Journal of Physical Chemistry & Biophysics
Open Access
ISSN: 2161-0398
ISSN: 2161-0398
Review Article - (2018) Volume 8, Issue 1
Photovoltaic effect is the emergence of a voltage between electrodes attached to a solid or liquid system up on shining light on to this system. Conjugated polymer is a molecular entity whose structure is represented as a system of alternating single and double bonds which give rise to their semi-conductor properties. Conjugated polymers are used for photovoltaic devices because, intrinsically stable up on photoexcitation with visible light, High absorption cross-section for photon harvesting, Tunable band gap with in the entire visible spectral range and High yield of charge generation when mixed with electron acceptor materials. The important physical process in the energy conversion process that take place in polymers for photovoltaic cells are; Absorption of a photon of light by photoactive material and generation of excitons, diffusion of excitons in conjugated polymers, dissociation of charge carriers (electron-hole pair) at the donor-acceptor interface in to free carriers, transport of free carriers towards the electrodes, and extraction of the charge carriers at the respective electrode interfaces. The efficiency of converting solar to electrical energy by a solar cell depends on the band gap of the light absorbing semiconductor. Band gap (Eg) is the difference in energy between the HOMO and LUMO and there by the maximum amount of energy required for an excitation or is the energy difference between the edges of the conduction band and valence band. The power conversion efficiency is a function of band gap. For device architectures of conjugated polymer based photovoltaic cells; there are three types Single layer photovoltaic cell, Bilayer hetero junction photovoltaic cell and Bulk hetero junction Photovoltaic cell.
<Keywords: Photovoltaic effect; Conjugated polymers; Band gap; Bulk hetrojunction device; Photon harvesting
Photovoltaic effect or photoconductive effect
Is the emergence of a voltage between electrodes attached to a solid or liquid system up on shining light on to this system? A material or device is said to be "photovoltaic" when exposure of the material to light that can be absorbed by the material is able to transform the energy of the light photons in to electrical energy in the form of a current and voltage by harvesting light [1].
Conjugated polymers as photovoltaic devices
Conjugated polymer is a molecular entity whose structure is represented as a system of alternating single and double bonds which give rise to their semi-conductor properties. From Figure 1 below, the highest occupied molecular orbital (HOMO) and the lowest unoccupied molecular orbital (LUMO) are constructed from the overlapping of pz-orbitals and therefore, the HOMO is filled with the π-electrons in the conjugated polymer. The π-system can undergo all kinds of optical and electronic transitions and interactions, while the δ-bonds preserve the structure of the molecule by providing the chemical bonding. From the π-orbitals the valence band emerges, bordered by the HOMO; while the π*-orbitals form the conduction band which is bordered by the LUMO. Between the LUMO and the HOMO an energy gap exists, also called the band gap of the material. This gap originates from being a forbidden zone for electron transitions. In the case of metallic conductors this zone is absent, leading to half-filled bands and hence to intrinsic condition [1-4]. The semi conducting properties of a conjugated polymer has been shown in the figure below [5].
Reasons that conjugated polymers are used for photovoltaic devices
Conjugated polymers are used for photovoltaic devices because:
• Intrinsically stable up on photoexcitation with visible light. Excitation of conjugated polymers with visible light leaves the primary structure (backbone) formed by the δ-bonds intact, which should make them intrinsically stable against photodegradation in an inert atmosphere.
• High absorption cross-section for photon harvesting.
• Tunable band gap with in the entire visible spectral range. Absorption of light over a broad range of the solar spectrum, i.e., a good overlap of the polymers absorption spectrum with the solar spectrum to have high efficiencies.
• High yield of charge generation when mixed with electron acceptor materials.
Charge generation is facilitated by:
• Strong coupling of the fundamental photoexcitations to the lattice (electron- phonon) interaction facilitates charge separation against the attractive coulomb (electron-hole) interaction. Phonon is a quantum of energy associated with a compressional wave such as a vibration of a crystal lattice or a quantum of vibrational or acoustic energy in a crystal lattice.
• A hetrojunction created between two materials with different affinity also facilitate charge dissociation that overcome coulomb barrier to give free charge carriers.
Photovoltaic cells are known as excitonic solar cells, which are characterized by strongly bound neutral electron-hole pairs (excitons) that are formed after excitation with light. Exciton dissociation occurs almost exclusively at the interface between two materials of differing electron affinities (and/or ionization potentials) [1,3-5]:
• The electron donor (Donor-D).
• The electron acceptor (Acceptor-A).
The Energy Conversion Process
The important physical process that take place in polymers are shown in Figure 2 below;
Absorption of a photon of light by photoactive material and generation of excitons: To reach a high efficiency, the active layer of a photovoltaic cell should capture a large fraction of the incoming sunlight. When a crystal is illuminated with photons hν>Eg electrons are excited to a higher energy levels by absorbance of a photon. When a photon with energy beyond the absorption edge are incident on a semi-conducting specimen of the polymer, an electron and hole with opposite spin are created, bound by their coulomb attraction in a singlet exciton state. An excited electron leaves behind a hole. The columbic potential acts to bind the electron-hole pair to produce exciton.
Diffusion of excitons in conjugated polymers: Also called electron transfer from the donor to the acceptor. Conjugated polymers have high exciton binding energy, which influence the dissociation of excitons. In order to overcome this a donor-acceptor system must be employed. When the exciton reaches the donor-acceptor interface the electron will transfer to the material with the larger electron affinity and the hole will be accepted by the material with the lower ionization potential. For this electronic optimization a soluble fullerene (generally a C60 derivative) acceptor and a polymer donor that can be processed in solution. Fullerenes are used as the ideal acceptor because of:
• have an energetically deep lying LUMO-which endows the molecule with a very high electron affinity.
• have triply degenerate LUMO of C60 -which allows the molecule to be reversibly reduced which up to six electrons, thus illustrating its ability to stabilize negative charge.
• have a very high electron mobility-ultrafast photo induced charge transfer.
Dissociation of charge carriers (electron-hole pair) at the donoracceptor interface in to free carriers: Conducting polymers have low dielectric constants (from 2 to 4) which result a photo generated electron and hole at the donor-acceptor interface the coulomb binding energy can be very strong. The bound electron-hole dissociates in to free charge carriers when the carriers are able to escape their mutual coulomb attraction. Photogeneration of charge carriers results from field and temperature assisted dissociation of a singlet excitons. (assisted by thermal and electric field built up by the difference of the work functions of the electrodes).
Transport of free carriers towards the electrodes: Once charge separated state is formed, the free charges are transported through the device by diffusion and drift processes, to the electrodes in order to produce photo current.
Extraction of the charge carriers at the respective electrode interfaces: The transported free electrons and holes are collected at the electrodes. Two factors mainly govern the efficiency of charge collection:
• The carrier life time.
• The charge carrier mobility.
The elementary steps in the process of photoinduced charge separation for donor (D) and an acceptor (A) are [2,4,6,7]:
D+A → D*+A (Photoexcitation of the donor)
D*+A → (D.....A)* (Excitation delocalization(diffusion) on the donoracceptor complex=exciplex)
(D.....A)* → (Dσ+........Aσ-)* (Partial charge transfer)
(Dσ+........Aσ-)* → (D.+........A.-) (ion radical pair formed =germinate)
(D.+........A.-) → D.++A.- (Charge separation).
Photovoltaic Device Architectures
At the interface between two different materials possessing different electron affinities lead to creation of a potential difference that an electric current pass through it, a junction is formed [1-4].
For device architectures of conjugated polymer based photovoltaic cells; there are three types as shown below in Figure 3a and 3b [2,7,8]:
The electron-donor and acceptor with different electron affinity are mixed to form a hetrojunction active layer (charge generator interface) by vacuum deposition of molecular components. A bilayer hetrojunction inserted between a high work function electrode matching the HOMO level of the donor and a low work function electrode matching the LUMO level of the electron acceptor should function as a diode with rectifying current voltage characteristics [1,8]. Figure 4 shows the Essential energy levels and energy state [1,2,6,8].
Key: AA= affinity of acceptor, AD= affinity of donor, EL2=low work function of cathode(acceptor), EL1=high work function of anode(donor).
Under forward bias (the low work function electrode (EL2) is biased negative in respect to the high work function electrode (EL1)) the electron injection in to the LUMO of the acceptor layer from the low work function electrode as well as the electron extraction out of the HOMO of the donor by the high work function electrode (EL1) is energetically possible and a high current can flow through the bilayer when the injected charges can move in their respective thin films [1,2,8].
Under reverse bias (the low work function is biased positive in respect to the high work function electrode), the electron removal from the electron donor and electron injection to the electron acceptor is energetically unfavorable. Bilayer hetrojunction devices have been fabricated based on a spin coated layer of conjugated donors and different acceptors such as evaporated C60 layers or high electron affinity conjugated polymers sandwiched between ITO coated glass and evaporated gold electrodes [1,3,9,10]. Figure 5 gives the Schematic representation of bulk hetro junction photovoltaic cell [4,6,8,10].
The acceptor and donor components are mixed and processed simultaneously in to the active film to form a donor/acceptor architecture bulk hetrojunction composite. Bulk hetrojunction device is formed by a bicontinous composite (network) of donor and acceptor phases; there by maximizing all important interfacial area between the donors and acceptors [2,4,11]. Figure 6 shows the donor-acceptor components in bulk hetrojunction photovoltaic cell architecture [2,6,12].
Advantage of bulk hetrojunction device
Which can be processed in solution over, vacuum deposition is the ability to process the composite active layer from solution in a single step, by using a variety of techniques that are spin coating, the doctor blade technique, screen printing or evaporative spray deposition technique. The exciton can travel a shorter distance to meet the electron acceptors before recombination occurs.
Criteria for an efficient bulk hetrojunction solar cell polymer
For a conjugated polymer to suit in organic photovoltaic bulk hetrojunction solar cell, it should posses favorable physical and chemical properties in order to achieve reasonable efficiency. These include:
Large absorption coefficient: To increase the solar absorption of the photoactive layer:
• increase the thickness of the photoactive layer.
• increase the absorption coefficient, and
• matching the polymer absorption with solar spectrum.
Low band gap to absorb at long wave length: Low band gap polymer is that once a polymer absorbs at longer wave length, there will be one absorption hollow at the shorter wave length range, leading to a decreased incident photon to electron conversion efficiency at that range.
High charge carrier mobility: Higher charge carrier mobility of the polymer increases the diffusion length of electrons and holes generated during photovoltaic process and at the same time reduces the photocurrent loss by recombination in the active layer, hence improving the charge transfer efficiency from the polymer donor to the acceptor.
Favorable blend morphology with fullerene derivatives: A polymer blend is created by composing equal amounts of the hole acceptor and the electron acceptor and dissolving them in a solvent. Ordered structure results enhanced conjugation length.
Environmental stability: The air stability of the solar cell device, result longer shelf life time and longer operation life time. The air instability of solar cell device is caused by:
• Polymer degradation in air,
• Oxidation on low work function electrode and
• The degradation of the morphology of the photoactive layer.
For a conjugated polymer to achieve such long-lasting life time, it should have intrinsic stability towards oxygen. Oxidation which requires the HOMO energy level below the air oxidation threshold (5.27 eV) against vacuum level
Suitable (or desired) HOMO/LUMO level: The HOMO and LUMO of the polymer should be carefully tuned.
Example: To achieve high Voc in the device, HOMO level of the polymer should be reasonably low.
To ensure efficient electron transfer from polymer donor to acceptor in the bulk hetrojunction device blend, the LUMO energy level of the polymer material must be positioned above the LUMO energy level of the acceptor at least 0.2-0.3 eV.
Solubility: Polymer prepared for solar cell application should posses reasonable solubility so that it can be analyzed by solution based characterization methods such as NMR A spectroscopy. Polymer with poor solubility will be found inappropriate for solution processing and device performance is normally low due to unfavorable microscopic morphology of the thin film formed by spin coating.
Aliphatic chains attached to the polymer backbone are essential to ensure solubility of the polymer. To increase solubility of a polymer:
• Longer chain is better than shorter chain to solubilize polymer.
• Branched chain is better than linear chain to solubilize polymer.
• The more rigid, or planer the polymer back bone is, the more or longer alkyl chains are needed [4,6,12].
Photon Harvesting Via Band Gap Engineering
Band gap engineering refers to the ability we have to tailor the electronic and chemical properties of semiconductor materials by varying the composition and impurity concentrations throughout the body of a semiconductor device.
Limitations to power conversion efficiency
The efficiency of converting solar to electrical energy by a solar cell depends on the band gap of the light absorbing semiconductor. The fraction of absorbed photons of the solar photon flux increases with decreasing band gap, and reaches maximum when the band gap is zero.
The power conversion efficiency is a function of band gap. The photovoltaic power conversion efficiency ηe is defiined by;
• increase the thickness of the photoactive layer,
• increase the absorption coefficient, and
• matching the polymer absorption with solar spectrum.
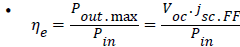 .............(1)
.............(1)
• because Pout.max=Voc.jsc.FF
• Where, Pout: the electric power at the maximum power point of the cell.
Pin: the incident optical (light) power per area.
Voc: the open circuit voltage.
FF: the fill factor.
jsc: the short circuit current.
The open circuit voltage (Voc): the voltage where the current equals zero. It is the voltage of the cell delivers under illumination in absence of a current flow. It is determined by the maximum splitting of electromechanical energy of the electron-hole pair is zero when the band gap is zero, and increases with the increasing band gap.
Fill factor (FF): is the ratio of maximum rectangle fitted in the fourth quadrant I-V curve and the product of Voc and jsc which is;
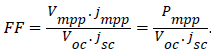 ...............(2)
...............(2)
Where jmpp and Vmpp are the current and voltage at the maximum power point in the fourth quadrant of the current voltage characteristics.
The short circuit current (jsc) is determined by the amount of absorbed light and the internal conversion efficiency. It is also measured up on electro conductive connection of the electrodes under illumination. The Efficiency factors for power conversion has been shown in Figure 7 [1,6,8,12].
The characteristic intersections with the abscissa and ordinate are the open circuit voltage and the short circuit current respectively. The largest power output (Pmax) is determined by the point where the product of voltage and current is maximized. Division of Pmax by the product of jsc and Voc yields the fill factor. Solar cells are operated between open circuit and short circuit condition (fourth quadrant in the current-voltage characteristics). In the dark, there is almost no current flowing, until the contacts start to inject heavily at forward bias for voltages larger than the open circuit voltage. Under illumination, the current flows in the opposite direction than the inject currents.
An experimental accessible value is the external quantum efficiency or incident photon to current efficiency (ICPCE), which is defined as:
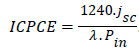 .................(3)
.................(3)
Where λ (nm): is the incident photon wave length.
jsc (μA. cm-2): is the photocurrent of the device.
Pin (W/m2): is the incident power.
Main energy loss mechanisms in donor-acceptor bulk hetrojunction solar cells: Main loss mechanisms leading to maximum theoretical power conversion efficiency are attributed to:
• Unabsorbed photons of the polychromatic light at energies lower than the band gap of the semiconductor.
• Loss of the energy of the electron-hole pair due to thermalization.
• Thermodynamic losses in converting the chemical energy of the electron-hole pair in to entropy free electrical energy and
• Device electrical losses that are usually defined by the filling factor [1-4,8,12].
Low Band Gap Polymers
Band gap (Eg) is the difference in energy between the HOMO and LUMO and there by the maximum amount of energy required for an excitation or is the energy difference between the edges of the conduction band and valence band. Decreasing the band gap allows light with longer wave length, thus less energy, to be absorbed and increases the total amount of photons that can be harvested from the solar spectrum.
Narrowing of the polymeric band gap will eventually result in a decrease in power conversion efficiency (PCE) due to a decrease in open circuit voltage (Voc).
Band gap tuning of conjugated polymers
The magnitude of the band gap and energy positions of the HOMO and LUMO energy levels are the most important characteristics for determining the optical and electrical properties of a given conjugated polymer which influence photovoltaic performance. It is highly desirable to develop conjugated polymers with broader absorptions through narrowing their optical band gap. Band gap of conjugated polymers with in a non-degenerate ground state can be tuned and modified by changing different parameters in the conjugated polymer.
Most conjugated polymers used for photovoltaic purpose have a non-degenerate state with:
Aromatic form:
• energetically more stable.
• dominate the structure of the chain and is the most important resonance contributor.
Quinoid form: has higher energy and lower energy gap.
These mesomeric forms are obtained by the flip of the double bonds which are not energetically equivalent. Minimizing the energy difference between the aromatic form and the quinoid form is the most important design/tool to narrow the band gap of conjugated polymers having aryl groups in the back bone.
Aromaticity (RE): Most conjugated polymers have aromatic units as monomers; the aromaticity is preserved in the polymer structure. The aromaticity energy (or resonance energy) is the energy difference between the aromatic structure and a hypothetical reference consisting of isolated double bonds. Aromaticity leads to a confinement of the π- electron on the ring and competes with the delocalization. The higher the value the broader the band gap of the aromatic unit will be, (because it prevents delocalization). Low resonance energy value results a smaller band gap.
Bond length alteration (EBLA): Figure 8 shows the difference in bond length between single and double bonds.
Delocalization of the confined π-electrons along the conjugated chain converts double bonds in to single bonds and synchronously transforms single bonds in to double bonds, leading to a resonance structure referred to as the quinoid form. The ratio of the aromatic to quinoid population in a polymeric conjugated system can be correlated and represented by a geometrical parameter, i.e., bond length alteration (BLA); which is the average of the difference in length between adjacent carbon-carbon bonds in a polyene chain. As the quinoid contribution increases, the carbon-carbon single bonds between two adjacent rings adopt more double bond character and the bond length alteration (BLA) starts to decrease, which decreases HOMO-LUMO band gap. Planarization between adjacent aromatic units allows parallel P-orbital interactions to extend conjugation and facilitate delocalization, which decreases bond length alteration (BLA) and reduce band gap.
Conjugation length (EROT): Band gap decreases with increasing conjugation length, approaching a finite value for infinite conjugation length. Torsion between the adjacent rings partially interrupts the conjugation and leads to an effective increase of the band gap.
Substitution effects (ESub): Electron donating groups such as alkoxy or amine, raise the energetic position of the HOMO (or the density of the electron is pushed in to the π-system raising the energy of the corresponding orbitals and making it easier to remove an electron from the HOMO). Electron withdrawing groups such as cyano, or triflouro methyl lower the energetic position of the LUMO (or lowers the LUMO because the reduction potential is lowered, making it easier to push an electron in to the LUMO).
Intermolecular interactions (Eint): Conjugated polymers in the solid state generally show lower band gap as compared to the solution phase, which is attributed to an increased interaction between the chains.
Ordered phases< disordered (band gap)
Bulky side chains can hinder intermolecular interactions between the backbones.
Torsion angle (Eθ): Reducing the tilt angle by using either small side groups, non-proton carrying atoms, or bridging via covalent bonds or via H-bonds, the torsion angle can be reduced; in a polymer there by enhancing the back bone planarity, and reduces the band gap of the polymer. Generally
Eg=EBLA+ERE+ERot+ESub+Eint+Eθ……………….(4)
The discovery of metal-like electrical conductivity of polyacetylene in 1976 by Shirakawa with Heeger and MacDiarmid opened the field of research of polymers [2-7,9,12].
Avdantages of organic solar cells:
• Relatively cheap in production and purification.
• Materials can be tailored for the demand.
• Can be used on flexible substrate.
• Can be shaped or tinted to suit architectural applications.
Disadvantages of solar cells:
• Do not reach the energy conversion efficiency exceeding 24% in inorganic materials.
Renewable energy sources are of major interest to the world today. Providing energy without producing vast amounts of greenhouse gasses such as CO2, is required to prevent global warming which might induce irreversible climate changes. Increasing energy consumption and rising energy prices in the world forces to look for energy alternatives, one of the most promising being the photovoltaic solar energy conversion. Converting sunlight into electrical energy, photovoltaics (PV), is a fast-growing technology and is very likely to play an important role in renewable energy-supply in future years. Crystalline silicon solar cells have nowadays reached efficiencies up to 25%, but material requirements are high making them rather expensive. The power conversion efficiency of the inorganic solar cells can be further enhanced by constructing multi-junction devices that convert the sunlight into electricity covering a large part of the solar emission spectrum. Polymer solar cells employ a nanoscopic phase separation or bulk heterojunction (BHJ) between two complementary molecular based organic semiconductors to convert sunlight directly into electricity. The operational principle involves a complex sequence of events, starting with the absorption of light, followed by creation, separation, transport, and collection of charges. Reducing the band gap of the p-conjugated polymer, allocating a good overlap between the polymer absorption and the solar emission spectrum, potentially increases the number of absorbed photons and hence photovoltaic performance. By varying the chemical nature of the building blocks to control the position and separation of the energy levels, lead to efficient bulk heterojunction solar cells.