Journal of Clinical & Experimental Dermatology Research
Open Access
ISSN: 2155-9554
ISSN: 2155-9554
Research Article - (2020)Volume 11, Issue 6
Aim: A new eye-contour balm product containing melatnin 0.1%, Butyrospermum Parkii (Shea) butter, a tetrapeptide and hyaluronic acid has been recently developed. So far, no objective data are available regarding the anti-wrinkle effect of topical melatonin in the eye contour area. We performed an open, prospective, 2-month, trial in 20 women (mean age 52 years) with mild-to-moderate wrinkle in the eye-contour area.
Subjects and methods: Participating subjects were instructed to apply a melatonin-based eye contour balm (MECB) twice daily (morning and evening). An Antera 3D evaluation of fine wrinkle volume and skin roughness of the crow’s feet area was performed at baseline and after 2 months. The software of the device automatically calculated the roughness index (a parameter linked to fine wrinkles) expressed in mm. Primary outcome was the evolution of skin roughness in the target area. Secondary outcome was the evaluation of skin local tolerability.
Results: All the 20 enrolled subjects completed the trial. The product was very well tolerated. At baseline the eye- contour roughness index mean(SD) value was 19.5(3.0) mm. After 2 months of MECB treatment this parameter was reduced to 13.5(2.8) mm, an absolute difference of 6 mm, representing a 31% reduction. This difference was statistically significant (P=0.001).
Conclusion: A melatonin-based eye-contour balm has demonstrated in this prospective Antera 3D trial a clinically significant anti-wrinkle effect in women with mild-to-moderate eye contour fine wrinkles with very good skin tolerability.
Topical melatonin; Antera 3D; Crow’s feet wrinkles
In 2005 Slominski and co-workers demonstrated that skin is a full and independent melatoninergic system [1]. Melatonin in the skin is a potent antioxidant substance protecting mitochondrial [2] and cell functions [3]. Tan and co-workers [4] have shown that melatonin is a free radical scavenger and it could protect skin from UV damage [5]. Melatonin is able also to express anti-inflammatory actions [6]. Several researches [7,8] support the concept that skin melatonin can have an anti-aging and geroprotector function. In aging skin, a progressive reduction of local melatonin production has been documented [9]. Melatonin, in an animal model of UV-induced skin damage, has shown to have anti-wrinkle effect [10]. Clinical trials, conducted in women with mild-to-moderate skin aging, have demonstrated that topical melatonin in cream or serum formulations has shown to improve clinically skin aging appearance [11,12]. Crow-feet area is commonly involved in chrono and photoaging with the formation fine wrinkles [13]. Crow’s feet wrinkles (dynamic and static) are characterized as fine lines around the lateral aspect of the eyes [14]. These wrinkles develop with aging and their cosmetic treatment could be challenging [15]. A new eye- contour balm (Nutriage Eye Balm, Cantabria Labs Difa Cooper) product containing melatonin 0.1%, karitè butter, hyaluronic acid and a tetrapeptide is available. No objective data are available regarding the anti-wrinkle effect of this topical melatonin product in the eye contour area (the so-called crow’s feet area). The aim of the present study was to evaluate the efficacy of this melatonin- based eye balm in reducing crow’s feet wrinkles evaluated by means of Antera 3D system.
We performed an open, prospective, 2-month, trial in female subjects (mean age 52 years) with mild-to-moderate wrinkles in the eye-contour area. A group of 20 healthy Caucasian women with Fitzpatrick skin phototype I-III, aged 45-70 years and having visible crow’s feet wrinkles were recruited from two Dermatologic Clinics in Italy (Modena and Bolzano). The study was conducted following the Good Clinical Practices (GCP) regulations in accordance with the Declaration of Helsinki [16]. Before patients’ enrollment, the study protocol has been approved by each Institute Review Bord of the two participating centers (approval Code: NEB-01-2019). All clinical and instrumental procedures utilized in the study were described in detail to the volunteers and a written informed consent was obtained from all the enrolled subjects before the start of the treatment. Subjects were told to apply a melatonin-based eye contour balm (MECB) twice daily (in the morning and in the evening) for 8 consecutive weeks. The formula of this product contains also a tetrapeptide, (acetyl tetrapeptide 5), Butyrospermum Parkii (Shea) butter and hyaluronic acid. An Antera 3D evaluation of fine wrinkle volume and skin roughness of the crow’s feet area was performed at baseline and after 2 months. This diagnostic tool utilizes a technology related to shape from shading, photometric stereo measurements, with the aim to reconstruct the skin in 3D thanks to multiple images taken under different light sources with 7 different wavelengths spanning most of the visible spectrum [17,18]. The software of the device automatically calculated the roughness index (a parameter linked to fine wrinkles) expressed in mm. We planned to have as the primary outcome of this trial the evaluation of the evolution of skin roughness in the target area (eye contour). Secondary outcome was the evaluation of skin local tolerability. Statistical analyses were performed using GraphPad Prism ver. 5 (San Diego, California, US) statistical software. All data are presented as mean ± standard deviation (SD). The Wilcoxon test for paired measurements was used to evaluate the primary endpoint of the trial (i.e. evolution of eye-contour roughness index values) comparing baseline with after treatment values. A P value less than the significance level 0.05 (p<0.05) was considered as statistically significant. The present trial should be considered as a pilot study. For this reason, not a formal sample size calculation was done; however, we hypothesized that a total of 20 evaluable subjects could be considered a sufficient number.
The trial was conducted between March 2019 and November 2019. All the enrolled subjects completed all the phases described in the study protocol. At baseline, the eye-contour roughness index means (SD) value was 19.5(3.0) mm. After 2 months of MECB application, this parameter was reduced to 13.5 (2.8) mm, an absolute difference of 6 mm, representing a 31% reduction. This difference was statistically significant (P=0.001; Figure 1). Figures 2 and 3 show Antera 3D pictures of eye contour zone at baseline and after treatment of two subjects (subject A and subject B). The tested product was very well tolerated. All evaluated subjects reported no side effects.
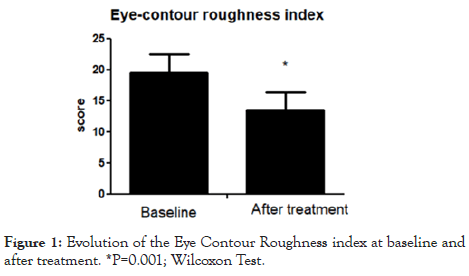
Figure 1: Evolution of the Eye Contour Roughness index at baseline and after treatment. *P=0.001; Wilcoxon Test.

Figure 2: Antera 3D evaluation of eye contour wrinkles at baseline and after treatment in one treated subject (A). The shaded area is the evaluated target zone.
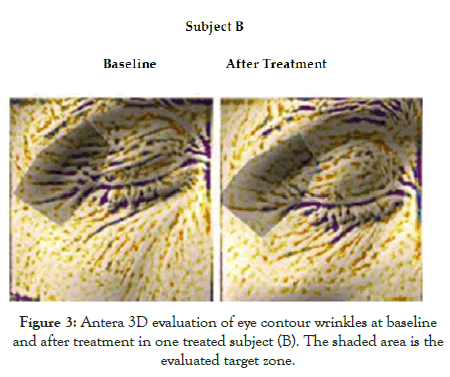
Figure 3: Antera 3D evaluation of eye contour wrinkles at baseline and after treatment in one treated subject (B). The shaded area is the evaluated target zone.
Melatonin, from the chemical point of view, is an N1-acetyl-5- methoxytryptamine [19]. This molecule was isolated for the first time from bovine pineal tissue [20]. In mammals, the blood levels of melatonin increase during the nocturnal phase and are reduced during the day [21]. This circadian rhythm is almost exclusively a result of its nighttime synthesis and secretion from the pineal gland. Melatonin biological actions are defined as pleiotropic, mediated by specific membrane and nuclear receptors, or mediated by non-receptor chemical interactions [22]. Melatonin, in addition to the well-known secretion of pineal gland, is also produced in a greater amount by other non-endocrine organs like gastrointestinal system, bone marrow and ovary [23]. Interestingly, melatonin is also produced by the skin [3]. Melatonin modulates skin functions and structures through actions mediated by cell-surface and nuclear receptors expressed in skin cells [24]. In vivo and ex-vivo experiments have shown that exogenously applied melatonin exerts antioxidant and anti-inflammatory effects [25]. Melatonin, thanks to its amphiphilic chemical structure, can reduce oxidative damage in both lipid and aqueous cellular compartments [26]. Melatonin protects and maintains mitochondrial homeostasis, reduces free radical generation, and preserves mitochondrial ATP production [2]. Therefore, melatonin could express powerful anti-aging actions [27,28]. Different from the pineal melatoninergic system, skin melatonin is exposed directly to numerous environmental stressors, such as ultraviolet radiation (UVR) [29] and pollutants [30]. Melatonin, present in the skin, is able to attenuate ultraviolet radiation-induced skin damage [31]. Therefore, cutaneous melatonin could fight against photoaging mechanisms [32]. For these reasons, skin melatonin is considered a relevant anti-aging and geroprotector molecule [33,34]. In aged skin production of melatonin is progressively reduced [35,36]. Therefore, increasing skin melatonin levels could be considered a relevant anti-aging therapeutic strategy [37]. However, exogenous melatonin must be used topically rather than orally. The reason for this is related to the fact that orally administered melatonin reaches rather low levels in the blood due to a strong first-pass effect [38]; as a result of this aspect, oral intake of melatonin is not able to increase its concentration in the skin. Topical melatonin administration can bypass this problem. Topical melatonin application is considered as meaningful pharmacological strategy since it can easily penetrate the stratum corneum, reaching the lower epidermis and dermis [39,40]. Two recent clinical trials [11-12] conducted in a total of 40 women with mild-to-moderate photoaging have shown that topically applied melatonin, in cream and serum formulations, improves clinical and instrumental-assessed signs of skin aging. In the present study, we demonstrate that a specific eye-contour topical product containing melatonin can improve wrinkles of the crow’s feet area in middle age women. Some limitations should be taken in account in evaluating the results of this trial. First, this is a proof-of-concept, pilot study. Future trials with larger sample size are warranted to evaluate the real clinical effects of this topical product in the treatment of skin aging of the eye contour area. A second limitation of this study is that this is not a double-blind trial. However, to enhance the internal validity of our results, we evaluated the primary endpoint by an instrumental, non-operator dependent approach (Antera 3D).
A melatonin-based eye-contour balm has demonstrated in this Antera 3D prospective pilot trial a clinically significant anti-wrinkle effect in women with mild-to-moderate eye contour fine wrinkles. This formulation has shown also very good skin tolerability. Further studies are warranted to compare the clinical efficacy of this product with other non-melatonin eye-contour products.
Conceptualization, M.P. and M.M.; methodology, M.P; software, M.M.; validation, M.P, M.M., and K.E.; formal analysis, M.M.; investigation, M.P and K.E; writing—original draft preparation, M.M.; writing—review and editing, M.P.; All authors have read and agreed to the published version of the manuscript.”, please turn to the CRediT taxonomy for the term explanation. Authorship must be limited to those who have contributed substantially to the work reported.
This research received no external funding.
The Authors want to acknowledge the non-medical staff involved in the trial for their support in conducting the study.
The authors declare no conflict of interest.
Citation: Puviani M, Eisendle K, Milani M (2020) Anti-Wrinkle Efficacy of a Melatonin-Based Eye Contour Balm: An Antera 3D Prospective 2-Month Pilot Trial. J Clin Exp Dermatol Res. 11:534.
Received: 09-Sep-2020 Accepted: 23-Sep-2020 Published: 30-Sep-2020 , DOI: 10.35248/2155-9554.20.11.534
Copyright: © 2020 Puviani M, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.