Clinical & Experimental Cardiology
Open Access
ISSN: 2155-9880
ISSN: 2155-9880
Review - (2023)Volume 14, Issue 2
Chronic Heart Failure (CHF) is the end-stage of various cardiovascular diseases in which cardiac output is severely deficient and cannot meet the body's metabolic needs. This article focuses on three main issues surrounding exercise rehabilitation in cardiovascular disease, particularly in patients with CHF: 1. Why do patients in CHF experience two regressions after exercise rehabilitation: exercise tolerance and exercise intolerance? 2. What exercise modalities are appropriate for patients with CHF to achieve exercise tolerance? 3. The adaptive population and evaluation indicators of exercise tolerance of patients with CHF patients (NYHA I~III) with CHF who receive Exercise Training (ET) in combination with left ventricular systolic dysfunction can improve the exercise safety of CHF patients by strengthening the four indicators of 6 Minute Walking Exercise Test (6MWT), Peak oxygen consumption (peak VO2), Brain Natriuretic Peptide (BNP) and Heart Rate (HR). This paper also provides new ideas in the application and study of exercise rehabilitation in patients with CHF.
Chronic heart failure; Exercise rehabilitation; Exercise tolerance; Exercise intolerance; Evaluation indicators
NYHA: New York Heart Association Heart Function Classification; MET: Metabolic Equivalents; RER: Respiratory Exchange Rate; VO2 AMT: Anaerobic Metabolic Threshold Oxygen Consumption; NT-proBNP: Amino-terminal pro Brain Natriuretic Peptide; LVEDD: Left Ventricular End-Diastolic Internal Diameter; MSNA: Muscle Sympathetic Nerve Activity; AT: Anaerobic Threshold
The global prevalence and mortality of cardiovascular disease are still on the rise CHF, as the end stage of various cardiovascular diseases, has not significantly improved the overall survival rate of patients despite the increasing treatment options. In addition to primary pharmacological treatment, the combined implementation of health management therapies such as exercise rehabilitation, psychology and diet is significant in preventing and treating various cardiovascular diseases. In recent years, exercise rehabilitation has received increasing attention in rehabilitating cardiovascular diseases. Aerobic exercise at moderate intensity is a core component of cardiac rehabilitation, which not only enhances the relative adaptability of cardiac function but also improves the blood supply capacity of the coronary arteries of the heart to a certain extent and reduces cardiovascular mortality by 20% to 30% [1]. According to the latest cardiac rehabilitation guidelines, exercise intensity can be maintained at 60% to 70% of HR reserve 3-5 times a week [2]. During the home-based exercise rehabilitation phase, patients could take 40 minutes of aerobic exercise (using a power bicycle or walk, with 10 minutes of warm-up and stretching to be completed before and after exercise); In patients with persistent atrial fibrillation or frequent ventricular ectopic beats, prescribed based on HR reserve, and if the use of a HR monitor was ineffective, sensory exertion grading to guide exercise intensity; Patients in cardiac rehabilitation could adopt a variety of exercise modalities, which include: aerobic exercise, brisk walking, Tai Chi, power cycling exercise, Inspiratory Muscle Training (IMT), etc; strength training, dumbbell, elastic strap and other resistance exercises. Exercise intensity, velocity, and slope both increased, guided by the Borg scale and HR reserve.
Although more studies have affirmed the effectiveness of exercise rehabilitation in preventing and treating CHF, some scholars still dispute it. There are three main areas of controversy; 1. Why do patients with cardiovascular disease experience two outcomes after exercise: improvement in cardiac function and reduction in symptoms after exercise, and deterioration in cardiac function and worsening of symptoms after exercise? 2. What types of patients are suitable for exercise rehabilitation? 3. How to evaluate the suitability of exercise rehabilitation for CHF patients? This paper will discuss these three controversial issues to further clarify the role of exercise rehabilitation in preventing the development of cardiovascular disease, especially CHF; controlling risk factors and reducing cardiac events; improving exercise tolerance and providing a basis for the health management of CHF patients from an exercise perspective [3].
Why do CHF patients experience two disease regressions after exercise rehabilitation interventions?
Overview and classification of CHF: CHF is a global healthcare problem characterized by signs of dyspnoea, fatigue and volume overload, ultimately triggering a decline in heart pump function with a high morbidity and mortality rate [4]. The European Society of Cardiology (ESC) classifies CHF into three types according to ejection fraction: ejection fraction ≥ 50%, defined as Preserved Heart Failure (HFpEF); Ejection fraction between 41% and 49%, called Heart Failure with Intermediate Ejection Fraction (HFmrEF); and ejection fraction ≤ 40% as Heart Failure with reduced Ejection Fraction (HFpEF), where HFpEF accounts for more than half of the patients with either type of heart failure [5-7]. After exercise rehabilitation, two regressions of the disease occur: exercise intolerance and exercise tolerance.
Patients with CHF who underwent exercise rehabilitation intervention showed increased exercise tolerance and delayed disease progression
CHF and exercise tolerance: Guidelines recommends Exercise Training (ET) for people with CHF [7,8]. It can improve exercise capacity and Quality of Life (QoL), reduce depressive symptoms, improve survival and reduce the risk of hospitalization. Exercise-based cardiac rehabilitation can be provided in different ways: intermittent aerobic training, resistance and IMT. The experimental results show that lack of exercise affects exercise capacity and increases the risk of complications such as constipation and blood clots in patients with CHF [9]. Bed rest does not positively affect the recovery of cardiac function in patients with CHF. An exercise-based study included 3872 (60 ± 11 years) patients with Myocardial Infarction (MI) and showed that exercise-based Cardiac Rehabilitation (CR) reduced hospitalization, cardiovascular mortality and all-cause mortality by 31%, 26% and 20%, respectively [10]. In another trial combining an exercise-based Cardiac Rehabilitation (CR) intervention with >6 months of follow-up in a study population dominated by HFrEF and HFpEF, the results of the exercise group found that: CR appeared to not affect mortality in the short term compared to usual medical care (12 months of followup) [11]. Again, the data suggest that CR may reduce the risk of all-cause hospitalization and may reduce hospitalization rates specific to CHF in the short term (12 months). ET increases exercise tolerance in CHF and improve cardiopulmonary function symptoms, including increases in VO2 peak, ventilatory threshold, and 6MWT distance [2]. Therefore, for patients with CHF, cardiac rehabilitation exercise is used as a non-pharmacological intervention for cardiovascular health and active treatment of the primary disease. The main symptoms in clinically stable CHF patients have reduced exercise tolerance and peak VO2, which are associated with reduced QoL and survival [12]. Exercise-Based Cardiac Rehabilitation (EBCR) is a safe and effective intervention that improves peak VO2, muscle strength, physical function performance and QoL. It is associated with a reduction in overall hospitalization and HF-specific hospitalization rates in patients with clinically stable CHF. ET is pleiotropic and can act on multiple systems (cardiovascular, immune, skeletal muscle, etc.) through various pathways to exert its beneficial effects in the CHF setting.
Endurance exercise can slow the progression of CHF
Many national and international guidelines encourage ET in patients with CHF and affirm its unique role in slowing disease progression [13]. Endurance ET positively impacts modifiable cardiovascular risk factors and functional capacity. The traditional view of rest as one of the conventional tools in treating CHF is that rest reduces myocardial oxygen consumption, decreasing the burden on the heart and reducing symptoms. However prolonged bed rest has many potential dangers, such as deep vein thrombosis in the lower limbs, bedsores, disuse muscle atrophy, osteoporosis and loss of appetite [9]. Studies in recent years have found that low-intensity exercise not only delays the onset of CHF but also extends the life span of people with congestive CHF. A trial aimed assessing endurance ET on endotheliumdependent Flow-Mediated Arterial Dilation (FMD) and carotid artery stiffness showed that 16 weeks of ET in elderly HFpEF patients with impaired endothelial function or arterial stiffness improved Peak VO2 [14]. A study was conducted on patients with HFpEF to examine the effects of exercise intervention [15]. The study's results showed that 3-6 months of aerobic training alone is a safe and effective therapy. Symptoms of discomfort or poor sleep during exercise indicate high exercise or activity levels that need to be reduced or modified.
Mechanisms of the protective effect of endurance exercise on CHF
Enhancing skeletal muscle adaptability: CHF is a systemic disease with profound effects on a wide range of peripheral tissues, including skeletal muscle [16]. In the setting of CHF, perturbations in skeletal muscle physiology, structure and function can lead to the development of exercise intolerance and skeletal muscle-related disorders. There is growing evidence that CHF leads to specific pathological changes in skeletal muscle, causing muscle dysfunction and tissue atrophy [17]. The literature reports that Sympathetic Hyperactivity (SH) contributes to HF-induced skeletal muscle lesions. Skeletal muscle plays a central role in the dysfunction and prognosis of patients with CHF. Recent evidence suggests that skeletal muscle contraction produces actin, which actively counteracts the inflammatory state of CHF [18]. In skeletal muscle, CHF causes morphological changes, including fibrous atrophy, an increased proportion and dependence of type II fibrosis, and reduced mitochondrial bulk density and capillary degeneration, resulting in a reduced capillary-to-fibre ratio [19]. Endurance ET increases the ability of the skeletal muscle to oxidize fat by increasing mitochondrial density, engaging beta oxidase activity, and delivering oxygen to the muscle [20]. CHF causes various pathological changes in skeletal muscle function. Ventura-Clapier R, et al. showed that reduced skeletal muscle mitochondrial Adenosine Triphosphate (ATP) production during CHF leads to impaired energy synthesis [21]. The metabolic disorders without significant features occur in CHF, resulting in abnormal skeletal muscle contraction and diastole. In patients with CHF, endurance training reduced phosphocreatine depletion, increased Adenosine Diphosphate (ADP) during exercise, and increased the rate of phosphocreatine resynthesis after exercise, indicating significant improvements in skeletal muscle oxidative capacity [22]. Jolly et al. conducted an irregular controlled trial within which long-run regular aerobics through home viscus rehabilitation enlarged musculus action, reducing respiratory organ stasis and assuaging dyspnea symptoms; ET will improve exercise tolerance through peripheral mechanisms, thereby up musculus gas uptake, with no critical impact on left chamber pulse operate [23]. There was no significant effect on LV diastolic function. Another study has shown that ET can produce a variety of beneficial skeletal muscle adaptations, including an increased percentage of oxidized fibres, oxidase activity and capillary density [22]. The literature reports animal experiments on Left Anterior Descending Artery Occlusion (LAD) 4 weeks after surgery and endurance training in the form of a moderate-intensity treadmill for 10 weeks in observation rats [24]. The results showed that endurance training improved, to some extent, the reconstitution of skeletal muscle fibre types in the fast and slow hind limbs of rats with CHF by increasing capillary density in the slow muscle and small artery density in the tight muscle.
In summary, exercise rehabilitation for CHF patients to achieve endurance exercise and improve inflammation and energy metabolism disorders can be an effective strategy to increase skeletal muscle mass and functional capacity.
Enhances cardioprotective effects: ET can reduce the cardiovascular risk of diseases such as Atherosclerosis (AS), the lifetime risk of CHF and cardiovascular mortality [25]. Long-term moderate ET can improve cardiac function, reduce cardiovascular disease incidence and slow ageing. Exercise induces preconditioning effects in two ways: first, preconditioning caused by relative ischemia due to myocardial ischemia or activity itself; Second, exercise-induced cardioprotection is a stressor that triggers several other endogenous factors [26]. The heart relies on its systolic and diastolic functions to complete blood circulation in the body, with systolic function ensuring the expected cardiac output and diastolic function, providing sufficient blood reserve for cardiac ejection.
A Meta-analysis study that included 19 randomized controlled of the effect of exercise therapy on Left Ventricular Remodelling (LVR) in CHF showed that exercise significantly improved Left Heart Ejection Fraction (LVEF), left ventricular end-diastolic and end-systolic volumes, beats per minute and cardiac output in patients with CHF [27,28]. Yuan etc. showed that the administration of exogenous Mitochondria-Derived Peptide (MOTS-c) increased the level of endogenous MOTS-c in the myocardium and improved the mechanical efficiency of the myocardium [29]. Mechanical efficiency enhanced cardiac contractile function and improved diastolic function during ET. Myocardial mechanics and enhanced cardiac function were more remarkable in exercising rats that received MOTS-c. Exercise decreases sympathetic activity, and vagal tone at rest increases Nitric Oxide (NO) bioavailability and the increased stress that occurs during exercise, which is vital for improving endothelial function and arterial vasodilatory capacity, resulting in potent vascular anti-atherogenic effects.
From the above, it is clear that endurance exercise has a positive significance in delaying myocardial remodelling, reducing sympathetic nerve activity, improving coronary endothelial function and bringing relief to CHF symptoms, further increasing exercise tolerance and improving cardiac function in patients with CHF.
Protection of the peripheral vasculature: Moderate exercise has a positive effect on the enhancement of vascular function [30]. Exercise induces remodelling of the structure and function of vascular function, mainly in the vascular endothelium and Vascular Smooth Muscle Cells (VSMC). Endothelial dysfunction is closely related to the development of CHF, and improving vascular endothelial function significantly affects the prevention and treatment of CHF [31]. Exercise can promote the development of collateral circulation and induce improved blood flow and oxygen supply to the ischaemic limb [32]. It can increase the release and synthesis of Nitric Oxide (NO) and prostaglandins from the vascular endothelium, leading to vasodilation and increased blood flow. A prospective study has shown that exercise improves asymptomatic walking time in patients with PAD [33].
Exercise can also be used to slow vascular ageing [29]. Arterial stiffness is a common feature of patients with CHF, and arterial stiffness-related arterial tonometry is a novel and effective method of great value in the diagnosis and prognosis of CHF. ET is considered adjunctive therapy in improving arterial stiffness in CHF patients and a helpful tool for diagnosis and prognosis. A Meta study including 18 articles with a total of 876 participants concluded that patients with HFrEF had a lower basal status of Flow-Mediated Dilatation (FMD) in the brachial artery compared to the healthy population, with significant improvements after exercise. A study of over 100 people reported beneficial effects on central blood pressure and arterial stiffness. Exercising also improves endothelial function and has an antiinflammatory effect on chronic inflammation. In a randomized controlled trial investigated the effect of physical training on cardiovascular control in patients with CHF (NYHA I and II) and normal controls, 20 min/time, 5 days/week, after 5 weeks of training, exercise found that in CHF, physical exercise improves overall management of the vegetative nervous system, including the vagus nerve over the heart and sympathetic nerves over the peripheral vasculature [34]. Wang Wei, et al. concluded from clinical trials that regular exercise prevents the occurrence of CHF, the vicious cycle caused by excessive neurohormonal activation, and improves exercise tolerance by improving diastolic endoth elial function and enhancing the aerobic metabolic capacity of skeletal muscle, resulting in lower peripheral vascular resistance [35]. In patients with NYHA I -II, rehabilitation training can improve vascular elasticity to improve pulmonary circulation function and increase blood volume to improve blood concentration and flow rate [36]. ET was also associated with improvements in vasodilation and endothelial function, skeletal muscle morphological characteristics and function, ventricular filling pressures, exercise performance and Quality of Life (QoL) in patients with CHF [37].
In conclusion, ET can counteract many of the injuries caused by CHF. Endurance exercise encompasses a positive impact on up tube physical properties and proper pressure, regulation of the vegetative nervous system, counteracting chronic anti-inflammation and delivering relief to CHF symptoms, thereby up internal organ circulatory operation and increasing blood concentration and rate by increasing blood volume, so enhancing QoL in CHF patients.
Mechanisms of exercise intolerance and exacerbation of disease progression in patients with CHF after exercise intervention
Overview of CHF patients and exercise intolerance: Exercise intolerance refers to impairment of Physical Activity (PA) ability with obvious symptoms, including dyspnea [38]. It appears in the early stages of CHF, progresses with the severity of cardiac insufficiency, and is associated with reduced QoL and increased mortality. The pathogenesis of exercise intolerance in CHF is multifactorial. These include impaired heart and lung reserves, decreased peripheral blood flow, and skeletal muscle abnormalities, all of which may harm exercise rehabilitation in patients with chronic heart failure.
Impairment of exercise intolerance in patients with CHF: Exercise intolerance occurs in patients with CHF for a variety of reasons. The impairment of oxygen delivery and extraction at the tissue level is the critical determinant of exercise intolerance. In some cases, exercise intolerance is one of the main limiting factors of exercise and functional ability [39]. In addition, it is related to the decrease of heart and lung reserve and the damage to the peripheral vascular system and skeletal muscle.
Mechanisms of exercise intolerance in patients with CHF Decreased Cardiac Reserve (CR): CR refers to the ability to upregulate cardiac function. Typical classifications include systolic reserve, diastolic reserve, and HR reserve. Decreased CR is the primary mechanism of exercise intolerance. A decrease in cardiac addition occurs early in CHF, and the degree of the decline reflects the state of cardiac function. Research results show that 20-fold difference in CR between subjects with the most severely impaired cardiac function and those with the best physical performance [40]. CR capacity is a weakening of reserve capacity or compensatory capacity, while other parts remain intact and are equivalent to the size of the compensatory capacity of the heart [41]. Chronotropic Incompetence (CI) is generally defined as the inability to adequately increase the HR during exercise to match cardiac output to metabolic demands. Nevertheless, it has been demonstrated that CI in CHF is associated with reduced functional capacity and poor survival. The average heart increases stroke volume and HR during exercise, whereas contractility reserve is lost in failing hearts, thus rendering cardiac output increases primarily dependent on cardio acceleration. Consequently, poor cardio acceleration because of CI may be considered a major limiting factor in the exercise capacity of patients with CHF. Recent findings suggest that myocardial ischemia and injury may lead to limited systolic and diastolic functional reserve, even in the absence of epicardial coronary artery disease. In a Meta-analysis, 1396 clinical HFpEF subjects, randomly assigned in 5 cohorts, underwent studies including supine exercise testing, upright or semi-erect exercise testing inclusion, and peak VO2 and exercised haemodynamic assessments [42]. When the central nervous system is damaged, the variable time reserve response to exercise decreases, which is related to exercise intolerance. In a study, 142 patients (58.36 ± 3.17 years) with cardiac rehabilitation sequential training in combination with Electrocardiogram (ECG) for 8 weeks [43]. All patients underwent ECG, ultrasound and other routine investigations. The results showed that the LVEF, FVC, FEV1/ FVC, and end PP levels in the observation group increased, and the AT, 6MWD and peak VO2 end levels increased significantly. Conclude that patients with coronary artery disease showed a significant improvement in cardiac reserve function and exercised tolerance using an eight-week cardiac rehabilitation programme combined with ECG. It is worth noting that men have higher cardiac reserve function than women [44,45]. Traditional risk factors for coronary heart disease and cardiovascular disease (eg: age, dyslipidaemia) can hurt cardiac reserve function.
Thus, exercise intolerance is closely related to reduce CR capacity and reduced CI due to CNS damage. In addition, the presence of exercise intolerance in patients with CHF is, to some extent, related to patient age, gender, and lipids.
Peripheral vascular injury: Peripheral vascular dysfunction leads to an obstruction of oxygen delivery to skeletal muscle at the onset of exercise, increased time spent on anaerobic energy use and increased energy expenditure in later exercise phases. Enhanced sympathetic stimulation due to desensitization of muscle metabolic receptors leads to a decrease in endothelial vasodilator bioavailability and a significant reduction in oxygen supply to active muscles as exercise intensity increases. In addition, the impaired peripheral vasodilatory response to exercise alters the timing and amplitude of the reflected pulse wave, leading to increased load on the heart during the later stages of contraction and ultimately to exercise and functional intolerance. Thus, disease-related changes in the peripheral circulation may be an actual cause of exercise intolerance in patients with HFpEF. Peripheral Arterial Disease (PAD) has been reported in the literature to similarly lead to exercise intolerance and severely limit mobility [46]. To isolate the central and peripheral effects on blood flow to the exercising limb [47]. A study using a knee extensor model in patients with HFpEF found that leg blood flow was reduced by 15- 20% in the observation group when faced with similar central haemodynamic changes. The above suggests that atherosclerosis and vasodilatory abnormalities during exercise are directly related to elevate filling pressures and impaired cardiac output reserve in patients with CHF, again with important implications for exercise intolerance. Borlaug et al. who used a cycling exercise modality found impaired systemic vascular resistance, particularly during exercise with variable timing and reduced cardiac output reserve in patients with HFpEF [48].
In conclusion, patients with reduced cardiorespiratory reserve capacity require more professional assessment during exercise. At the same time, peripheral vascular disease can exacerbate atherosclerosis and vasodilatory abnormalities during activity associated with increased filling pressures and impaired cardiac output reserve in patients with CHF, and a significant reduction in oxygen supply during exercise in patients with CHF, increasing myocardial load during exercise and thus causing the development of exercise intolerance.
Skeletal muscle damage: Skeletal muscle damage is an essential aetiology of exercise intolerance in CHF patients, and the development of exercise intolerance, particularly in HFpEF patients, is thought to be due to reduced oxygen delivery or impaired oxygen utilization by exercising skeletal muscle. Peak VO2 depends on oxygen delivery (i.e. CO2 and arterial oxygen content, peripheral vascular function) and consumption (i.e. diffusion and extraction). At maximal exercise, oxygen consumption by skeletal muscle peaks, shifting towards anaerobic metabolism and exacerbating skeletal muscle fatigue and ventilatory dyspnea. In addition, systemic inflammation, oxidative stress and sympathetic nervous system activity similarly reduce oxygen delivery to active muscles. Systemic oxidative stress is associated with exercise intolerance and skeletal muscle abnormalities in patients with CHF. Yokota et al. in 17 patients with CHF, showed that serum Thio-Barbituric Acid Reactive Substances (TBARS) were high and serum Superoxide Dismutase (SOD) activity was low in the experimental group, suggesting increased systemic oxidative stress in CHF [49]. VO2 Peak and AT VO2 serve as features of CHF, reduced wholebody aerobic capacity, and impaired skeletal muscle energy metabolism as assessed by muscle Phosphocreatine (PCR) loss and Intra-Myocyte Lipid (IMC) content during exercise.
It is important to note that anemia is also a cause of exercise intolerance. Anaemia is common in people with CHF. The leading cause of anaemia is iron deficiency. It is worth noting that exercise intolerance during ET in CHF patients does not mean that they cannot exercise, but instead, that exercise, to some extent, is based on eligibility on the Borg scale score. Similarly, patients with exercise tolerance in CHF who exceed their tolerance range can move from exercise tolerance to exercise intolerance, worsening disease progression. From the above mechanisms, it is clear that (1) improving the efficiency of skeletal muscle energy metabolism and oxidative capacity, (2) enhancing the systolic-diastolic function of the heart and thus cardioprotection, (3) improving the remodelling of the functional structure and function of blood vessels and thus protecting the peripheral vasculature, and (4) improving the efficiency of blood-myocyte oxygen transfer and increasing oxygen utilization capacity are also correspondingly effective ways to improve exercise tolerance (Figure 1).
Figure 1: Schematic diagram of the different transitions and their causes in patients with CHF.
What type of exercise is appropriate for a patient with CHF to achieve exercise tolerance?
Exercise tolerance for CHF recovery: Exercise tolerance refers to the ability to tolerate aerobic exercise and achieve the expected age-related level or duration of practice [50]. In healthy people, exercise tolerance mainly reflects cardiorespiratory function; in patients with cardiovascular disease, exercise patience reflects the overall operation of the heart, lungs and skeletal muscles. The WHO recommendation for exercise in the general population is at least 150 minutes of moderate-intensity or 75 minutes of vigorous aerobic activity per week [51]. Numerous studies have shown that: Exercise therapy has some effectiveness in cardiac rehabilitation in patients with CHF, but further research is needed to determine the optimal exercise mode, duration, and activity combination. Common exercise modalities include (1) Aerobic exercise programs: power bicycles, jogging, pedal cycles, and callisthenics. (2) Strength exercises: resistance exercises, exercise plank training, Tai Chi exercises, etc. (3) General breathing exercises: IMT slow breathing exercises [52]. Exercise modalities often advocated for CHF patients can also combine aerobic, resistance, and flexibility exercises to achieve optimal exercise results.
Power cycling facilitates exercise rehabilitation in patients with CHF (NYHA II-III): Power cycling is one of the aerobic training programs, with high safety and easy blood pressure and HR monitoring during exercise. Liao Yuejun et al. seventy patients with CHF were selected to establish an aerobic cardiac rehabilitation exercise program consisting of two phases: the first phase was performed with 30% peak VO2 power cycling for 30 min each time, 3 times a week for two weeks; the second phase was completed with 50% peak VO2 power cycling for 30 min each time, 3 times a week for 6 weeks; the results showed that: after 8 weeks, the BNP, LVEDD and 6MWT of the observation group were significantly improved compared with those at the time of admission [53]. The observation group's BNP, LVEDD and 6MWT were significantly improved after 8 weeks compared to the entrance, with BNP decreasing by 44% and 6MWT increasing by 48% in the exercise group. One study used the New York Classification of Cardiac Function to guide exercise with power cycling as the primary mode of exercise intensity: an individualized Δ50%W intensity exercise rehabilitation prescription was developed based on the cardiopulmonary exercise test results [54].
Δ50%W=(anaerobic threshold measured power-power increment rate × 0.75)/2+(extreme exercise measured powerpower increment rate × 0.75)/2 cardiac function.
Those in class II passed power cycling for 40 min/d (2 min warm-up first, 30 min maintenance after reaching target power), those in NYHA III for 15 min/d initially, then increased by 5 min/d per week as appropriate until target power time of 30 min was maintained, and the rest as in NYHA II, 5 times/ week for 3 months after the intervention, where 6MWT and METmax increased by 70% and 29.1% respectively. Chasland LC, et al. studied 11 participants (NYHAI-III) with an ejection fraction <45%, and after one week of Eccentric Cycling (ECC) training, participants rode at 40rpm with resistance adjusted every 30 seconds and found that respiratory variables were lower in PeakVO2 than in concentric cycling throughout the exercise period [55]. That eccentric cycling exercise enhanced peripheral adaptation and functional capacity. The use of power cycling [56] enables the development of an individualized exercise plan tailored to the patient's condition and, thus, effective rehabilitation exercise. Power bicycle exercise rehabilitation allows CHF patients to establish collateral circulation and myocardial blood supply, improving (NYHA II~III) cardiac function indicators, lung function, exercise tolerance, and QoL. If a patient has worsening cardiac function or a HR of more than 150 beats per minute, withdraw from ET.
Resistance training combined with aerobic exercise is beneficial to the rehabilitation of patients with class IIIII chronic heart failure: Resistance exercise, also known as strength or resistance training, usually refers to practices that allow the body to overcome resistance to achieve strength or muscle growth. Currently, resistance training has been gaining popularity in rehabilitation in recent years [57]. Resistance training significantly benefits patients with motor dysfunction and allows for controlling various cardiovascular risk factors. Studies have shown that both resistance and aerobic training significantly affect health promotion, and resistance training has unique advantages in some areas, such as increasing or maintaining lean body mass and bone mineral density [58]. Wang Yingying et al. included 214 patients with HFpEF and resistance training combined with 8,000 skelp steps [59]. The results of the study group showed improved measures of cardiac function in patients with HFpEF after 12 weeks of intervention, with a 35% increase in peak VO2 and a 9.6% increase in peak VO2 decrease in Left Ventricular End-Diastolic Internal Diameter (LVEDD) in the study group. Zhu Hui et al. performed conventional exercise-based training combined with exercise planking in the study group based on NYHA IIIII [57]. The starting HR was 50% reserve HR, and the target HR was 60% to 70% reserve HR, calculated as: 50% reserve HR=Resting Heart Rate (RHR)+(maximal exerciseHR - RHR × 50%). Both maximal exercise HR and RHR base on cardiac exercise test measurements. The exercise duration was 30-60 min, and the patient was trained twice a week for 8-12 weeks. The results showed a 28% decrease in NT-proBNP levels and a 16% increase in maximum HR after 12 weeks. Wang Huijie et al. gave the observation group of 127 elderly CHF patients with preserved Left Ventricular Ejection Fraction (LVEF) resistance ET based on an exercise tablet, using 50% reserve HR as the starting target HR and gradually increasing the intensity of exercise to 70% reserve HR after adaptation, with exercise time controlled at 30-60 min [60]. After 3 months, cardiac function improved significantly, with 6MWT increasing by 62% respectively. Ejection fraction enhanced by 27%. In a study with 98 patients with CHF, the experimental group was given a home tele-health ET programme based on usual care, consisting of 32 exercise sessions over 8 weeks [61]. The ET programme consisted of 2 phases: weeks 1-4 focused on endurance exercise, 3 times a week for 20 minutes. The training consists of walking and jogging 3 times a week. 5-8 weeks include resistance and muscle strengthening exercises 5 times a week for 30 minutes. Training modalities include walking, jogging and aerobics for muscle training. The experimental results show that 6MWT is improved by 5%. RHR, NYHA classification and LVEF were all positively affected. Hu et al. included 60 patients with CHF in their study [62]. Observation group members participated in resistance training. Their patients with central function class II were treated with single arm bending training, knee lift+leg abduction training, anterior panel training for arching, flexed resistance training for prone leg, and gastrocnemius resistance training, 20-30 times in each group within 30 min, 3 times per week; Standing leg abduction training was increased in patients with class III cardiac function compared to those with class II. For patients in cardiac function class III, there is additional training for leg abduction in a standing position, 10-20 reps per set, completed within 10-20 minutes, 2 times/week. The exercise intensity increased from 30% to 40% of the maximum repetitions completed in a single exercise (1-RM) to 60% of 1-RM for the upper limbs. In the observation group, 6MWT increased by 22% after 12 months of training, and BNP decreased by 34.7% over the same period. Bai et al. the experimental group carried out resistance exercises based on conventional drug therapy [63]. Resistance exercise was performed once every 2 days for 12 weeks: weeks 1-4: 70-80 steps/min, walking for 15 min, resting for 1 min every 2 min, gradually increasing the walking time to 30 min according to the patient's physical condition; weeks 5-8: 80-90 steps/min, walking for 30 min, resting for 1 min every 4 min; weeks 9-12: 90-100 steps After 12 weeks of ET, the results showed that the 6MWT and EF increased by 20% and 20% respectively.
In conclusion, aerobic exercise combined with resistance exercise rehabilitation can significantly improve endothelial function, enhance exercise function and improve some cardiac function indicators, thus increasing QoL. Notably, the step size keeps in the range of 30-50 cm for 12 weeks. The exercise stopped if the HR increased by more than 20/min. If symptoms such as weakness, dizziness or chest tightness occur.
Tai Chi facilitates rehabilitation training for CHF: Tai Chi is one of the most famous traditional exercise methods in China. Tai Chi has a unique theory and practice pattern: gentle and slow movements, high concentration of thought, and a combination of aerobic, resistance, stretching and balance exercises [64]. A meta-analysis of 15 studies, including 1,236 participants, showed that. Compared to usual care alone, tai chi combined with regular care improved the Minnesota CHF Patient Questionnaire, 6-minute walk test, LVEF and B-type natriuretic peptide [65]. Numerous studies have concluded that tai chi exercise is beneficial in improving myocardial contraction strength, cardiac reserve HR, improving cardiac pumping function, and providing a comprehensive improvement in cardiopulmonary function [66,67]. Tai chi exercise is safe and feasible and has good compliance for CHF patients, especially those who do not like exercise and refuse to seek cardiac rehabilitation. Tai Chi is safe for stable elderly patients with congestive CHF (NYHAII) while building exercise capacity and muscle strength [68]. It is a recommended method of rehabilitation exercise. One study compared the effects of an exercise rehabilitation program of different training times on cardiac function in CHF, the results showed that the experimental group trained 60 min per session with better results in 24-week cycles, and the 6-min walk test improved by 25.6% [69,70]. Furthermore, the experimental group had a 58% decrease in brain natriuretic peptide levels; this study suggests that TaiChi can improve cardiac function and exercise endurance in patients with CHF. Patients in NYHA class I-III were included in the study by Zhao S, et al. and meeting the diagnostic criteria for myocardial infarction, aged 35-80 years, will be selected, in which the experimental group will keep 24 simplified Tai Chi styles based on conventional treatment for a total duration of approximately 20 minutes, twice a day for 24 weeks [71]. The results showed an improvement in the overall efficiency 6-minute walk test, brain natriuretic peptide, and left ventricular ejection fraction Tai Chi group. Wang et al. included 80 patients with HFpEF (NYHA II-III), the observation group was given tai chi exercise based on conventional medication with a 5-10 min warm-up in the preparation phase [72]. The exercise intensity was: target HR (%)=(maximal HR-RHR) × (30%-60%)+RHR, and also combined with Borg self-exertion rating of 11-13 as the maximum exercise intensity; the exercise duration was about 30 min each time, 3-5 times a week, and after the tai chi exercise, 5-10 min for 12 weeks. The results showed a 3% increase in LVEF and a 35.5% decrease in pro- BNP. 6MWT increased by 10%.
There is a literature report 127 elderly patients with CHF (67.32 ± 3.12) years old were studied [73]. The observation group was based on conventional rehabilitation training combined with tai chi. If the patients' cardiac function was class II, they were instructed to walk indoors for 500 m/d; if the patient's cardiac function was class III, they were guided to move and stand at the bedside for 6 min, 4 times/d, and adjusted accordingly according to their actual condition, and then switched to indoor walking when their condition was relatively stable. Both groups continued the intervention for 2 months. The results revealed a 17.7% increase in VO2, a 34% increase in Anaerobic Threshold (AT) and a 43% decrease in BNP.
In conclusion, Tai Chi exercise effectively improves serum-free fatty acid levels and oxidative stress in CHF patients, improves cardiac function and gains more benefits with time spent exercising.
IMT facilitates the rehabilitation of CHF patients: IMT improve respiratory muscle function, reduce dyspnoea and improve exercise capacity in patients with CHF [74]. IMT can correct abnormal respiratory patterns, provide an excellent foundation for cycling, and promote pulmonary blood circulation. Highintensity interval training and peripheral training can enhance the body's aerobic metabolism and expand the ratio of capillaries to muscle fibres, increasing myocardial contractile function. Set the inspiratory muscle inspiratory load at 30% of the maximum static inspiratory pressure and the weekly training load at 30% of the maximum inspiratory pressure (PImax). One study found that high-intensity interval training with peripheral training and inspiratory muscle resistance training may be more beneficial for patients with CHF [75]. IMT may improve exercise and functional capacity in HFpEF. Meta-analysis showed that IMT significantly improved VO2 peak and 6MWT, so IMT may be a treatment option to improve cardiorespiratory function in patients with stable CHF. Dall'Ago P, et al. subjected 32 patients with CHF and inspiratory muscle weakness to a 12-week IMT programme [76]. Patients received IMT for 30 minutes seven times a week, maintaining diaphragmatic breathing at a respiratory rate of 15-20 breaths/min for 12 weeks. Analysis showed that slow breathing training increased ejection fraction and 6MWT by 3% and 4%, respectively.
Trevizan PF et al. in a study of CHF patients with reduced ejection fraction, including NYHA II-III (30-70 years), the experiment group was based on moderate aerobic ET given with IMT, where moderate aerobic ET consisted of 60 min, 3 times a week for 4 months, and IMT consisted of 30 min sessions, 5 times a week for 4 months [77]. Results found: reduced muscle sympathetic nerve activity (MSNA) in patients. Forearm blood flow was increased, and VO2 was increased by 12% in the observation group of the aerobic training group. A study included 100 patients with CHF (NYHA class II-III), aged 55-69 [4]. The combined exercise group performed moderateintensity continuous aerobic exercise: 3 times/week, 45 min/ time, exercise target HR=HR at anaerobic valve-10. Respiratory muscle training three times a week: (1) lip retractor breathing training: the patient lies flat or sits, relaxes and then inhales slowly and deeply with maximum effort. The mouth becomes whistle-like and then exhales gradually, breathing gas time. The training was for 10-15 min/d; (2) abdominal breathing: the patient was placed in a supine position, relaxed, and inhaled slowly through the nose. The therapist placed the palm under the patient's rib cage to feel the rise and fall of the thorax and exhaled slowly in a lip-retracting manner, repeated the training for 10-15 min/d. The above training was carried out for 12 weeks. Results found that Peak VO2 improved by 8.3% and 6MWT by 14.2% (Figure 2).
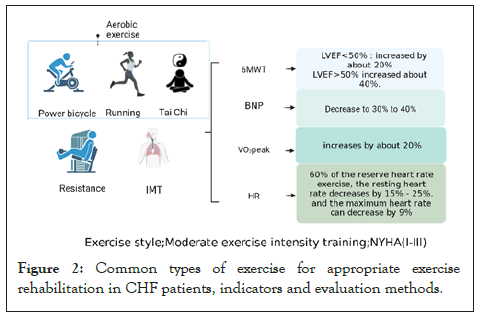
Figure 2: Common types of exercise for appropriate exercise rehabilitation in CHF patients, indicators and evaluation methods.
Indication of exercise tolerance in patients with CHF and evaluation indicators
Indications for exercise rehabilitation in patients with CHF: Cardiac rehabilitation can be attempted by heart patients in general, including patients with acute myocardial infarction, CHF, patients who have undergone Coronary Artery Bypass Grafting (CABG), Percutaneous Coronary Intervention (PCI), heart valve surgery, pacemaker surgery, heart transplantation and patients with chronic stable angina, hypertension, hyperlipidemia, diabetes and its metabolic syndrome. Patients should be monitored and treated differently at different stages. Some patients are also temporarily unsuitable for cardiac rehabilitation exercises, such as unstable angina, severe arrhythmias, hypertension resting systolic blood pressure >180mmHg (1mmHg=0.133kPa) or resting diastolic blood pressure >100mmHg and cardiac function class IV, hyperthermia or severe infection, cachexia, multi-organ failure or inability to cooperate, or patient refusal. These conditions are contraindications to cardiac rehabilitation exercise.
Indication of exercise tolerance in patients with CHF and evaluation indicators
Overall assessment of cardiac function: Cardiac assessment is carried out throughout the cardiac rehabilitation process, and it is vital to carry out a thorough evaluation, especially for cardiac exercise rehabilitation interventions in patients with CHF. The whole process includes: starting with the first contact with the patient and continuing throughout the cardiac rehabilitation process. Specific components include biological history, lifestyle habits, risk factors, cardiovascular function and exercise risk. The assessment is used to understand the patient's overall status, risk stratification and impact.
Evaluation of exercise tolerance in patients with CHF: Numerous indicators have been reported in the literature and clinical practice regarding the evaluation of exercise tolerance. However, many are relatively subjective and comprehensive in the literature and clinical practice. We focus on four indicators, Peak VO2, brain natriuretic peptide, 6MWT, and HR.
Peak VO2: PeakVO2 is the maximum capacity of the cardiovascular system to deliver oxygen to exercising skeletal muscle and for exercising muscle to extract oxygen from the blood. It is common for clinically stable CHF patients to be measured using a peak VO2. ET to improve peak VO2 helps to improve central and peripheral adaptation in clinically stable CHF patients [78]. Peak VO2 is the best indicator of aerobic capacity and cardiorespiratory adaptation. A study that included 175 (mean age 30 ± 11 years) patients with NYHAI-III Congenital Heart Disease (ACHD) had a peak VO2 of 26.4 ± 9.8 ml/kg/ min. Peak VO2 based on NYHAI class:26.6 ± 6.1 ml/kg/min, NYHAII: 21.1 ± 5.5 ml/kg/min, NYHA class III 16.8 ± 4.5ml/ kg/min [79]. The difference between NYHA I and III were significant. Chen YC et al. found that an experimental group of 39 patients with NYHA I-III CHF exercised at moderate exercise intensity (approximately 60-85% HRR) for 12 weeks or more extended 55-70% peak VO2 3-7 times per week for 20- 60 min, the results showed that there was also a significant increase in Peak VO2 of 3.47 mL/kg/min, a finding that suggests: abnormal peripheral blood flow, and skeletal muscle, leading to increased oxygen utilization by skeletal muscle [80]. Shen Yuqin et al. observed 50 patients with CHF with LVEF <49% and performed a Cardiopulmonary Exercise Test (CPET) after 3 months of aerobic exercise rehabilitation, Peak VO2 increased by 3.4 ± 1.2 ml-kg/min, concluding that 3 months of aerobic exercise rehabilitation could increase peak VO2 and improve exercise tolerance in patients with CHF [81]. Erbs S et al. included 37 patients aged ≤ 70 years with CHF in NYHAIII, and exercise tests were performed on an electronic braking bicycle with workloads starting at 25 W and increasing progressively every 3 min in 25 W steps for 12 weeks, results revealed a 16.3% increase in VO2 max, a 19.3% increase in VO2 AT and a 2% increase in RHR in the experimental group [82]. In conclusion, ET improves the central and peripheral adaptability of Peak VO2 in patients with LVEF <49% and NYHAI-III, and by moderate exercise intensity (about 60-85% HRR) for 12 weeks, the peak VO2 in the cardiac function level increased by about 20%, the patient's oxygen consumption during exercise increased. The accumulation of lactic acid decreased, so the exercise endurance increased, and the patient's fatigue improved significantly. The prognosis was good, which has a specific reference value for the clinical care of these patients.
6MWT: The 6MWT, a subpolar exercise tolerance test is economical, simple, consistent with the amount of exercise and daily life, and correctly assesses the patient's cardiac function [83,84]. It reflects the functionality of multiple systems during training, including the body, cardiovascular, peripheral, and neuromuscular units. Bittner et al. classified the 6MWT into 4 classes according to the condition of CHF patients: <300m as Class I; 300-375m as Class II; 375-450m as Class III; and >450m as Class IV [85]. It is also negatively correlated with the New York Heart Association (NYHA) CHF class. A BIOSTATCHF study showed patients who walked less than 300m had a higher incidence of adverse events than those who walked at least 450m [86]. Thus, a decrease in the 6-minute walk test distance was associated with a poorer prognosis. A study of 100 patients NYHA class II-III, experimental group based on moderate-intensity continuous aerobic exercise guided by cardiopulmonary exercise test, 3 times/week, 45 min/time, exercise target HR (beats/min)=HR at anaerobic valve-10, showed a 14.2% improvement in 6-minute walk distance after treatment [84]. Eighty-two patients with HFpEF with LVEF >50% were selected [87]. The observation group was given exercise therapy based on conventional medicine, gradually accelerating to a moderate walking speed of approximately 30 min per day. After 6 months of comparing the two groups, the Result displayed that the patients in the observation group, where the 6-minute walking distance increased by 47%. The study also found that with Moderate Intensity Exercise (MICT) Training, LVEF <50% six-minute walking distance increased by 10% and LVEF >50% CHF increased by 40%. Chen et al. included 72 patients with CHF (NYHA II-III), and the intervention group was given rehabilitation training with medication, and the results showed that the 6MWT distance increased by about 20% [88]. According to the findings above, cardiac function class negatively correlates with 6MWT and in patients with cardiac function class II-III, if LVEF<50%, moderate intensity ET can increase the six-minute walking distance by about 20%. In patients with LVEF>50% or mild CHF, the increase can be 40%.
HR: Therapeutic goal in treating chronic heart failure controls HR because high HR is a marker of incident CHF [89]. It is well known that an elevated RHR increases the risk of Cardiovascular Morbidity and Mortality (CVM) [90]. There was a strong correlation between tachycardia (>100 beats/minute) and CVM. Recent studies have shown that in the general population, there is a 14% increase in mortality for every 10 beats/min increase in HR; the maximum HR is mainly age-related, with maximum HR (beats/min)=220 - age (years). Therefore, for different age groups, divide the intensity of exercise appropriate for them: HR at or above 85% of the maximum HR corresponds to heavy exercise; an HR controlled in the range of 60% to 85% of the maximum HR corresponds to moderate exercise; an HR held in the field of 50% to 60% of the maximum HR corresponds to low-intensity practice [91]. One study published in the European Journal of preventive cardiology included 460 healthy individuals (24-62 years) in an exercise stress test and found that setting the peak HR at 85% was safe. It is also one of the medical auxiliary diagnostic indicators of regional myocardial ischemia (coronary artery disease). 150 CCHF cases, aged 32 to 57 years, all with a cardiac function classification of II to III, were used as study subjects [92]. The experimental group kept the training frequency at 3-5 times/week, 30-40 min/time, and the results showed that the RHR was reduced by 15%. 162 patients with CHF, the observation group was given fast inhalation and slow expiration training based on conventional cardiology care [93]. The training method for rapid inhalation and slow expiration was as follows: inhalation time was 0.8~1.0s, inhalation time was 3s, then exhalation time was 3~4s. Training intensity: 6 cycles of fast inhalation and slow expiration were performed within 1 min. Each training lasted 10 min. The training is from day 1 to 3 months after discharge. The results showed a 25% reduction in RHR in the observation group. A randomized clinical trial included 73 patients (58 ± 11 years) with coronary artery disease, NYHAI-II patients [94]. The ET modality was circuit ergometry, with 40 minutes per session, 3 times per week in the experimental group (24 sessions over 2 months). The results showed that High-Intensity Interval Training (HIIT) was more suitable for low-risk chronic patients with ischaemic heart disease. The results showed that HIIT was ideal for lowrisk chronic patients and improved post-exercise HR recovery. A total of 60 patients with CHF (NYHA II-III) aged 62.9 ± 4.13 years were trained on an exercise plank at 60% reserve HR for 12 weeks in the experimental group [95]. The study found that: starting at 20% of the reserve HR and finally reaching 60% of the reserve HR, performed the training.3 times a week for 12 weeks. Cai et al. studied 65 patients with CHF, and the observation group underwent home ET based on pharmacological treatment [96]. The symptom-limited continuous cycling load increment method was used, with load amplitude increments of 10-24 W/min, and patients maintained 60-65 r/min for the cycling exercise test until maximal HR occurred. The results found that the anaerobic sub-threshold HR control group decreased by 9% and 7% before and after the intervention. For patients in cardiac function classes II-III with appropriate exercise at 60% HR reserve, their RHR can be reduced by 15-25% and their maximum Peak HR by 9%.
BNP: Brain Natriuretic Peptide is one of the markers of myocardial injury and is commonly used to assess the degree of myocardial injury in patients with CHF [97]. In 82 patients with HFpEF and normal or mildly abnormal left ventricular systolic function (left ventricular ejection fraction, the exercise therapy intervention in the observation group from slow to moderate walking speed for about 30 min per day, and compared the two groups' treatment effects after 6 months [94]. The results were significantly better than those of the control group, with BNP decreasing by 28.4% in the observation group. Li et al. selected 136 elderly CHF patients given conventional antiheart failure drugs, and the observation group was assigned aerobic ET at peak VO2 [98]. We compared the patient's cardiac function and NT-proBNP levels after 6 months of exercise. After the movement, the observation group had a 40% drop in NT-proBNP levels. A study included 80 patients with CHF (NYHA class II-III) and the experimental group was given individualized exercise rehabilitation based on pharmacological treatment, with individualized cardiac function and Metabolic Equivalents (METs)={[speed (m/min) 0.1]+[incline (expressed as a decimal) speed (m/min) 1.8]+3.5}/3.5 according to the patients' different, the exercise rehabilitation program was as follows: (1) NYHA II while the target metabolic equivalent was 4-5w, twice a day, walking 500m each time and going up one flight of stairs (ladder height 9cm); (2) NYHA class II while the target metabolic equivalent was 5-6w, twice a day, walking 500m each time and cycling 10min each time; (3) NYHA class III metabolic equivalent was 2-3w and the rehabilitation exercise modality was 10min of bedside standing and 10min of indoor walking per day; (4) NYHA cardiac function class III metabolic equivalent of 3 to 4w, twice a day, walking 250m each time and going up one flight of stairs (ladder height 9cm) [99] (Table 1).
| Movement style | NYHA | Method of exercise (frequency; duration) | Results | References |
|---|---|---|---|---|
| Power bikes | Class I-III | Method I (phased approach): Weeks 1-2: 30% peak VO2 power cycling, 30min each, 3 times a week for 2 weeks. Weeks 3-8: 50% peak VO2 power cycling, 30min each time for 6 weeks; | After 8 weeks, BNP decreased by 44%, 6MWT increased by 48% | [58] |
| Class II-III | Method II (Grading of Cardiac Function): Power cycling for 40 min/d for NYHA II: (a warm-up for 2 min, followed by a gradual increase to Δ50% W over 5 min1) maintained for 30 min, followed by a reduction in exercise power to 0 over 2 min, ending with 1 min of empty pedaling). | 6MWT and METmax increased by 70% and 29.1%, respectively | [59] | |
| NYHA III: 15 min/d initially, followed by an additional 5 min/d per week as appropriate, Δ50% W for 30 min, and the rest as for NYHA cardiac class II, 5 times/week for 3 months. | ||||
| Method III (eccentric cycle method): ECC training power is increased to 70% of maximum capacity for 5 minutes. Rotation speed of 40 rpm with resistance adjustments every 30 seconds, followed by 3 minutes of recovery time, keeping the legs rotating without resistance for one week of training. | PeakVO2 and RER were reduced by 12.7% and 4.1%, respectively, in the eccentric bicycle, compared with the concentric cycle | [60] | ||
| Resistance training | Class II-III | Method I (resistance training + brisk walking): 50%-60% of single maximal load test (IRM) continuous training for 8 weeks + walking 60-80min/day | PeakVO2 was increased by 35%, and LVEDD was decreased by 9.6%. | [64] |
| Method II (regular ET combined with exercise planking): The starting HR is 50% reserve HR, gradually increasing to 60%-70% reserve HR, calculated as 50% reserve HR2). A cardiac exercise test measures maximum exercise HR and RHR. The exercise duration was 30-60 min, and training was performed twice a week for 12 weeks. | NT-pro BNP levels decreased by 28%, and the Max HR raised by 16%. | [65,66] | ||
| Method III (phased approach): Weeks 1-4 focus on endurance exercise, with training consisting of walking and jogging for 20min 3 times a week. 5-8 weeks focus on resistance and muscle strengthening exercises, including walking, jogging and aerobics for 30min 5 times a week. 32 exercise sessions in total for 8 weeks. |
6MWT was increased by 5%. RHR, NYHA classification and LVEF were all positively affected. | [67] | ||
| Method IV (Grading of Cardiac Function): Class II: Single arm curl training, gastrocnemius resistance training, knee lift + leg abduction training, lunge front planks training, prone leg curl resistance training, 20-30 reps per set, completed in 30min, 3 times/week, 5-10min in the closing phase, with appropriate stretching. | After 12 months of training, 6MWT increased by 22%, and BNP decreased by 34.7% | [68] | ||
| Class III: Single arm curl training, gastrocnemius resistance training, standing leg abduction training, 10-20 reps per set, completed in 10-20min, practiced 2 times/week. The intensity of the exercise is gradually reduced, and stretching is performed. | ||||
| Tai Chi | Class I-III | Method I (Tai Chi+Rehabilitation): Rehab at least 5 times a week for 24 weeks. Rehabilitation exercises with 24 simplified Tai Chi, 5 times/week for 60 min. A target HR of 120 beats per minute will terminate the activity. | 25.6% increase in 6MWT and BNP decrease of 58% levels. | [75] |
| Method II: TaiChi: Warm up for 5 to 10 min in the preparation phase. Slow exercise to target exercise intensity 3; exercise duration of approximately 30 min each, 3-5 times per week, with a closing stage of 5-10 min of stretching for 12 weeks. | The results showed a 3% increase in LVEF and a 35.5% decrease in pro-BNP. 6MWT increased by 10%. | [78] | ||
| Method III (Cardiac function grading method): In Level II, the patients walked 500 m/d indoors; in Level III, the patients moved and stood at the bedside for 6 min, 4 times/d. The patients performed twenty-four simplified TaiChi exercises, including preparatory exercises, training and finishing exercises, 25 min/time, 2 times/d. Both groups continued the intervention for 2 months. | The results found a 17.7% increase in VO2Peak, a 34% increase in AT and a 43% decrease in BNP. | [79] | ||
| IMT | Class II-III | Method I (MICT+IMT): (1) Moderate intensity continuous aerobic exercise: 3 times/week for 45min/time to achieve the exercise target HR4. On top of this, respiratory muscle training 3 times a week, including (1) lip retraction breathing training: patient lying flat or sitting, relaxed, inhale slowly and deeply with maximum effort, mouth into a whistle-like, then exhale slowly, breathing time ratio of 2-3:1, repeat training, 10-15min/d; (2) abdominal breathing: patient lying supine, relaxed, inhale slowly through the nasal cavity, therapist place the palm on the patient (3) abdominal breathing: the patient is lying supine, relaxed, inhaling slowly through the nose, the therapist places the palm under the patient's rib cage to feel the rise and fall of the thorax, exhale slowly with the lips contracted, repeat the training, 10-15 min/d. The above last for 12 weeks. 2) Moderate aerobic ET for 60min, 3 times a week for 4 months and IMT for 30min, 5 times a week for 4 months. | VO2 Peak increased by 8.3% | [84] |
| Decreased MSNA in patients. Increased forearm blood flow and 12% increase in Peak VO2 in the observation group in the aerobic training group. 12% increase in Peak VO2 and 10%-20% increase in 6MWT. | [83] | |||
| Class I-III | Method II (ventilator training): IMT for 30min, during which the patient maintains diaphragmatic breathing at a rate of 15-20 breaths/min, 7 times/week for 12 weeks | EF and 6MWT increased by 3% and 4%, respectively. | [82] |
Table 1: CHF patients can take the appropriate exercise, exercise method, frequency and evaluation index.
1. Δ50% W=(anaerobic threshold measured power-incremental power rate × 0.75)/2+(extreme exercise measured power-cumulative power rate × 0.75)/2;
2. 50% reserve HR=RHR+(maximal exercise HR-RHR) × 0%; 3. Exercise intensity: target HR (%)=(max HR-RHR) × (30%-60%)+RHR, combined with a
Borg score of 11-13 for maximum exercise intensity; 4. Target HR (beats/min)=HR at anaerobic valve-10.
Exercise rehabilitation can reduce BNP levels in patients with CHF (NYHA II-III) to varying degrees, and the moderate exercise intensity (50% of peak power) can be reduced by as much as 30% to 40%. In addition, appropriate metabolic equivalents depending on the level of cardiac function, can be used as a targeted exercise rehabilitation programme.
This article reviews ET and the impact on prognosis related to the development of CHF, addressing the role of exercise in CHF through three questions: the causes of exercise tolerance in CHF patients, the causes of exercise intolerance, and the type of exercise to be undertaken, and the indicators to be used to assess the appropriate and suitable exercise rehabilitation for CHF patients. One point to note is that exercise tolerance and exercise tolerance are inter-convertible and regular exercise is safe in CHF patients with left ventricular systolic dysfunction in stable phase (NYHA I-III), improving four indices including 6MWT, peak VO2, BNP, HR. Additional histological techniques are also expected to identify which patient subgroups may benefit most from exercise and determine the optimal intensity, duration and frequency of movement required to maximize clinical benefit and reduce fatigue. Finally, improving patient compliance is necessary to ensure that as many patients as possible benefit and further research are needed to determine the extent to which ET are useful in treating CHF. ET will provide a rich clinical basis for clinical treatment.
Not applicable
The authors declare that there is no conflict of interest.
Not applicable
Not applicable
Not applicable
Not applicable
Not applicable
These authors contributed equally to this work.
Thanks to Dr Guo for her insightful suggestions, valuable comments on this article, and all the authors' support and efforts.
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
Citation: Jiaqi Z, Yuli W, Yun D, Qiaoling C, Shuangcui W, Jingfang L, et al (2023) Application of Exercise Rehabilitation in Patients with Chronic Heart Failure: A Review. J Clin Exp Cardiolog.14:773.
Received: 06-Mar-2023, Manuscript No. JCEC-23-22047 ; Editor assigned: 10-Mar-2023, Pre QC No. JCEC-23-22047 ; Reviewed: 27-Mar-2023, QC No. JCEC-23-22047 ; Revised: 03-Apr-2023, Manuscript No. JCEC-23-22047 ; Published: 11-Apr-2023 , DOI: 10.35248/2155-9880.23.14.773
Copyright: ©2023 Jiaqi Z, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.