Journal of Biomedical Engineering and Medical Devices
Open Access
ISSN: 2475-7586
ISSN: 2475-7586
Research Article - (2021)Volume 6, Issue 9
Background: The wound healing process comprises of highly complex and dynamic events which allow the reestablishment of skin's structural integrity. To overcome the drawbacks associated with this process, researchers have been focused on the development of new therapeutics. To meet this demand, an interesting option is Chitosan based biopolymers. This study develops and evaluates a combination of Chitosan and bioactive Spirulina microalgae, extract hydrogel as skin serum to enhance the wound healing process.
Methods: Crude extract of dry S. platensis was prepared using three solvents (methanol, ethanol and water). Spirulina Extract-Chitosan Hydrogels (SECH) was synthesized using an ionic dilution method. SECH was characterized using Dynamic Light Scattering (DLS) and TEM microscopy. In vitro cytotoxicity of nano hydrogels on HFF-1 fibroblast Skin Normal cells was determined using MTT assay. Scratch assay was performed to evaluate the wound healing properties of SECH. QReal Time PCR was applied to evaluate the effect of SECH on gene expression of TNF-ß and PDGF.
Results: Aqueous SECH (SECH-W) showed higher proliferation activity compared with the control group by 1.78- fold and 2.55-fold increase of cell viability after 24 and 48 hr respectively with 3 mg/ml concentration. Aqueous SECH enhanced wound healing process on HFF-1 cell line with 100% wound closure after 48 hrs. TNF-ß and PDGF gene expressions were up-regulated by 3-fold and 4.2-fold after 24 h incubation, respectively.
Conclusions: Our findings showed that the SECH-W increased the proliferation of HFF-1 cells significantly and promotes the wound healing process in vitro. Therefore, the Spirulina-chitosan hydrogel could be of potential value in cosmeceutical and biomedical applications.
Spirulina microalga; Chitosan hydrogel; Cell viability; Wound healing; TNF-ß; PDGF
Skin, the largest organ of the body, is the barrier which separates the body from the outer environment. The skin is considered as the first layer of the innate immune system which prevents the body from water loss and infections [1,2]. The skin comprises of a multilayer structure with specific properties. The epidermis, mainly consisting of keratinocytes, is the mainly represent a barrier against external factors including but not limited to ultraviolet radiation (UV), pathogens, and mechanical disturbances. Dermis, a connective tissue of fibroblasts and rich in collagen; is a thicker layer comprise of Extracellular Matrix (ECM), living cells, nerve endings and blood vessels, which provides nutrition to the epidermis and responsible for mechanical strength and elasticity of the skin. Hypodermis, the deepest layer, is responsible for thermal isolation and mechanical protection of the body [3,4].
A wound is damage or any disorder in the healthy structure and function of the skin. The wound can be caused by injury, genetic disorders, trauma (either acute or thermal), and surgical interventions [5]. Wound repair is a complex process including haemostasis, inflammation, proliferation and remodelling. Haemostasis, the first immediate response to the injury, is the process in which the blood loss stops at the wound site [6]. Right after inflammation occurs which takes from 24 hr to 4–6 days. The inflammatory phase initiated with the release of proteolytic enzymes and pro-inflammatory cytokines over invaded immune cells to the wound zone. These inflammatory cells produce Reactive Oxygen Species (ROS) which limited the growth of bacteria at wound site and thus preserve infection in low concentrations [7]. Nevertheless, ROS in high concentrations have destructive effect on wound healing process by associating with the chronic pathogenesis, and wounds, which leads to enhanced oxidative stress, lipid peroxidation, and severe cell damage [8]. In the inflammatory phase, the wound site is rinsed from all foreign particles and tissue debris by neutrophils and macrophages [9].
In addition, fibroblasts and my fibroblasts are stimulated by emitting of cytokines and enzymes [10,11]. Inflammation is followed by proliferation to generate new granulation tissue on the wound area, making new Extracellular Matrix (ECM). Finally, remodelling step in which the composition of matrix changes and type III collagen is replaced with type I to increase the new tissue tensile strength [12]. Wound healing is one of the most complex processes in multicellular organisms, associated with numerous intra and intercellular signalling pathways in various cell types. Several growth factors are involved in wound healing, of which transforming growth factor beta (TGF-β) is of particular importance for all phases of this procedure. TGF-ß, a cytokine which involved and positively regulate the biosynthesis of collagen, regulates cell proliferation, differentiation, extracellular matrix production, and modulating the immune response and thereby if in excess it may results in over healing and adverse outcomes, like hypertrophic scarring and keloid [13,14].
Platelet-Derived Growth Factors (PDGF) are expressed by several cell types during tissue repair [15] and there is prevailing evidence to suggest a decisive role for PDGF and their receptors in primary myelofibrosis [16]. PDGF is involved in the epithelialization stage of wound healing by up-regulating the production of growth factors, including Insulin Growth Factor (IGF)-1 and thrombospondin-1. In turn IGF-1 enhances the motility of keratinocyte cells while thrombospondin-1 inhibits proteolytic and enzymatic degradation of PDGF [17].
Wound dressings are applied as a barrier to protect the wound which should be applicable, flexible, stable, and biodegradable to promote the healing process and decrease the chances of infection [18]. Chitosan, a poly-cationic biopolymer, possess desirable unique properties like antimicrobial, anti-inflammatory, low immunogenicity, maintained tissue-adhesive and antioxidant and therefore has been used as a wound dressing material [19]. Furthermore, Chitosan and its derivatives are well recognized to accelerate the wound healing process by affecting the haemostasis phase and blood clotting, this leads in reduction of the pain [20].
Spirulina platensis, a unicellular cyanobacterium, is classified as blue-green filamentous algae and has been commonly used in skin care products because of its therapeutical value with bioactive compounds like antioxidants, pigments, unsaturated lipids, UVscreens and vitamins [21,22]. Multiple studies report that S. platensis produce varying bioactive secondary metabolites with antioxidant [23], antiviral [24], antibacterial [25], antifungal [26], anti-inflammatory [23], hypocholesterolaemia [27], anti-diabetic [28] and anticancer activities [29]. Moreover, the polysaccharide content of S. platensis extract enhances cell nucleus enzyme activity and DNA repair synthesis in vitro [30].
S. platensis also produce ECM-like bioactive molecules which may form tissue-like matrices and therefore can mimic ECM [31]. An interesting application of S. platensis extract is to incorporate it into biomaterials like chitosan. Regarding the broad application of chitosan as wound dresser and desirable properties of S. platensis extract, here we synthesized a SECH using ionic dilution method. The SECH was characterized by DLS and TEM microscopy and investigate its wound healing potential on human fibroblast cells, HFF-1, by MTT and scratch wound healing assays. Further, we studied the alteration in expression patterns of two important genes involved in wound healing process, TGF-ß and PDGF.
Materials
All the chemicals were obtained from either Sigma Aldrich (St. Louis, Missouri, USA) or Merck (Kenilworth, New Jersey, USA) with appropriate grade. All of the solvents were provided by Merck. Cell lines were purchased from the Pasteur Institute, Tehran, Iran. Cell culture medium and FBS were provided by Gibco (Thermo Fisher Scientific, Waltham, Massachusetts, USA). The powder of S. platensis was provided from Iranian Biological Resource Centre.
Spirulina extract preparation: The dry spirulina was extracted using three distinct solvents (Methanol, ethanol and water). Briefly, 5 g of dry spirulina mixed with 25 ml of solvent and vortex vigorously for 20 min, followed by centrifugation at 8000 rpm, 30 min, twice. The solvents were evaporated using a rotary evaporator under vacuum condition. The organic and aqueous extracts were kept at -80ºC [32].
Chitosan hydrogel synthesis: Chitosan hydrogel was synthesized by an ionic dilution method using sodium tripolyphosphate (TPP) as cross-linking agent. Briefly, 96 mg of low molecular weight Chitosan (viscosity 35 cps, deacetylation degree, 91.5%) dissolved in acetic acid solution 1% under gentle stirring for 24 h. The pH was adjusted to 4.7–4.8 using 20 wt.% aqueous sodium hydroxide solution. Then the appropriate amount of spirulina extracts synthesized by three different solvents -EtOH, MeOH and distilled water-separately and in three different reactions was added to the solutions and sonicated for 10 mins. TPP was dissolved in ultrapure water at a concentration of 0.5 mg/ml. TPP solution was quickly added to the Chitosan solution drop by drop further sonicated for 10 mins and then rinsed by ddH2O twice and centrifuged at 8000 rpm for 30 min. The resulting suspension was subjected to further analysis.
Chitosan hydrogel characteristics: The z-average particle size, particle size distribution and Polydispersity Index (PDI) of the Chitosan/TPP nanoparticles were measured at 25ºC by Dynamic Light Scattering (DLS) on a high-performance particle Sizer (Scatteroscope I, Qudix, South Korea). The morphological characteristics of the hydrogels were studied utilizing a highresolution Transmission Electron Microscope (TEM, Tecnai G20, FEI and Netherland).
Cell culture: HFF1 (Human Fibroblast) was maintained in a humidified atmosphere containing 5% CO2 at 37°C in Dulbecco’s Modified Eagles’ Medium (DMEM) supplemented with 2 mm l-glutamine, 100 units mL−1 penicillin, 100 μg mL−1 Streptomycin and 10% foetal bovine serum (GIBCO, USA). The cells were serially passaged twice a week.
MTT assay
The cell viability and proliferation assays were performed using the MTT method [33]. Briefly, exponentially growing cells was seeded into 96 well flat bottom plates and incubated for 24 h at 37ºC in the presence of 5% CO2. Then a defined number of cells were exposed to Spirulina, Chitosan and Spirulina-Chitosan hydrogel, in different concentrations (0, 1, 2 and 3 mg/mL) in a period of 24 and 48 hr. Afterwards, the MTT (3-(4,5-dimethylthiazol-2-yl)-2,5- diphenyltetrazolium bromide) solution was added to achieve a final concentration of 0.45 mg mL-1 and a further incubation for 4 h at 37°C. Finally, 100 μl of DMSO was added to each well to dissolve formazan crystals and the absorbance was recorded at 570 nm and 630 nm (turbidity assessment) using the SPECTROstar® Nano microplate reader (Eppendorf, Germany). All the experiments were performed in triplicates. The cell viability percentage was calculated by following equation:
Cell viability % = [AT (sample)/AT (control)] × 100
AT = A570-A630
Scratch wound healing assay
HFF1 cells were plated and grown to confluence of 90% in 24 well plates. Then, the cell layer in each well was scratched using a 100 μL pipette tip and once the scratch was made, medium was removed and replaced with fresh medium supplemented with Spirulina extract, Chitosan and SECH-W and incubated for 48 hr. Images were taken after the scratch was induced and also 24 and 48 hr after treatment. The migration distance was quantified with Image J software and the wound closure distance calculated as follows:

RNA extraction
Exponentially growing cells were treated with chitosan and SECH-W for 24 hr respectively, tripsinized and suspend in 1 ml of RNX solution. Total RNA was extracted using phenol chloroform method [26]. The Optical Density (OD) of each sample at 260 and 280 nm (measured using Ultrospec 2100, Biochrom, USA) was used as criteria to determine the amount of each sample. Reverse transcription of RNA was performed using Pars Toos- Revers Transcription Kit (Iran) at 37°C for 1 hour in a 20 μl reaction mixture comprising of: first strand buffer, 200 units of Moloney murine leukemia virus reverse transcriptase, 20 units of RNasin, 10 mM DTT, 4.75 μM random hexamers, and 500 μM deoxynucleotides (all from Promega, Madison, WI). Following cDNA synthesis, the resultant mixture was heated at 95°C for 5 min before storage in -80°C.
Primer design
All of the primers were designed using gene runner software and further analysed with Primer-BLAST and oligo analyser. All the primers are listed in Table 1. GAPDH gene was used as internal control. These sequences were checked subsequently for their specificity, using the Check-Probe function of the Ribosomal Database Project software package and the BLAST database search program.
Quantitative gene expression assay by Real time Qpcr
Real-time qPCR was performed by the Rotor-Gene 6000 real-time PCR cycler (Qiagen Corbett, Hilden, Germany) with the following program: one step at 95°C for 10 minutes, followed by 40 cycles at 95°C for 15s, 62°C for 30s, 72ºC for 15s. Melting curve analysis was performed at 60 to 95ºC s/degree. For each reaction, 20 μl of the reaction mixture was used comprising: 0.4 μl of forward primer, 0.4 μl of reverse primer, 12 μl of 2x Master Mix (Takara Bio, Shiga, Japan), 1 μl of template cDNA and 6.2 μl sterilized ultra-pure water. Negative controls included all compartments of the reaction mixture except for the template cDNA. The negative controls had no detected amplified DNA products and were used during the analysis. The presented data are the mean values of triplicate Realtime PCR analysis.
Statistical analysis
The experiments were repeated at least three times and outputs were reported as mean ± standard error of the mean. A comparison of each group was assessed by One-Way Analysis Of Variance (ANOVA) and T-test. The differences were considered significant for *p <0.05.
SECH was synthesized using the Ion gelation liquid dilution method. The particle shape and hydrodynamic size distributions of SECH were analyzed by Transmission Electron Microscopy (TEM) images and particle size analysis by Dynamic Light Scattering (DLS). As illustrated from TEM images Chitosan hydrogel, EtOH and MeOH SECH are spherical shaped with core size of 150 ± 20 nm (Figure 1A-C). Furthermore, TEM image of aqueous SECH is, spherical shaped with core size of 150 ± 20 nm (Figure 1D). Particle size determination by DLS showed that the sizes of Chitosan hydrogel, aqueous, EtOH and MeOH SECH are 100 ± 20 nm, 130 ± 10 nm, 110 ± 20 nm and 100 ± 20 nm respectively. This difference in size can be attributed to the swelling effect of polysaccharides in the liquid phase and entrapment of spirulina extract among nano hydrogels.
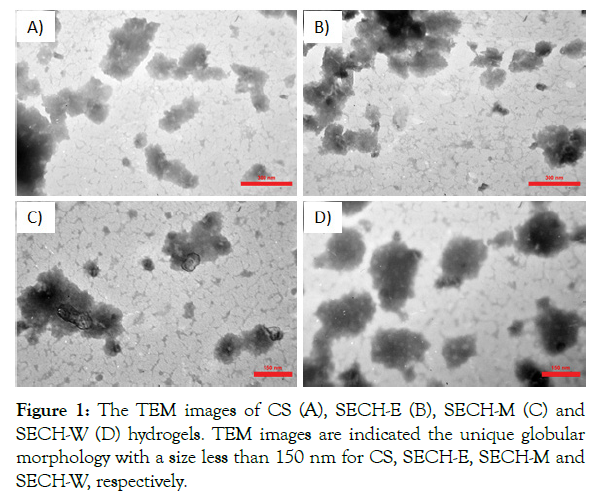
Figure 1: The TEM images of CS (A), SECH-E (B), SECH-M (C) and SECH-W (D) hydrogels. TEM images are indicated the unique globular morphology with a size less than 150 nm for CS, SECH-E, SECH-M and SECH-W, respectively.
SECH induce fibroblast cell proliferation
Before performing anti-aging tests, the basal cell toxicity of all types of SECH were investigated on HFF-1 cells at a high concentration of 3 mg/mL for 24 and 48 hr and no significant cytotoxic effect was observed. Further, proliferative impact of all types of SECH was investigated (Figure 2) by MTT assay. The cells treated with SECH showed increased cell viability/proliferation in a concentration and time dependent manner. In a time course of 24 hr, SECH increased cell viability up to 1.7 fold in comparison to the control sample. Interestingly, viability of cells treated with SECH for 48 hr increased more than 2.55-fold in comparison to control sample. However, the viability of cells treated with chitosan hydrogel and the other types of SECH-M and SECH-E showed near 100% in comparison to control treated cells. In comparison to the aqueous hydrogel, the EtOH and MeOH SECH were less effective on HFF-1 cells proliferation and viability. After 24 hr, the HFF-1 cell viability was 90 ± 10% and 95 ± 5% after treatment with the SECH-E and SECH-M respectively at the highest concentration of 3 mg/ml. The viability of treated cells is maintained after 48 hr too. As illustrated in Figure 3, the aqueous SECH was the most predominant and enhanced HFF-1 fibroblast cell proliferation up to 2.55-fold in the highest concentration of 3 mg/ml. Therefore, the SECH-W was chosen as the best candidate and was further investigated for its potential to promote and enhance the wound healing process.
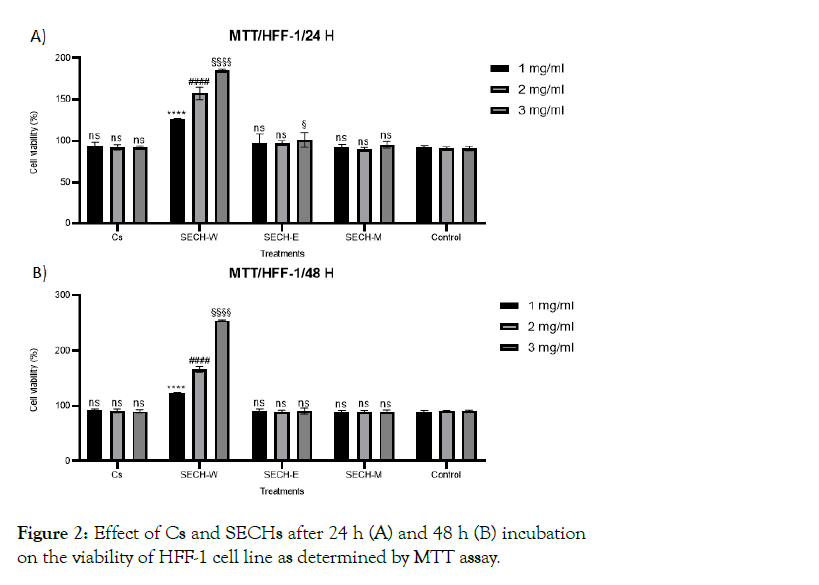
Figure 2: Effect of Cs and SECHs after 24 h (A) and 48 h (B) incubation on the viability of HFF-1 cell line as determined by MTT assay.
Chitosan- Spirulina hydrogel induce HFF-1 wound closure
Wound healing assay using the scratch assay revealed the induction of cellular proliferation and proper migration when treated by chitosan and SECH-W compared to the control group (cells without treatment). As illustrated in Figure 3A, in HFF-1 cells without treatments, no wound closure detected after 24 hr and only when hydrogels (3 mg/mL) were applied cell migration and wound closure is visible after 24 h and the wound is fully healed at the end of the experiments. The wound closure distance was calculated as 28.18% and 53.33% after 24 hr for cells treated by Chitosan and SECH-W respectively (Figure 3B). After 48 hr, the cell migration is raised to 45% without treatment (control) whereas; the wound is completely disappeared after SCECH-W hydrogel exposure. Moreover, in line with the MTT assay findings, the confluency of HFF-1 cells was significantly increased after treated with chitosan hydrogel and SECH-W for 48 hr. The density of cells in scratch line in cells treated with SECH-W is more than chitosan in 48 hr.
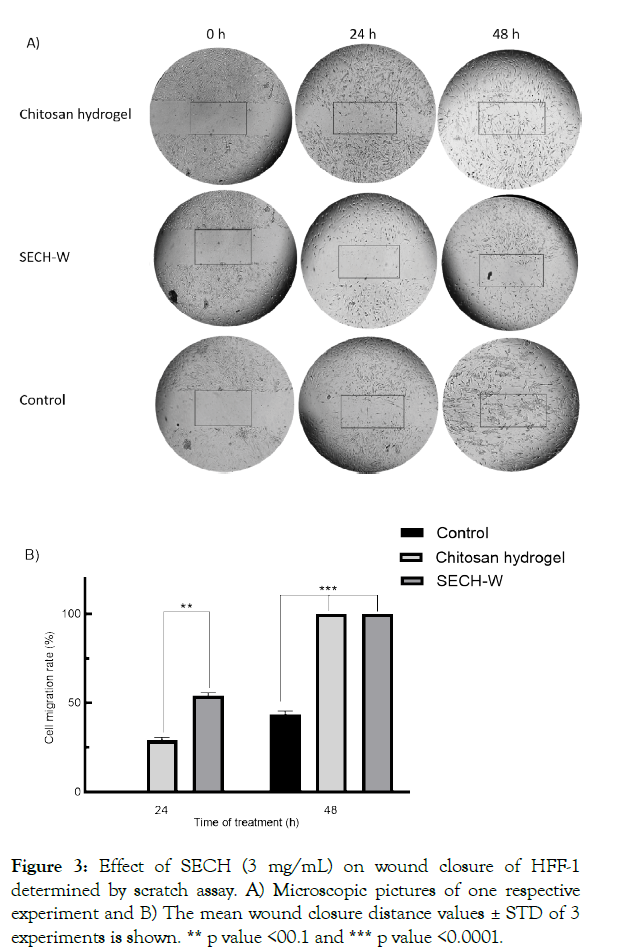
Figure 3: Effect of SECH (3 mg/mL) on wound closure of HFF-1 determined by scratch assay. A) Microscopic pictures of one respective experiment and B) The mean wound closure distance values ± STD of 3 experiments is shown. ** p value <00.1 and *** p value <0.0001.
Aqueous Spirulina-Chitosan hydrogel impacts on PDGF and TGF-ß expression in HFF-1 cells
Regarding the importance of PDGF and TGF-ß genes role in wound healing process, the mRNA level of these genes in HFF-1 cells treated with chitosan hydrogel and SECH-W were measured using qRT-PCR. The expression levels are illustrated in Figure 4. The expression pattern of TGF-ß gene after HFF-1 cells exposed to Spirulina extract, chitosan hydrogels and SECH-W (Figure 4A). Similar to PDGF (Figure 4B), a significant increase was observed in the TGF-ß mRNA levels in cells treated with SECH-W (p value <0.0001). The TGF-ß gene transcription significantly raised 3-fold when SECH-W applied. The aqueous spirulina extract also, up regulates TGF-ß expression by 1.7-fold. Despite from a slight increase in mRNA level (1.2-fold), Chitosan hydrogel could not alter TGF-ß gene expression pattern significantly.
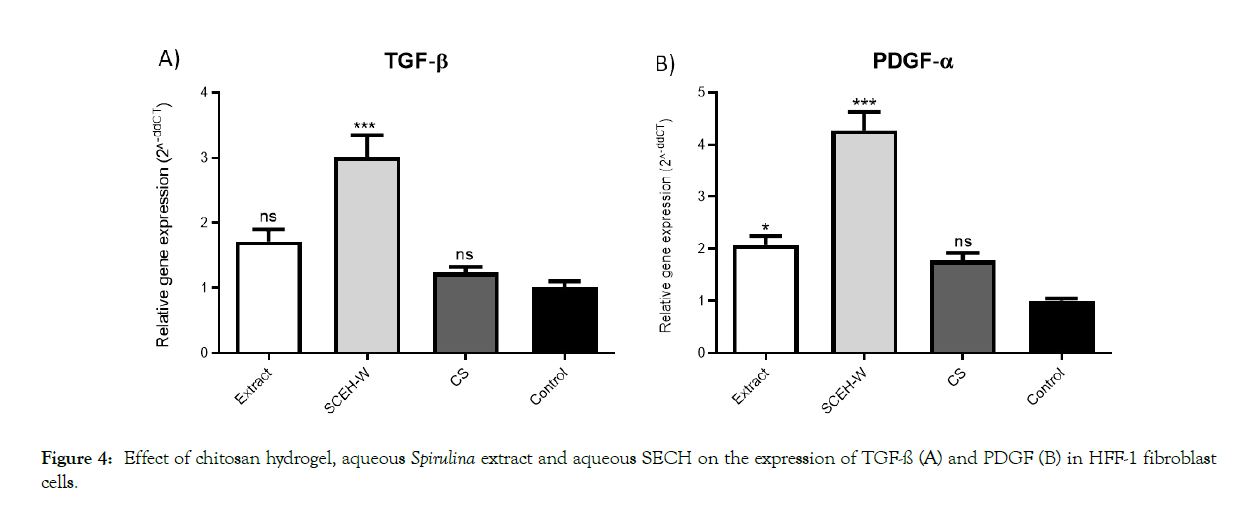
Figure 4: Effect of chitosan hydrogel, aqueous Spirulina extract and aqueous SECH on the expression of TGF-ß (A) and PDGF (B) in HFF-1 fibroblast cells.
We further investigate, the expression level of PDGF gene was up regulated and increased by 2, 1.5 and 4.2-fold in Spirulina extract, Chitosan and SECH-W respectively which the effect of SECH-W is more significant in comparison to Spirulina extract treated cells (p value <0.0001) (Figure 4B). These data indicate that although Spirulina extract and SECH-W positively affect PDGF gene expression, the combination (SECH) is much more effective.
We further investigate, the expression level of PDGF gene was up regulated and increased by 2, 1.5 and 4.2-fold in Spirulina extract, Chitosan and SECH-W respectively which the effect of SECH-W is more significant in comparison to Spirulina extract treated cells (p value <0.0001) (Figure 4B). These data indicate that although Spirulina extract and SECH-W positively affect PDGF gene expression, the combination (SECH) is much more effective.
Numerous researchers have been focused on the development of novel therapeutic techniques as a result of the complexity and drawbacks of the wound healing process. The innovation of this study was to develop a natural polymer matrix (Chitosan) entrapping Spirulina extract with potential application as sustainable wound dressing and/or anti-aging skin serum.
Chitosan, a bioactive, non-toxic, biocompatible and biodegradable polymer, is a deacetylated derivative of chitin, an abundant polysaccharide found in the exoskeleton of insects and crustaceans with many applications in agriculture, cosmetology, and pharmaceutical [34]. Chitosan is well known for its wound management properties. The main biochemical activities of chitosan‐based materials in wound healing are activating polymorpho-nuclear cell and fibroblasts, production of cytokine, migration of giant cell and stimulation of type IV collagen synthesis [35]. Moreover, nanosized chitosan hydrogels can improve the absorption of small molecules and essential elements into deep layer of skin.
Kumar V, et al. [25] described excellent film forming ability of Chitosan which can absorbed below 400 nm with potential application as sunscreens [36,37]. Moisturizing activity was reported in chitosan samples prepared by oxidative degradation with H2O2 [38,39]. Natural ingredients like chitin nanofibrils, lignin, and metalloproteinase have been employed to develop a safe, effective and eco-friendly anti-aging moisturizer mask for humans [40]. Chitosan membrane is an ideal coverage for entrapping bioactive compounds in particular antibacterial agents to enhance wound healing process [41]. Moreover, some chitosan-based wound dressings are already commercially available namely the HidroKi®, Axiostat®,Chitopack®, Tegasorb®, and KytoCel® [42].
Moghadas B, et al. [43] developed a chitosan membrane using non‑toxic crosslinkers for potential wound dressing applications. Kenawy E, et al. [44] described fabrication of biodegradable gelatin/ chitosan/cinnamaldehyde crosslinked membranes for antibacterial wound dressing applications. Here we succeed to synthesis a chitosan hydrogel using ionic gelation method to entrap S. platensis extract. This membrane structure showed excellent wound healing properties in molecular and cellular levels. S. platensis comprise of high amino acid content (62%), fatty acids, polysaccharides, multivitamins and minerals [45]. Moreover, S. platensis produced a wide range of phytochemical compounds, with strong antioxidant properties, through secondary metabolism like phenolic acids, tocopherols and b-carotene.
The biological activities of the S. platensis extract are highly associated with phenolic compounds content such as chromogenic acid, caffeic acid and ellagic acid. S. platensis possesses considerably high amounts of catechins (5799 mg/kg extract), which could also promote photo-chemo protective activity [46]. It is well stabilized that solvent used for extraction is of most importance in extract contents. Water and alcohols (e.g. Ethanol and methanol) are powerful solvents which are widely used as extracting agent. Ethanol is stated as the best solvent for extracting antioxidant compounds like phyocyanin from S. platensis [47]. Herrero M, et al. [48] reported that ethanol and closely followed by water are the best solvents to obtain S. platensis extract with high antioxidant activity. Nevertheless methanol is considered to be the best solvent for lipid extraction [49]. Our findings showed that the most effective hydrogel on induction of fibroblast proliferation is the SECH-W which is containing more effective metabolites, presented in aqueous extract, can induce fibroblast proliferation. Moreover, the synthesized Chitosan and SECH-W showed no cytotoxicity on Fibroblast Cells (HFF-1) at the end of experiment (48 hr) and increased cell proliferation by 1.7 and 2.55 fold after 24 and 48 hr respectively.
It has been reported that vitamins and trace mineral elements as well as C-phycocyanin and carotenoids present in S. platensis extract are involved in the proliferation of keratinocytes and fibroblasts [50]. Liu Y, et al. [39] reported that a crude protein extract of Spirulina, induce fibroblast proliferation through activating EGFR/ MAPK signalling pathway. Gunes S, et al. [21] reported that 0.1% S. platensis aqueous extract exhibited higher proliferation activity on HS2 keratinocyte cell line compared with the control group with 198% of cell viability after 72 hr and promoted wound healing (74.9% wound closure) after 48 hr [21]. In line with our data, the S. platensis extract showed no toxic effect on human fibroblast and PBMCs [51]. In agreement to their findings, our data showed that SECH-W and chitosan hydrogel closed the wound by 100% by the end of experiment. These findings revealed that utilizing S. platensis aqueous extract in combination with Chitosan hydrogel increased its impacts on fibroblast cells migration and promote its effectiveness on the skin rehabilitation.
TGF-ß affect the wound healing process through regulation of cell proliferation, differentiation, ECM production and the immune response [52]. Moreover, PDGF plays a vital role in each phase of wound healing process. PDGF triggers an inflammatory response by stimulating mitogenicity and chemotaxis abilities of neutrophils, macrophages, fibroblasts and smooth muscle cells at the wound site [53]. Regarding the importance and vital role of TGF-ß and PDGF in all stage of wound healing process, the effects of chitosan and SECH-W hydrogels on both genes expression in fibroblasts were investigated. TGF- ß ligands and receptors are both co-expressed by dermal fibroblasts.
The TGF-ß/SMAD pathway triggers transcription of proteins involved in tissue regeneration and are vital for skin homeostasis maintenance and successful completion of cell generation after injury [14]. Mokoena D, et al. [54] report that photo-biomodulation promotes wound healing by activation of the Smad pathway via TGF-β. Bahei-Eldin I, et al. [55] found that Spirulina extract significantly promoted the rate of skin wound closure and increase the re-epithelialization promote the neovascularization process at the wound site. Liu P, et al. [56] showed that Spirulina protein promotes skin wound repair in albino rats. Werner S, et al. [57] reported secreted PDGF is of significant importance for activating keratinocytes and fibroblasts at the wound margins. Our findings revealed that Chitosan hydrogel and SECH-W both activate the transcription of PDGF. To our best of knowledge this is the first report that spirulina extract up regulated PDGF expression. Our findings indicate that SECH-W might promote wound healing in fibroblast cells by activating TGF-ß and PDGF pathways.
In conclusion, we succeed to design an effective multifunctional skin serum based on chitosan hydrogel entrapping spirulina extract. The SECH-W was spherical shaped with core size of 150 nm. This serum induced HFF-1 fibroblasts proliferation and promoted wound closure. Aqueous SECH up regulated two important growth factors involved in wound healing, TGF-ß and PDGF. Our data strongly suggest that SECH-W affects the fibroblast cells positively and more strongly than either chitosan hydrogel or spirulina extract separately. Therefore, SECH-W is an excellent candidate for wound healing and/or facial skin serum applications. However, further investigations are required to study the potential application of SECH-W skin serum as commercial product.
Citation: Khaleghi S, Majedi Z, Lohrasbi A, Rahbar M, Hajrasouliha S (2021) Application of Spirulina-Chitosan Nano Hydrogel for Enhanced Wound Healing through Alteration of Expression Pattern of TGF-ß and PDGF Genes. J Biomed Eng & Med Dev 9:185
Received: 20-Aug-2021 Accepted: 02-Sep-2021 Published: 09-Sep-2021 , DOI: 10.35248/2475-7586.21.6.185
Copyright: © 2021 Khaleghi S, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.