Gynecology & Obstetrics
Open Access
ISSN: 2161-0932
ISSN: 2161-0932
Research Article - (2013) Volume 3, Issue 5
Introduction: Cervical cancers are the second most frequent type of female cancer, responsible for about 5% of cancer deaths in females worldwide. Recently, parameters of cell proliferation and cell death have emerged as important diagnostic and prognostic tools.
Aims: The aim was to evaluate the role of Apoptotic Index and Ki-67 as proliferation marker in premalignant and malignant lesions of uterine cervix. Materials and Methods: The study included 179 patients of cervical dysplasias and malignancy. Evaluation of Apoptotic Index (using light microscopy) was performed on hematoxylin and eosin-stained sections. Ki-67 (MIB-1 antibody) expression was both graded as well as Labelling Index was calculated. Statistical evaluation was carried out using the Student t test (p<0.05).
Results: There was increase in mean Apoptotic Index with increasing grade of dysplasia and difference in mean values between CIN-I and CIN-II; CIN-I and CIN-III were found to be statistically significant. Also Apoptotic Index increased from well differentiated Squamous Cell Carcinoma (SCC) to poorly differentiated SCC. There was increase in mean Labelling Index with increasing grade of dysplasia and when the p value amongst these groups was statistically significant. Labelling Index was maximum in Poorly Differentiated SCC and minimum in Moderatly Differentiated SCC and p value amongst these groups was found to be statistically significant.
Conclusion: Both Apoptotic Index and Ki-67 expression could be used as a biomarkers in the evaluation of the proliferative activity and progressive potential of dysplastic and neoplastic changes.
Keywords: Cervical cancer, Lymph node, Metastasis, PEComa, Treatment, Systematic review, Survival
Cervical cancers are the second most frequent type of female cancer, responsible for about 5% of cancer deaths in females worldwide [1]. Having said this, however, no form of cancer better documents the remarkable effects of prevention, early diagnosis and curative therapy on the mortality rate than does cancer cervix. Papanicolaou (Pap) smear screening programmes and histologic interpretation of biopsy specimen by the pathologist have significantly reduced the mortality of cervical cancers. However, the Pap test is not very accurate due to subjective test criteria. This limits the present screening programmes and emphasizes the need for the identification of specific biomarkers for dysplastic epithelial cells to aid in primary screening and lesion diagnosis [2].
Apoptosis is genetically controlled death which enables the elimination of the cells that have been damaged [3]. As apoptotic tumour cells can be identified and counted by light microscopy, there has been interest in the application of the enumeration of apoptosis in malignant growths as a putative prognostic marker.
Ki-67 is a proliferative marker. In 1990, it was demonstrated that the MIB-1 antibody detects Ki-67 antigen in the G1, S, G2 and M phase, but it is absent in the G0 phase [4]. Therefore, this antibody may be a useful marker of proliferation in dysplastic lesions, particularly in cervical smears, and, in addition, can be of prognostic value [5].
The present study was carried out on 179 patients of cervical dysplasias and malignancy for a period of 2 years. A detailed clinical history and examination was carried out along with routine investigations. The post surgical specimens were then processed. All sections were routinely stained with Hematoxylin and Eosin (H&E) stain. Care was taken to have sections of uniform thickness (not greater than 5 mm). Protocol used for Immuno-histochemistry for Ki-67 (MIB-1 antibody) on paraffin embedded tissue sections using the kit, Ki-67 Diagnostic Biosystems in a dilution of 1:100.
Apoptotic Index (AI): The H&E sections were examined using a ‘40x’objective. From each section four areas devoid of any preservation or fixation artifact were selected. In each section, 1000 tumour cells were evaluated for the presence of apoptotic cells and apoptotic bodies; and apoptotic index was calculated as the number of apoptotic cells and apoptotic bodies expressed as a percentage of total number of tumour cells counted in each case [6].
Grading of Ki-67 expression
The sections stained for Ki-67 proliferation (revealed as nuclear staining) were evaluated using scores from 1 to 3 [7]:
1: “+++”- High proliferation->50% positive cells
2: “++”- Moderate proliferation-30%-50% positive cells
3: “+” - Low proliferation-10-30% positive cells.
Calculation of MIB-1 Labelling Index [8]
MIB-1 labelling index (LI) was calculated by the number of positive cells per 100 cervical epithelial cells in different areas under X400 magnification and the mean was calculated. Positive nuclei were expressed as the percentage of total nuclei counted. MIB-1 labelling index was calculated as follows:
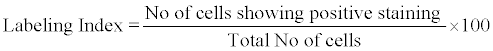
Statistical evaluation was carried out using the Student t test, with P<0.05 being significant.
A total of 179 cases of cervical lesions were divided into 4 broad categories: Cervical dysplasia- 78 cases (43.6%) [CIN-I-45, CIN-II-19, CIN-III-14], Squamous cell carcinomas (SCC)-94 cases (52.5%) [Well Differentiated (WD SCC)-20, moderately differentiated (MD SCC)-66, Poorly Differentiated (PD SCC)-8], Adenocarcinoma-4 cases (2.2%), Adenosquamous carcinoma-3 cases (1.7%).
The apoptotic cells showed certain well defined features which included cell shrinkage, condensation and deep eosinophilia of the cytoplasm and pyknotic, round to crescentric or irregular nucleus (Figure 1). Karyorrhexis was also observed frequently. Apoptotic bodies, which appeared as tiny, round and pyknotic nuclear fragments, were seen scattered among tumour cells and sometimes forming a cluster.
The mean value of Apoptotic Index increased progressively from dysplasia (0.178% ± 0.143) to SCC (0.652% ± 0.302) and the difference was found to be statistically significant. There was increase in mean AI with increasing grade of dysplasia i.e. from CIN-I ( 0.106% ± 0.084); to CIN-II (0.24% ± 0.106); to CIN-III (0.34% ± 0.184) , however the AI values between CIN-II and CIN-III were not found to be statistically significant but difference in mean values between CIN-I and CIN-II ; CIN-I and CIN-III were found to be statistically significant (p<0.05). An increase in apoptotic index was observed from well differentiated SCC to poorly differentiated SCC. The mean AI in WD SCC was (0.577% ± 0.274) ; MD SCC was (0.664% ± 0.31) and in PD SCC was (0.7% ± 0.3), but no statistical significance was observed on correlation. The mean AI in adenocarcinoma was (0.567% ± 0.153) and in adenosquamous carcinoma was (0.433% ± 0.208) (Table 1).
| S.No | Category | No. of Cases | Mean AI () ± SD | Range (Min-Max) |
|---|---|---|---|---|
| 1. | CIN-I | 35 | 0.106 ± 0.084 | 0-0.3 |
| 2. | CIN-II | 15 | 0.24 ± 0.106 | 0-0.4 |
| 3. | CIN-III | 10 | 0.34 ± 0.184 | 0.1-0.6 |
| 4. | Well Differentiated SCC | 13 | 0.577 ± 0.274 | 0.2-1.2 |
| 5. | Moderatly Differentiated SCC | 55 | 0.664 ± 0.31 | 0.2-1.7 |
| 6. | Poorly Differentiated SCC | 7 | 0.7 ± 0.3 | 0.5-1.3 |
| 7. | Adenocarcinoma | 3 | 0.567 ± 0.153 | 0.4-0.7 |
| 8. | Adenosquamous carcinoma | 3 | 0.433 ± 0.208 | 0.2-0.6 |
Table 1: Correlation of Apoptotic index with grades of cervical Dysplasia and cervical Cancer.
50 cases out of total cases of cervical dysplasias and carcinomas were subjected to immunohistochemical staining for Ki-67.
Out of 20 cases of dysplasias, 16 (80%) showed low proliferation, 3(15%) showed moderate proliferation and 1 (5%) high proliferation. Out of 26 cases of SCC 19 (73.1%) showed moderate and 7 (26.9%) showed high proliferation (Figure 2 and Table 2).
| S.No | Category | No.of Cases | Low | Moderate | High |
|---|---|---|---|---|---|
| A | Dysplasia | 20 | 16 | 3 | 1 |
| 1. | CIN-I | 10 | 10 | _ | _ |
| 2. | CIN-II | 6 | 6 | _ | _ |
| 3. | CIN-III | 4 | _ | 3 | 1 |
| B | SCC | 26 | 0 | 19 | 7 |
| 1 | WD SCC | 6 | _ | 2 | 4 |
| 2 | MD SCC | 17 | _ | 17 | _ |
| 3 | PD SCC | 3 | _ | _ | 3 |
| C | Adenocarcinoma | 2 | _ | 1 | 1 |
| D | Adenosquamous Carcinoma | 2 | _ | 1 | 1 |
Table 2: Ki-67 Expression in cervical dysplasias and carcinomas.
The mean value of LI (Labelling Index) was found to increase as the nature of the lesion changed from dysplasia (16.94 ± 14.871) to SCC (50.754 ± 12.625) and the difference was found to be extremely statistically significant ( p value <0.0001). There was increase in mean LI with increasing grade of dysplasia, from CIN-I (5.54 ± 2.185); to CIN-II (18.9 ± 2.491); to CIN-III (42.5 ± 7.937), and when the p value amongst these groups was evaluated, it was found to be statistically significant. The mean LI in WD SCC was 55.333 ± 7.789; MD SCC was 43.976 ± 3.152 and in PD SCC was 80 ± 5. Correlation between WD SCC and MD SCC; MD SCC and PD SCC; PD SCC and WD SCC (p value <0.05) was found to be statistically significant. The mean LI in Adenocarcinoma was 55 ± 21.213 and mean LI in Adenosquamous Carcinoma was 52 ± 8.485 (Table 3).
| S.No | Category | No. of Cases | Mean LI±SD | Range (Min-Max) |
|---|---|---|---|---|
| 1 | CIN-I | 10 | 5.54 ± 2.185 | 3-11.2 |
| 2 | CIN-II | 6 | 18.9 ± 2.491 | 15.2-22.4 |
| 3 | CIN-III | 4 | 42.5 ± 7.937 | 35-53 |
| 4. | WD SCC | 6 | 55.333 ± 7.789 | 47-68 |
| 5. | MD SCC | 17 | 43.976 ± 3.152 | 38-49 |
| 6. | PD SCC | 3 | 80 ± 5 | 75-85 |
| 7. | Adenocarcinoma | 2 | 55 ± 21.213 | 40-70 |
| 8. | Adenosquamous Carcinoma | 2 | 52 ± 8.485 | 46-58 |
Table 3: Correlation of LI with grades of cervical dysplasia and cancer.
Our study is based on the evaluation of Apoptotic Index in 179 cervical premalignant and malignant squamous and adeno carcinoma cell lesions on light microscopy. Apoptotic bodies were counted using ×400 magnification and similar to the views of Soini et al. [9], we observed that a fairly accurate assessment of apoptosis is possible by light microscopy. Apoptosis was high in areas of necrosis (Figure 3) and these areas were excluded from the evaluation, as well as areas with intense inflammatory infiltrate. Apoptotic cells in the stroma around the tumors should also be disregarded.
Apoptosis is morphologically identifiable and characterized by light and electron microscopy. Although it is accepted that electron microscopy is the best way to identify apoptotic cells, this method is not practical in most histological studies of specimens [10]. Several studies assessing the prognostic relevance of apoptosis have used the In-Situ End-Labelling (ISEL) or the TdT-mediated dUTP-biotin nick End- Labelling (TUNEL) techniques [11-14]. However, it has been argued that TUNEL and ISEL do not differentiate equivocally apoptosis from necrosis [15]. Moreover, it is a specialized technique and so its set up and standardization is not available at every institute. Keeping this in mind and due to economic constraints, we have used light microscopy in our study.
We observed an increased AI as the nature of the lesion progressed from dysplasia to SCC. The results are in accordance with Nam et al., [12] Shoji et al. [14]. The mean apoptotic indices were found to increase with increasing degrees of dysplasias. Similar results were observed by Vijaya et al. [6] and Nam et al. [12,14]. We observed an increase in apoptotic index from well differentiated SCC to poorly differentiated SCC. The results are in concordance with the study done by Nam et al. [12] who also found an increase in AI with increase in grade of SCC.
Ki-67 is detected in the nucleus of proliferating cells in all active phases of the cell division cycle, but is absent in non-proliferating cells indicating that the Ki-67 antigen could be used as a marker for cells of the growth fraction [16]. In the present study 50 cases of cervical dysplasia and carcinomas were subjected to immunohistochemical staining for Ki-67. It comprised of 20 cases of dysplasia and 30 cases of carcinomas.
The mean value of LI was found to increase as the nature of the lesion changed from dysplasia to SCC. Similar results were observed by Mehrotra et al., Nam et al., Shoji et al. and Natália et al. [8,12,14,17]. Also the mean Labelling Index (LI) in cervical dysplasias increased with increasing grade of dysplasia. Similar results were obtained by Nam et al. [12], Harmsel et al. [18], Pablo Conesa et al. [19] and Simionescu et al. [20]. who also showed an increase in mean basal Labelling as we move from CIN-I to CIN-III.
The mean LI in WD SCC was 55.333 ± 7.789; MD SCC was 43.976 ± 3.152 and in PD SCC was 80 ± 5. The study conducted by Pahuja et al. [21] showed an increase in LI from WD SCC to PD SCC. The Study showed maximum mean value in PD SCC similar to ours. The study conducted by Nam et al., also showed an increase in LI from WD SCC to PD SCC [12].
The increasing prevalence Apoptotic Index and Ki-67 expression with increasing grade of dysplasia and increase in progression from dysplasia to carcinoma shows that both of these could be used as biomarkers in the evaluation of the proliferative activity and progressive potential of dysplastic and neoplastic changes.