Journal of Nanomedicine & Biotherapeutic Discovery
Open Access
ISSN: 2155-983X
ISSN: 2155-983X
Research Article - (2017) Volume 7, Issue 1
Hybrid Liposomes (HL) composed of 90 mol% L-α-dimyristoylphosphatidylcholine (DMPC) and 10 mol% polyoxyethylene (25) dodecyl ether (C12(EO)25) was examined to study the stimulation of apoptosis and antiinvasive actions against in vitro cultured human breast cancer (MCF-7) cells. The results obtained from the study reveals that HL suppressed the growth of MCF-7 cells without any drugs. Apoptosis for MCF-7 cells after subjecting them to HL treatment was noticed. To study the non-invasive actions of HL on the drifting of MCF-7 cells, we exercised Wound scratch assay. Non-invasive effects of HL on the migration of MCF-7 cells were obtained on the basis of wound scratch assay. HL suppressed the pseudopodium formation of MCF-7 cells. The study to determine the non-invasive effects of HL against MCF-7 cells were done by matrigel invasion assay which gave positive results due to inhibition of MT1MMP/MMP14 by HL.
Keywords: Hybrid liposome; Nanotherapy; Breast cancer; Apoptosis; Anti-invasive effects
Breast cancer is among the most prevalent cancer in females worldwide. Most of breast cancer causes invasion to neighbourhood lymph node and organs. The human epidermal growth factor receptor 2 (HER2) is tumour marker of breast cancer, and overexpression of HER2 is found in approximately 30% of patients with breast cancer [1]. Trastuzumab which is monoclonal therapeutic antibody against HER2 is applied to the treatment for patients with breast cancer [2-4]. However, the efficacy of Trastuzumab for patients with HER2-negative breast cancer and trastuzumab-resistant HER2-positive breast cancer is limited [4,5]. Therefore, novel nanomedicine against HER2-negative breast cancer is needed.
Hybrid Liposomes (HL) made of vesicular and micellar molecules are nano-sized liposomal particles and can be processed without contamination of the organic solvent unlike traditional liposomes [6]. Studies of HL made of DMPC and polyoxyethylene (n) dodecyl ethers (C12(EO)n) on the in vitro [7-9] and in vivo [9-12] development of lymphoma cells, and clinical applications [12] without drugs have already been reported. In chemotherapy for HER2-negative breast cancer, invasive suppression and the apoptotic induction of breast cancer cell are important treatment strategies. However, apoptotic and anti-invasive activities of HL against HER2-negative breast cancer of have not yet been confirmed.
We measured the apoptotic and anti-invasive actions of HL composed of L-α- dimyristoylphosphatidylcholine (DMPC) and polyoxyethylene(25)dodecyl ether (C12(EO)25) for metastatic breast cancer of HER2-negative cells in our study.
Hybrid liposomes preparation
HL was prepared by sonicating a mixture of 95 mol% 3 L-α- dimyristoylphosphatidylcholine (DMPC, NOF, Tokyo, Japan) and 5 mol% polyoxyethylene(25)dodecylether (C12(EO)25, Nikko Chemicals, Tokyo, Japan,) in 5% glucose solution in a bath type sonicator (VS-N300, VELVO-CLEAR, Tokyo, Japan) at 45°C and 300 W. Using a membrane filter (0.20 micron, Advantec, Tokyo, Japan), the filtration of the sample solutions were performed and stored at 25ºC. The same protocol was followed to prepare DMPC liposomes.
Dynamic light scattering measurements
An electrophoretic light scattering spectrophotometer (ELS-Z0, Otsuka Electronics, Osaka, Japan) was used to gauge the diameter of HL. Stokes-Einstein formula (Equation 1) was used to calculate the diameter (dhy), where T is the absolute temperature, κ is the Boltzmann constant, D is the diffusion coefficient and η is the viscosity and:
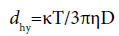 (1)
(1)
Cell culture
Human breast cancer (MCF-7) cell lines were acquired from ATCC (Manassas, VA, USA). MCF-7 cells were sustained in RPMI- 1640 medium (Gibco, Gaithersburg, MD, USA) enriched with 100 U/mL of penicillin, 50 μg/mL of streptomycin, and 10% fetal bovine serum (HyClone Laboratories, Logan, UT, USA). A 5% CO2 humidified incubator was used to culture the cells at 37°C.
Assessment of 50% inhibitory concentration of HL
The 50% inhibitory concentration (IC50) of HL on the development of MCF-7 cells was evaluated with respect to WST-8 [2-methoxy-4- nitrophenyl-3-(4-nitrophenyl)-5-(2,4-disulfophenyl)-2H-tetrazolium, monosodium salt] assay (Cell Counting Kit-1, Dojindo Laboratories, Kumamoto, Japan). MCF-7 cells were implanted in 96-titer well plates at concentration of (8.0 × 104 cells/ml) and then cultured at 37°C for 24 h in a 5% CO2 humidified incubator. After addition of DMPC (0.1-5 mM) or HL (0.1-2 mM depending on DMPC concentration), cells were cultured for an additional 48 h after which the cells were incubated for 3 hrs after WST-8 addition. At 450 nm, the absorbance was measured by using a spectrophotometer (Emax; Molecular Devices Co., California, USA). The thwarting effects of HL on the development of MCF-7 cells were determined by Amean and Acontrol, where Acontrol and Amean denote the absorbance of water-soluble formazan, with and without HL, respectively.
Assessment of apoptotic induction using annexin-V binding assay
Detection of early apoptotic cells was performed by the Annexin-V binding assay using Annexin-V-FLUOS Staining kit (Roche Diagnostics K.K., Basel, Switzerland). After addition of HL ([DMPC]=0.5 mM), MCF-7 cells were cultured for 3 h at a concentration of (8.0 × 104 cells/ ml). The cells were subjected to washing with phosphate buffered saline in absence of Ca and Mg (PBS (−)) and then stained with Annexin- V-fluorescein and Propidium Iodide (PI) and then scrutinized under a confocal laser microscope (TCS-SP; Leica Microsystems, Wetzlar Germany) enhanced with 488 nm Ar laser. The signals from Annexin- V-fluorescein and PI were detected at 500-562 nm and at 638-693 nm respectively.
Scratch wound assay in vitro
Motility of MCF-7 cells was examined using a scratch wound assay. At a concentration of 6.0 × 105 cells/ml, the cells were implanted into tissue culture dishes and cultured in a medium fortified with 10% FBS to nearly confluent cell monolayers. A sterile pipette tip was used to cautiously wound and any cellular debris was discarded by PBS (-) wash. A medium containing 10% FBS and treated with or without 100 μM HL was used to incubate the wounded monolayer. The images of the cells were taken after 24 h under a light microscope (EVOS fl, Life Technologies, CA. USA). The migration area was determined using ImageJ (Version 1.46r, National Institutes of Health, Bethesda, MD, USA) which is an image analysis software.
Formation of filopodia detection by fluorescence microscopy technique
The filopodia formation in the MCF-7 cells was examined using confocal laser microscopy. For 3 h, cells (8.0 × 104 cells/mL) were treated with HL (50 and 200 μM). After 10% formaldehyde fixing, incubation of the cells were done for 30 min with rhodamine phalloidin (molecular probes). The stained cells were observed by confocal laser microscope with a 488 nm Ar laser. Rhodamine phalloidin-fluorescein signals and PI signals were detected at 500-562 nm and 638-693 nm respectively.
In vitro invasion assay study
Matrigel invasion chamber (BD Biosciences, NJ, USA) was used to measure the cancer cell invasion in the present in vitro study. Cultured cells were transferred to 8 μm pore size membrane embedded in the matrix formed with thin layer of Matrigel. Freshly prepared serum-free medium containing either 5% glucose solution as a control, DMPC and HL (50 and 200 μM), was added to prepare cell suspension of MCF-7 cells (2.2 × 104 cells/ml) [2]. The very same composition of medium was mixed with 10% FBS to function as chemo-attractant in the outer chamber. After successfully completing the previous steps, the cells were subjected to prolong incubation (24 h) in room temperature (37°C). Further, cells were humidified with 5% CO2 for similar duration. To quantify the total number of tumour cell invasion, a cotton tipped swab was employed with mild scrubbing, which helped to discard the noninvading cells from the whole cell aggregates. The cells attached within the lower surface of membrane were then accessed for ethanol fixation and stained with crystal violet. Light microscopic technique (ECLIPSE TS100, Nikon, Tokyo, Japan) was used for the visualization of the cells. Necessary photographs were taken as documentary evidences.
Evaluation of MT1-MMP (MMP-14) by flow cytometer
At a concentration of 1.6 × 105 cells per well, MCF-7 cells were added to dishes and then was subjected to 24 h incubation, HL (200 μM) treated for 24 h, PBS(-) washed, trypsinized, centrifuged for 5 min at 1500 rpm and then 10% formaldehyde fixed for 30 min in the wells. Cells were made permeable using cold methyl alcohol in PBS(-) in ice for half an hour and then the cells were twice washed. Further 30 min incubation with Human MMP-14/MT1-MMP 18 Alexa Fluor 488 MAb (R&D systems, Minneapolis, MN, USA) (5 μg/ml) at room temperature was done and then in a humidified box maintained at 4°C for 1 h. Washed stained cells were then gauged by flow cytometer.
Statistical analysis
Student’s t-test was used to evaluate the data. p<0.05 represent a substantial statistically difference.
Physical properties of HL
On the basis of dynamic light scattering measurements, the morphology of HL comprising of 95 mol% DMPC and 5 mol% C12(EO)25 was done. The hydrodynamic diameter (dhy) of HL stored at 25°C was less than hundred nanometres with a distribution being narrow and single. Drawing a comparison between HL and DMPC liposomes, HL was found to be stable in even after one month while DMPC liposomes were found to be unstable and after 14 days, they precipitated. Dhy of HL can be retained at 25°C as it can be stored for a longer time span before its application in in vivo and clinical field. There is a possibility that HL below 100 nm in diameter might evade Reticular Endothelial System (RES) [13] and also be the appropriate candidate for the in vivo and clinical intravenous administration.
Thwarting actions of HL on the growth of MCF-7 cells
In vitro evaluation of the 50% inhibitory concentration (IC50) of HL on the progression of human breast cancer (MCF-7) cells using WST-8 assay was performed (Figure 1) [14]. The IC50 values of HL (200 μM) against MCF-7 cells were significantly less in comparison to the values of the DMPC liposomes (200 μM). It is remarkable that HL has high inhibitory effects on the growth of human breast cancer (MCF-7) cells without usage of drugs.
Stimulation of apoptosis for MCF-7 cells treated with HL
Determination of early apoptotic cells (Figure 2) was exercised by Annexin-V binding assay [15]. A green color signifying early-stage apoptosis was noticed in fluorescence micrograph of MCF-7 cells using Annexin-V binding assay after HL treatment for 3 h comparatively the intensity of green color was less in fluorescence micrograph of MCF-7 cells treated with DMPC liposomes. These results mean that the HL induces apoptosis in MCF-7 cells.
Suppression effects on migration of MCF-7 cells by HL
The determination of anti-migration effects of HL on the drifting potential of cells was done by scratch wound assay (Figure 3) [16,17]. The migration of MCF-7 cells were compared with control cells, which differed significantly (p<0.05). The antimigration effects of HL were scrutinized at low concentrations (200 μM) without affecting growth-inhibition below IC50 values (200 μM). Additionally, pseudopodium development on the exterior of tumour cells has a chief responsibility in the invasion and migration into the neighbouring tissue [18-20]. The suppression actions of HL on the pseudopodium formation of MCF-7 cells were scrutinised by using confocal laser microscopy (Figure 4). Scarce pseudopodium on the exterior of MCF-7 cells HL treated (40 μM) was under observation for 3 h, even though several pseudopodium on the cell surface in the control and after the DMPC treatment were obtained. These results reveal that HL can stop migration of MCF-7 cells by blocking the actin cytoskeleton.
Inhibitory actions of HL on invasion
The present study is dealing with the investigation on anti-invasive effect of HL on MCF-7 cell line. The same we have employed in vitro invasion assay in Matrigel invasion chamber (Figure 5) [16,20-22]. Reduced rate of invasiveness was evident in the present study after HL treatment in dose dependent manner. At lower concentration of HL appreciable anti-invasive effect was observed, which did not alter the growth-inhibition below IC50 values (238 μM). Further, flow cytometric experimentation with membrane type 1 metalloprotease (MT1-MMP/ MMP14) showed that, successive activation took place in MCF-7 cells, after HL administration. Reports have been shown that, MT1-MMP/ MMP14 is a transmembrane metalloprotease play effective role in pericellular proteolysis [2] and invasion, which is also evident in the present study (Figure 6). Expression analysis of MT1-MMP/MMP14 in MCF-7 cells has shown that, HL significantly decreased the protein level while compared with control and DMPC. Together, the result suggests that HL is capable of inhibiting the invasion process in MCF-7 cell.
The study confirms the role of HL in apoptosis as well as in the anti-invasive property on MCF-7 cell line. Moreover, The potential findings comes out of the present study highlighted that, HL functions as an inhibitory agent on the development of MCF-7 and at the same time initiates the process of apoptosis, which is evident via Annexin-V binding assay. HL also inhibits the migration of MCF-7 cells and hinders in the pseudopodium formation in the said cell line. Lastly, anti-invasive action of HL was evident through Matrigel invasion assay and inhibitory effect was also observed in MT1-MMP/MMP14 protein expression. The possible application of the study is promising and could be assistive in the patient with HER-2 negative breast cancer and with a prehistory of chemotherapeutic treatment.
We thank Arisa Kikumoto and Yumi Nakashima for their technical assistance. This work was supported in part by a Grant-in-Aid for Science Research from the Ministry of Education, Science, and Culture of Japan (Nos. 17K01383 and 17K05944).