirmed in the tumour cell lines AGP01 (human gastric adenocarcinoma), CAL-27 (oral cavity), SKMEL-103 (human melanoma) and murine sarcoma (Sarcoma-180) by cell viability bioassays such as MTT and Alamar Blue (Table 1, Figures 1 and 3). Other studies have also reported similar activity, such as, which demonstrated significant antitumor effects of bromelain against human breast cancer cell line MCF7, showed that pre-treatment with bromelain reduced the number and volume of skin tumours in mice and revealed anti-proliferative effects of bromelain in oral cancer cells lines Ca9-22 and SCC-25 [23-25].
The cytotoxic mechanisms of bromelain in tumour cells evidenced in this study are likely related to two essential pathways in the regulation of cell growth and apoptosis, namely the Phosphatidylinositol 3-Kinase (PI3K)/Akt and MAP kinase pathways [26]. The results showed that bromelain, in a dose-dependent manner, negatively regulates the phosphorylation of Akt, Extracellular Signal-Regulated Kinase 1 (ERK1) and Extracellular Signal-Regulated Kinase 1 (ERK2) in Cancer coli-2 (Caco-2) cells, suggesting a possible involvement of these pathways in the actions of bromelain [11]. Specifically, bromelain appears to promote cell death by apoptosis in tumour cells and reduce cell proliferation of tumour cells by inhibiting the phosphorylation of Akt and ERK1/2, respectively. These results are consistent with our fluorescence-based cell viability results (Figures 4 and 5), where we observed that this enzyme complex induced early apoptosis in the sarcoma 180 cell line. Furthermore, these molecular mechanisms observed by the authors mentioned align with previous observations that reported an inhibitory effect of bromelain on the activity of cell survival regulators such as Akt and ERK [27,28].
Apoptosis can be regulated by BCL-2 (B-Cell Lymphoma-2) family proteins and their interactions with mitochondria. The BCL-2 family proteins can be divided into three functional groups: pro-apoptotic effector proteins BAK (Bcl-2 antagonist/killer) and BAX (Bcl-2-associated X protein) [29]. Lee, et al., [25] demonstrated that treatment with bromelain increased the expression of BAX and BCL-2 in a dose-dependent manner. This result suggests that an increase in the BAX: BCL-2 ratio may be the molecular mechanism by which bromelain induces apoptosis in oral cancer cells. In the same study, bromelain induced the degradation of caspase-3 and caspase-7 and generated cleavage products. Caspase-9 expression decreased in a dose-dependent manner compared to the control. Therefore, these results indicate that mitochondria-mediated caspase activation is involved in bromelain-mediated apoptosis in oral cancer cells and may likely explain our findings in the studied tumour cell lines.
A study conducted by Chang, et al., [30] demonstrated that bromelain inhibited cell proliferation by inducing both autophagy and apoptosis-related proteins in Colorectal Cancer (CRC) cells. An analysis was carried out to identify the mechanism of action and proteins related to autophagy, such as ATG5/12, beclin, p62 and Light Chain 3 (LC3) I/II. Moreover, levels of ATG5/12, beclin and p62 increased. Furthermore, the conversion of LC3-I to LC3-II increased after treatment with bromelain, indicating its ability to induce autophagy.
By using the apoptotic biomarker levels, it was observed that the expression levels of Apoptosis-Inducing Factor (AIF), Endonuclease G (Endo G), caspases-3, -8 and -9 increased dramatically and the levels of caspase-3, caspase-8, caspase-9 and Poly-adenosine Diphosphate (ADP), Ribose Polymerase (PARP) increased significantly after treatment with bromelain in a dose-dependent manner in HCT116 (Colorectal Carcinoma) cells. These results indicate that bromelain can suppress Colorectal Carcinoma (CRC) proliferation by modulating gene expressions related to autophagy or apoptosis. Treatment with bromelain can inhibit the survival of CRC in three different CRC cell lines (HT-29 (Human colorectal adenocarcinoma cell line), HCT116 and DLD-1 (Colorectal adenocarcinoma cell line)) using the SRB assay.
Using specific fluorescent dyes from the CYTO-ID autophagy detection kit (NZ-51031) as observed by Chang, et al., [30] the green staining in autophagic vacuoles indicated that the positive signals of auto phagosomes increased 2 to 3 times in cells treated with bromelain. Related to lysosome formation, similar induction results were found after treatment, as measured by the LYSO-ID Green detection kit (ENZ-52405). These results suggest that treatment with bromelain can activate the autophagy pathway and promote lysosome formation.
Once the antitumor effect of isolated bromelain was established, this study also examined the potential anti-proliferative effect of combining this cysteine protease with the chemotherapeutic agent doxorubicin. The results of Alamar Blue and MTT viability assays showed that the combination BL+DOX continued to exhibit anticancer activity compared to the control, with IC50 of 26.29 μg/mL, 29.04 μg/mL, 2.68 μg/mL and 6.11 μg/mL for the AGP-01, SKMEL103, CAL27 and S180 cell lines, respectively. Additionally, a combination index study was conducted, demonstrating a synergistic effect of BL at concentrations of 200, 100 and 50 μg/mL with DOX at concentrations of 50, 25 and 12.5 μM for the SKMEL-103 and CAL-27 cell lines and at concentrations of 400, 200, 100, 50 and 25 μg/mL of BL for the AGP-01 cell line (Figure 2). In other words, this combination showed a synergistic effect at these concentrations, characterizing the cytotoxic capability of the combination.
Anti-neoplastic drugs affect the production of immune system cells, reducing white blood cells, which predisposes the body to the invasion and proliferation of microorganisms, increasing the risk of infections and inhibiting the inflammatory response DOX has been used in oncology since the 1960s [30,31]. Tumour regression is significant with the use of DOX alone and it is significantly greater when compared to other antitumor agents. Despite this, its clinical use is limited due to its potential for cardiotoxicity and the possibility of developing drug resistance [32].
The search for bioactive compounds or enzymatic complexes, such as bromelain, which have antitumor activity and, additionally, chemo protective ability, becomes interesting in oncological treatment to reduce systemic and cellular side effects caused by non-selective and toxic antineoplastic agents like DOX. Considering this, the results of our study demonstrate a perspective for combined use in chemotherapy, as we observed the cytotoxic potential of isolated BL and in combination with DOX, as well as its chemo protective ability in human blood cells observed in the Comet assay (Figures 5 and 6).
Chemo protection involves the use of vitamins, bioactive compounds and nutritional supplements to reduce the risk of developing or having a recurrence of cancer in other tissues. Functional food components can enhance the natural antioxidant defence system, scavenging reactive oxygen and nitrogen species, protecting and repairing DNA damage, as well as modulating signal transduction pathways and gene expression [33]. In the studies by Romano, et al., [11] it was observed that bromelain, but not proteolytically inactive bromelain, reduced the concentration-dependent production of Reactive Oxygen Species (ROS) induced by the Fenton reagent in differentiated Caco-2 cells. These data suggest that the inhibition of ROS by bromelain requires enzymatic activity. It can be speculated that proteolytic activity is necessary for bromelain to remove molecules from the cell surface, as reported in peripheral blood cells in which aligns with our study's observations [34].
In a study conducted by Park, et al., [35] that assessed cell viability in four lines of CRC cells, including wild-type Kras (Caco-2, NCI-H508) and mutant Kras CRC (HCT-116, DLD-1) exposed to treatment with the ferroptotic inducer erastin, it was observed that bromelain significantly reduced cell death compared to the DLD-1 treatment. Furthermore, treatment with bromelain-induced ACSL-4 and increased cell death induced by erastin in mutant Kras CRC cells (HCT-116, DLD-1).
Bromelain, in addition to its inhibitory action on certain CRC cell lines, also possesses anti-inflammatory properties. It reduces intestinal and spleen inflammation associated with this type of cancer and has been shown to increase the survival rate in mice [35].
The chemo protective ability observed in blood cells from bromelain can likely be attributed to its capacity to regulate the expression and activity of transcription factors, growth factors, inflammatory mediators and cell cycle intermediaries [36]. According to epidemiological data, various bioactive compounds inhibit different stages of carcinogenesis, including initiation, promotion and cancer progression, by reducing levels of ROS, as observed in the present study, where bromelain combined with DOX exerted modulatory activity on the damage caused by this chemotherapy drug when assessed independently (Figure 5 and Figure 6).
Finally, it is reported that an adequate nutrition pattern is important in helping prevent many diseases, including cancer. Several animal and human studies have indicated that bromelain may have some anticancer activity, but its potential chemo protective action against chemotherapeutic drugs such as DOX needs further exploration and investigation [20,22]. An example of bromelain’s chemo preventive potential was seen in the study by Romano, et al., [11] where it was reported that bromelain prevented the genesis of preneoplastic lesions, polyps and colon tumors induced in rats by the carcinogenic agent Azoxymethane. Further studies reporting the association between bromelain and chemotherapeutic agents should be conducted to improve anticancer efficiency while minimizing chemotherapy side effects.
In conclusion, the bromelain enzymatic complex, when administered in isolation, demonstrated cytotoxic effects, with the ability to induce apoptosis in human and murine tumour cell lines, as evidenced by a significant decrease in cell viability and fluorescent cell death labelling. Moreover, it did not exhibit genotoxic effects on normal human blood cells. Additionally, when combined with Doxorubicin, this enzymatic complex maintained its cytotoxic potential in tumour cells, as evidenced by synergistic effects in the combination index. In this same association (BL+DOX), bromelain modulated the genotoxic damage caused by the chemotherapy drug in human lymphocytes, displaying a chemo protective role in this context.
All authors contributed to the study. The design of the methodologies was defined by Joao Marcelo de Castro and Felipe Cavalcanti Carneiro da Silva. Preparation of reagents and performance of experiments, by Athanara Alves de Sousa, Taline Alves Nobre, Jorddam Almondes Martins, Kennyana Luz Miranda, Yanne Sousa Avelino and Marlene Gomes de Farias. Data analysis and writing review of the first draft of the manuscript were carried out by Raquel Carvalho Montenegro, Ana Rafaela Silva Pereira, Juan Carlos Ramos Goncalves and Felipe Pantoja Mesquita. The review of the results was evaluated by José Roberto Ferreira Junior Anderson Nogueira Mendes, Nicole Debia and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
The authors confirm that the data supporting the findings of this study are available in the article [and/or] in its supplementary materials.
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
Citation: De Sousa AA, Nobre TA, Martins JA, Miranda KL, Pereira AR, De Farias MG, et al. (2024) Antitumoral and Chemoprotective Evaluation of Isolated Bromelain and in Combination with Doxorubicin. J Clin Chem Lab Med. 7:281.
Received: 29-Nov-2023, Manuscript No. JCCLM-23-28297; Editor assigned: 01-Dec-2023, Pre QC No. JCCLM-23-28297 (PQ); Reviewed: 15-Dec-2023, QC No. JCCLM-23-28297; Revised: 22-Dec-2023, Manuscript No. JCCLM-23-28297 (R); Published: 04-Mar-2024 , DOI: 10.35248/2736-6588.23.7.281
Copyright: © 2024 De Sousa AA, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution and reproduction in any medium, provided the original author and source are credited.