Medicinal & Aromatic Plants
Open Access
ISSN: 2167-0412
ISSN: 2167-0412
Research Article - (2021)Volume 10, Issue 8
Considering the limitations of antivenom therapy towards toxicity following envenomation, investigators are probing for effective phytochemicals with promising antivenom effects. In the current study, Cardiospermum halicacabum leaf and callus methanol extracts were investigated for their ability to neutralize Indian cobra (Najanaja) venom. Soxhlet extraction using methanol was performed since leaf and callus extracts demonstrated significant neutralization of N. najavemon in preliminary studies. In vitro neutralizing potential of both extracts were evaluated against phospholipase A2, preoteases and hyaluronidases. In addition, alleviation of N. naja-induced characteristic edema inducing activity, myotoxicity and coagulation, hemolytic activity were performed in mouse models and human blood samples. Leaf and callus methanol extract contained high concentrations of phenols, alkaloids, steroids and saponins and demonstrated significant in vitro inhibition of N. naja PLA2, proteases and hyaluronidase activities at 30-40 µg concentrations. Significant neutralization of N. naja-induced indirect hemolysis, edema, anticoagulant effect and myotoxicity was attained using both extracts. Overall leaf and callus extracts (1mg/kg) postponed animal survival by 33 ± 9.3 and 50 ± 12.4 mins, respectively. C. halicacabum leaf and callus methanol extracts demonstrated significant neutralization of N. naja enzymes that contribute to grievous effects on various tissues/organs. The study scientifically supports the anecdotal use of C. halicacabum in complementary medicine and demonstrates that callus extract is more potent antidote than leaf. Further, callus extract is a potent candidate for isolation of phytochemicals towards management of snakebite.
Najanaja; Cardiospermum halicacabum; Protease; Hyaluronidase; PLA2; Callus extract
Snake venom is a complex mixture of many substances, such as toxins, enzymes, growth factors, activators and inhibitors with a wide spectrum of biological activities [1,2]. They are also known to cause different metabolic disorders by altering the cellular inclusions and enzymatic activities of different organs [3]. The outcomes of venom toxicity include nephro, neuro and cardiotoxicities, respiratory and circulatory collapse, necrosis, hemorrhage and edema [4,5]. Till date the most effective therapy against snakebite available is the antisnake venom (ASV) therapy and has appreciably reduced snakebite mortality rate [6]. However, ASV protects effectively againstsystemic venom-induced toxicity, it may not reach the local area of snakebite where focal necrosis occurs, and causes hypersensitivity problems [7-9]. It has been estimated that 5 million snake-bite cases occur worldwide every year, causing about 100,000 deaths. On an overage, nearly 2,00,000 persons fall prey to snake-bite per year in India and 35,000-50,000 of them die every year [10,11]. Despite the success of ASV, it is important to search for small molecules that are relatively non-toxic and effective inhibitors, which would complement the action of ASV, in particular neutralization of local tissue damage. Moreover, ASV have some disadvantages such as adverse reactions ranging from mild symptoms to serious (anaphylaxis) thus limiting their efficient use [12-14].
Medicinal plant extracts constitute an extremely rich source of pharmacologically active compounds [15] which can be confirmed by the successful use on snakebite wounds [16-18] as first aid treatment, which is particularly important in local areas where ASV are not readily available [14,19-22]. Thus, complementary therapeutics needs to be investigated, with plants being considered as a major source [23]. The exact mechanisms of action of the plant extracts remain largely underdetermined. However, a number of previous reports indicate that plant- derived compounds, such as rosmarinic acid [24,25], quercetin [26] and glycyrrhizin [27] have been shown to inhibit biological activities of some snake venoms in vivo and in vitro. Though in India numerous plants are used in ethnomedical practices as well as in Ayurveda [28], the pharmacological properties of these plants are not sufficiently evaluated for their venom neutralizing activity. One among such plants is Cardiospermum helicacabum Linn. (Sapindaceae), known as Karnasphota in Ayurveda. It is a herbaceous twiny climber found throughout India and used for the treatment of rheumatism, lumbago, earache and fever in Ayurveda and folk medicine [29,30]. C. helicacabum is also being used in India, China and South Africa against snake bite. Earlier, Chopra et al. [31] suggested its use in rheumatism, stiffness of limbs and snakebite. However, its usage in snakebite has not been systematically evaluated. The antivenin property thought to be attributed to wide variety of metabolites with diverse chemical structures. The extraction of these chemical compounds is often tedious and costly with sacrificing living plants. It is of the view that due to their excellent medicinal properties, plants have suffered constant extraction. This has lead to endangered perpetuation of native species to extinction, whose pharmacological activity is already confirmed or whose properties are still under investigation [23]. The use of plant cell culture methodology appears to be the best alternative solution, without sacrificing the whole plant. Kutney, [32] reported that plant cell culture achieves metabolite production significantly higher than in the living plant. There by we can obtain the required plant parts or metabolites without the sacrifice of medicinally important plants [23,33-36] . In this line, present study reports the comparative neutralization of N. naja venom using methanol extracts of C. halicacabum leaf and its callus.
Reagents
[14C] Oleic acid was obtained from Perkin Elmer life sciences Inc. Boston MA, USA. Fat free Bovine Serum Albumin (BSA) fraction V purchased from PAA Laboratories GmbH Haidmannweg, Austria. Casein, hyaluronic acid and Escherichia coli (lyophilized cells of strain W (ATCC 9637)) were purchased from sigma chemical laboratories, St. Louis, MO, USA. Lactate Dehydrogenase (LDH) and Creatine Kinase (CK) kits were purchased from Span Diagnostic Center, Bangalore, India. All other chemicals and reagents were of analytical grade. Blood samples for hemolytic activity and coagulant activity were obtained from the healthy volunteers following their consent.
Preparation of extract
Healthy, disease free mature leaves were collected from the university campus, Mysore, Karnataka (India) and used for the preparation of the extract. A voucher specimen of plant has been deposited in herbarium of Department of Studies in Botany, University of Mysore (Voucher no: HVG0311). About 10 g of dried C. helicacabum leaf and its callus powder separately were pretreated with petroleum ether in Soxhlet apparatus. Petroleum ether fractions were discarded and further extraction was carried out using methanol until the colorless extract was obtained. The resultant extract was concentrated and used as crude extract for neutralization studies.
Qualitative organic analysis
Qualitative organic analysis was carried out following standard procedures Indian pharmacopoeia by Anonymous [37], and Harborne [38].
Animals and venom
Swiss Wister mice (male/female) weighing about 20-25 g were obtained from the central animal house facility, Department of studies in Zoology, University of Mysore, Mysore, India. The animal care and handling were conducted in compliance with the national regulations for animal research. The animal experiments were carried out after reviewing, the protocols by the animal ethical committee of the University of Mysore. The lyophilized Najanaja venom was obtained from Haffkin`s Institute, Bombay, India and was preserved at 4°C. Before use, venom was dissolved in saline, centrifuged at 2000 × g for 10 min and the supernatant was used for antivenom studies and kept in freezer for further use. Protein concentration of venom was estimated according to the method of Lowry et al. using BSA as a standard [39].
Neutralization of lethal effects of N. naja venom
The median lethal dose (LD50) of N. naja venom was determined by injecting different concentrations of venom in a constant volume of 0.2 mL saline through i.p. The mice were constantly observed for symptoms/ signs of neurotoxicity and the survival time were recorded. The calculations were done according to the method of Meier and Theakston [40]. For venom neutralization studies, the 2 × LD50 was chosen as challenging dose and the potential of both plant and callus extracts were tested. Groups of mice (n=6) were injected with venom (2 × LD50) pre incubated for 15 min at 37°C with different concentration of extract (w/w) of both leaf and leaf derived callus to record the survival time (min).
Phospholipase A2 assay
Phospholipase A2 activity was assayed according to the method of Yamakawa et al. [41] as modified by Vishwanath et al. [42]. Venom sample (2 μg) was added in a final volume of 350 μL containing 5 mM CaCl2 and 100 mM Tris-HCl buffer (pH 7.5) incubated for 1 h in the presence of 30 μL of 14C oleate autoclaved E-coli membrane (equivalent to 64 nmoles of fatty acid). The reaction was terminated by adding 100 μL of 2 N HCl followed by 100 μL of 10% fatty acid free BSA. The samples were thoroughly mixed and centrifuged at 10,000 × g for 5 min at room temperature. An aliquot (140 μL) of the supernatant (containing released 14C Oleic acid) was mixed with scintillation cocktail and counted in a Hewlett Packard liquid scintillation analyzer TRI CARB 2100 TR. Activity expressed as nmoles of fatty acid released / min / mg of protein.
For inhibition studies, venom samples were pre incubated with or without different concentrations of both leaf and its callus extracts at 37°C for 30 min and further assay was carried out as described above. The inhibition is expressed in percentage taking activity of venom alone as 100%.
Neutralization of indirect hemolytic activity
Indirect hemolytic activity was assayed according to the method of Boman and Kalleta. Briefly, packed human erythrocytes, egg yolk and phosphate buffered saline (1:1:8, v/v) were mixed. One mL of this suspension was incubated for 15 min at 37°C with venom (10 μg) or with preincubated (30 min) venom: extract mixture (w/w) of both leaf and its callus extracts. After addition of 9 mL of ice cold PBS, the reaction mixture was centrifuged at 900 × g for 15 min and the released hemoglobin was read at 540 nm. The hemoglobin released in the suspension due to hemolysis of erythrocytes by adding 9 mL of distilled water to the control reaction mixture was taken as 100%.
Neutralization of edema inducing activity
The edema inducing activity was carried out according to the method of Vishwanath et al. [43]. Groups of three mice were injected in the right foot pads with different concentrations of venom in 20 μL saline. The left footpads received saline which, served as controls and the legs were cut at ankle joint after 45 min. The increase in weight due to edema was calculated as edema ratio, which equals the weight of edematous leg × 100/ weight of control leg. Minimum Edema Dose (MED) was defined as amount of protein concentration required causing an edema ratio of 120%. For inhibition studies venom (5 μg) was pre incubated for 30 min at 37°C with or without different concentrations of extracts and assayed for edema inducing activity as described above.
Protease activity
Protease activity was assayed according to the method of Murata et al. [44] using casein as substrate. 0.4 mL of casein (2%) was incubated with 150 μg of venom in a reaction volume of 1mL containing 0.2 M Tris-HCl buffer (pH 8.5) at 37°C for 2 h. The reaction was stopped by adding 1.5 mL of 0.44 M TCA and allowed to stand for 30 min to precipitate the undigested casein. The tubes containing reaction mixture were then centrifuged at 1500 × g for 15 min. An aliquot (1 mL) of the supernatant was mixed with 2.5 mL of 0.4 M sodium carbonate and 0.5 ml of folin reagent (1:2, v/v) and the color developed was read at 660 nm. One unit of enzyme activity was defined as the amount of enzyme required to increase an absorbance of 0.01 at 660 nm/h at 37°C.
For inhibition studies, venom samples were pre incubated with and without different concentration of extracts at 37°C for 30 min and further assay was carried out as described above. The inhibition is expressed in percentage inhibition taking activity of venom alone as 100%.
Hyaluronidase assay
Hyaluronidase activity was assayed by estimating the amount of N-acetyl glucosamine released according to the method of Ressign et al. [45]. Venom sample 200 μg was incubated with 50 μg of hyaluronan in 0.2 M Sodium acetate buffer (pH 6.0) and 0.15 M NaCl for 2 1/2 h at 37°C. The reaction was terminated by adding 50 μL of potassium tetra borate buffer (pH 9.1) followed by 1.5 mL of p-DMBA (1%) and the tubes were boiled in water bath for 3 min and the color developed was monitored at 585 nm. Activity was expressed as nmoles of N-acetyl glucosamine released /min/ mg protein.
For inhibition studies, venom samples were pre incubated with or without different concentration of extracts at 37°C for 30 min and evaluated for hyaluronidase activity. The inhibition is expressed in percentage inhibition taking activity of venom alone as 100%.
Neutralization of myotoxic effect of N. naja venom
Myotoxic activity of N. naja venom was determined by measuring serum levels of Creatine Kinase (CK) and Lactate Dehydrogenase (LDH) activities. Half the LD50 of N. naja venom (0.6 mg/kg body weight) was injected (i.m) into three groups of five mice each. Group I received 0.6 mg/kg body weight of venom alone in 50 μL of PBS. Group II received same amount of venom pre incubated for 30 min with different concentrations of extract (w/w) of leaf and callus and group III mice were injected with 100 μg of leaf and callus extracts alone. Mice from each group were anesthetized (Pentobarbitone 30 mg/kg, i.p) after 3 h and blood was collected by puncturing the heart and allowed to clot for 30 min. The collected blood samples were centrifuged at 2,000 × g for 15 min. The serum was diluted 1:25 times with saline and was used for the determination of CK and LDH values according to the method of Huges [46] and King [47] as provided in Auto Span diagnostics kit. Activity is expressed as units / liter.
Neutralization of coagulant activity
Recalcification time was determined according to the method of Condrea et al. [48] Fresh human blood was mixed with 0.11 M trisodium citrate (9:1, v/v). The mixture was centrifuged for 30 min at 500 × g and the supernatant obtained was used as Platelet Poor Plasma (PPP). To 300 μL of PPP, 30 μL of 10 mM Tris-HCl buffer (pH 7.4) was added and pre-warmed for 15 min at 37°C. To this plasma, 3 μg venom was added and clotting time was recorded (sec) after addition of 30 μL of 0.25 M CaCl2. For inhibition studies, venom samples were pre incubated with or without different concentrations of extracts (w/w) for 30 min at 37°C.
Statistical analysis
The results of experiments were expressed as mean ± SEM (n=3). Statistical analysis was carried out using Student's t-test. The comparison between the groups was considered significant if p ≤ 0.05. Data were analyzed using the statistical package Graph Pad Prism® (La Jolla, USA).
About 10 g dried powder of C. halicacabum leaf and its derived callus were used for extraction in Soxhlet apparatus. 10 g of each dried powder yielded 5.12% and 6.3% of the leaf and callus extracts in dry weight, respectively. Preliminary phytochemical analysis is being carried in both leaf and callus extracts is presented in Table 1. The result of preliminary phytochemical analysis shows that the plant is rich in chemical bases as steroids, phenolic compounds/ tannins, saponins and alkaloids. The presence of these secondary metabolites in plant is responsible for their usefulness as medicinal plant. N. naja venom is highly lethal with LD50 (i.p) of 1.2 mg/kg body weight of the mouse. Both leaf and its derived callus extracts at 1 mg significantly increased the survival time of the mice upon co-injection of different ratios of venom: extract (w/w). Leaf extract postponed the death time by 33 ± 9.3 min. On the other hand, callus extract was comparatively more potent and postponed the survival time by 50 ± 12.4 min (Table 2).
| Test for | Methanol Extract | |
|---|---|---|
| Plant | Callus | |
| Carbohydrates | + | + |
| Glycosides | + | + |
| Reducing sugars | - | - |
| Steroids | + | + |
| Phenolic compounds/tannins | + | + |
| Alkaloids | + | + |
| Saponins | + | + |
| Volatile oils | - | - |
| Gums & mucilage | - | - |
| Oils and fats | - | - |
Table 1: Preliminary phytochemical analysis of C. halicacabum leaf and its callus.
| Group | Treatment | No. of mice used | Death time |
|---|---|---|---|
| I | 2LD50 | 10 | 96.4 ± 12.9 |
| II | 2LD50+ Leaf extract (1 mg) | 10 | 129.1 ± 6.3 |
| III | 2LD50 + Callus extract (1 mg) | 10 | 146.5 ± 8.4 |
Table 2: Effect of C. halicacabum extracts on the lethal toxicity of N. naja venom.
The major lethal fractions of N. naja venom are PLA2s, which exerts various pharmacological actions in the victim as well as in experimental animals. Both leaf and callus extracts significantly inhibited the PLA2 activity. As in the case of lethality studies, callus extract was more potent in its action than the leaf extract. Leaf extract exerts 73 ± 4 % of inhibition at 40 μg/ 350 μL and the callus extract inhibited the venom PLA2 activity by 96 ± 2.3 % at the same amount of extract used (Figure 1). As PLA2 exhibits edema inducing activity, indirect hemolytic activity, anticoagulant activity and myotoxicity in vivo, the inhibition studies of these activities is beneficial in order to know the potency of the extracts. C. halicacabum leaf and its callus extracts neutralized the indirect hemolytic activity in a dose dependent manner. Indirect hemolytic activity was neutralized up to 75 % and 87 % by leaf and callus extracts, respectively at a maximum concentration of extract used (1:8, venom: extract w/w) (Figure 2A). Further, the extract of both leaf and callus neutralized the edema inducing activity of N. naja venom. However, the neutralization of edema induced by venom required higher concentration of extract compare to indirect hemolytic activity. The injection of 5 μg of N. naja venom into the mouse foot pad induces an edema ratio of 175 ± 8.8. Injection of pretreated venom with both leaf and its callus extracts reduce the edema ratio to 126 ± 1.5 and 113 ± 3.2, respectively at maximum concentration of extract tested (Figure 2B).
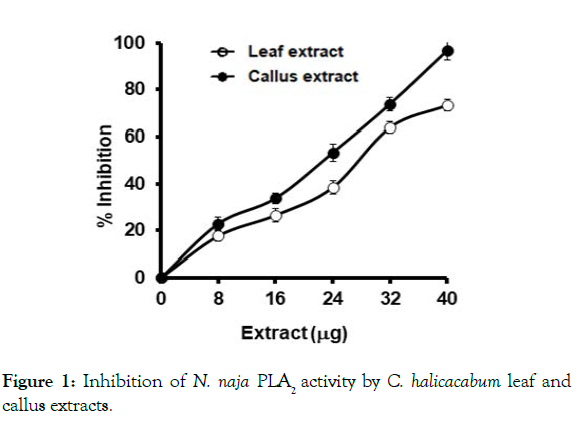
Figure 1: Inhibition of N. naja PLA2 activity by C. halicacabum leaf and
callus extracts.
The reaction mixture, 350 μL containing 2 μg of venom, 5 mM CaCl2 and
extracts pre-incubated for 30 min at 37°C. The PLA2 reaction was initiated
by adding 30 μL of substrate and incubated for 1 h. The percent inhibition
was calculated as described in Methods section. Data represents mean ±
SEM (n=4).
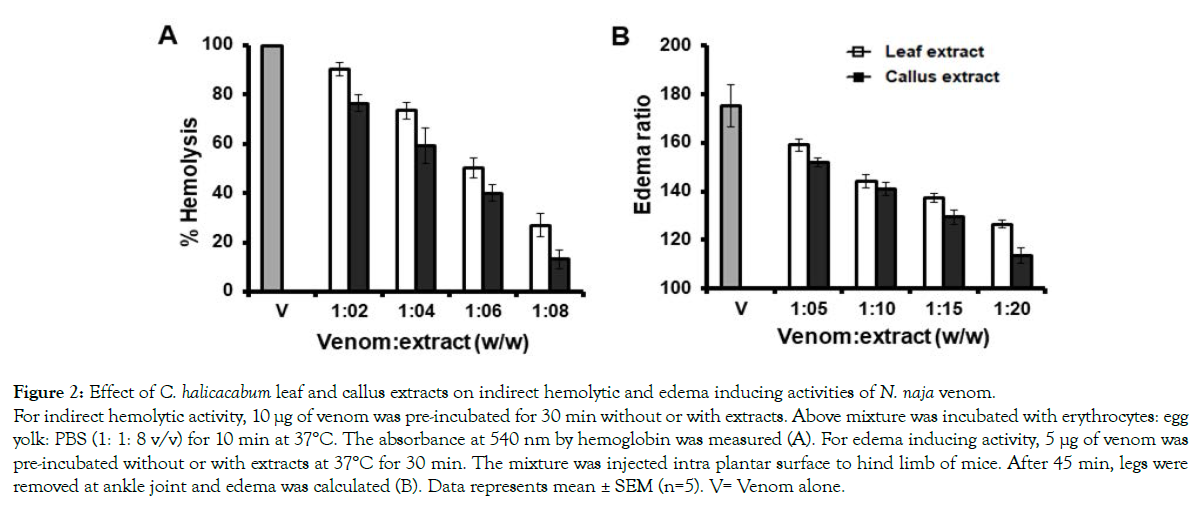
Figure 2: Effect of C. halicacabum leaf and callus extracts on indirect hemolytic and edema inducing activities of N. naja venom.
For indirect hemolytic activity, 10 μg of venom was pre-incubated for 30 min without or with extracts. Above mixture was incubated with erythrocytes: egg
yolk: PBS (1: 1: 8 v/v) for 10 min at 37°C. The absorbance at 540 nm by hemoglobin was measured (A). For edema inducing activity, 5 μg of venom was
pre-incubated without or with extracts at 37°C for 30 min. The mixture was injected intra plantar surface to hind limb of mice. After 45 min, legs were
removed at ankle joint and edema was calculated (B). Data represents mean ± SEM (n=5). V= Venom alone.
Although N. naja venom contains less concentrations of locally acting enzymes such as protease and hyaluronidase, they potentiate the distribution of systemic toxins into the circulation [49]. At a concentration of 30 μg/mL of both leaf and callus extracts, proteolytic activity caused by 150 μg of venom was inhibited upto 70 % and 86 %, respectively (Figure 3A). The extracts also inhibited the hyaluronidase activity in dose dependent manner and maximum inhibition of 56 ± 1.6 % and 73 ± 3.2 % was observed for plant and callus extract at 30 μg (Figure 3B). Since, N. naja venom has less concentration of protease and hyaluronidase it does not induce significant hemorrhage. However, N. naja venom induces significant myotoxicity by increasing the levels of serum CK an LDH in mice. Injection of 0.6 mg /kg body weight of venom into mice (i.p) elevated serum CK level from 500 ± 73 U/L to 1780 ± 130U/L and LDH values from 1255.7 ± 120 U/L to 6640 ± 217 U/L. Both leaf and its derived callus extract dose dependently inhibited the CK release in mice (Figure 4A). At the maximum concentration of extracts used, almost 90 ± 2.6 % of inhibition by leaf extract and 97.7 ± 1.9 % by callus extract were attained. Similar pattern of inhibition was observed in venom-induced serum LDH level in mice whereby, 92 ± 2.2 % and 99 % ± 1.5 of inhibition was observed for leaf and callus extracts, respectively (Figure 4B).
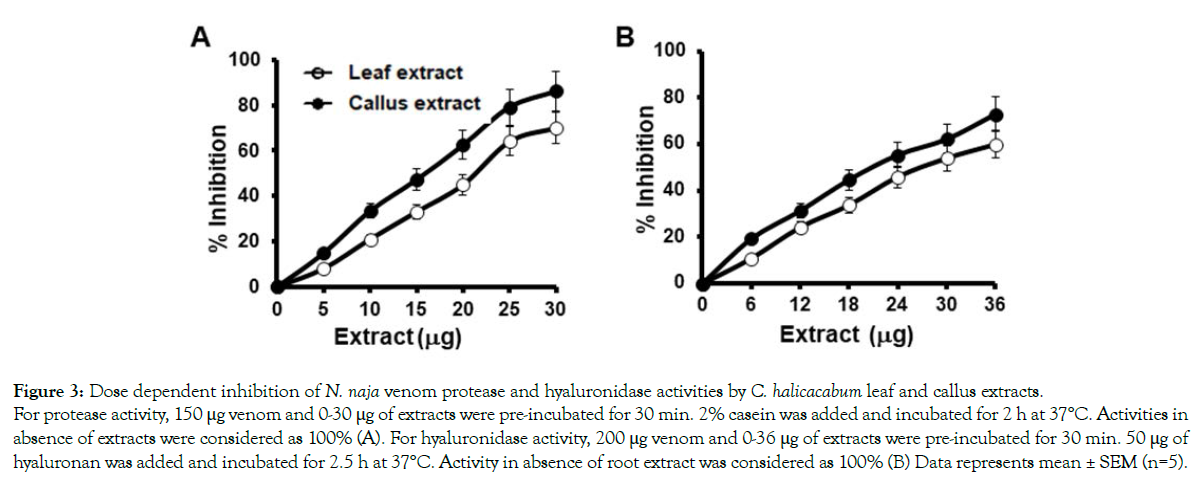
Figure 3: Dose dependent inhibition of N. naja venom protease and hyaluronidase activities by C. halicacabum leaf and callus extracts.
For protease activity, 150 μg venom and 0-30 μg of extracts were pre-incubated for 30 min. 2% casein was added and incubated for 2 h at 37°C. Activities in
absence of extracts were considered as 100% (A). For hyaluronidase activity, 200 μg venom and 0-36 μg of extracts were pre-incubated for 30 min. 50 μg of
hyaluronan was added and incubated for 2.5 h at 37°C. Activity in absence of root extract was considered as 100% (B) Data represents mean ± SEM (n=5).
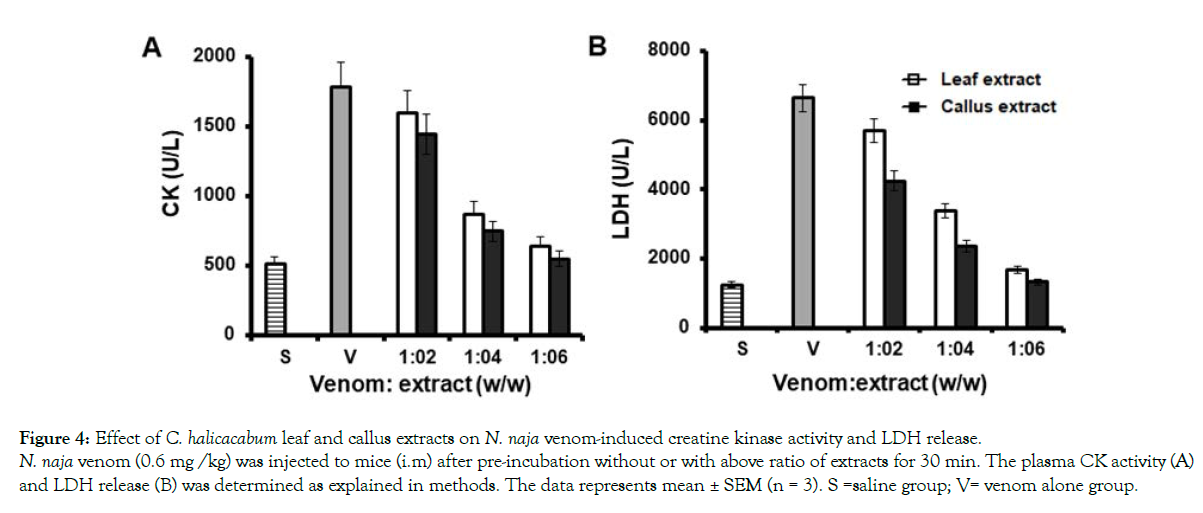
Figure 4: Effect of C. halicacabum leaf and callus extracts on N. naja venom-induced creatine kinase activity and LDH release.
N. naja venom (0.6 mg /kg) was injected to mice (i.m) after pre-incubation without or with above ratio of extracts for 30 min. The plasma CK activity (A)
and LDH release (B) was determined as explained in methods. The data represents mean ± SEM (n = 3). S =saline group; V= venom alone group.
Apart from these pharmacological actions, N. naja venom also exhibits anticoagulant effect. N. naja venom at concentration of 3 μg increases the clotting time from 175 to 271 sec. The anticoagulant activity of N. naja venom was neutralized by the extracts of both leaf and callus by 212 and 204 s, respectively at a ratio of 1:16 (venom; extract, w/w) (Figure 5). However, it was observed that none of the extracts neutralized the clotting time induced by Najanaja venom completely.
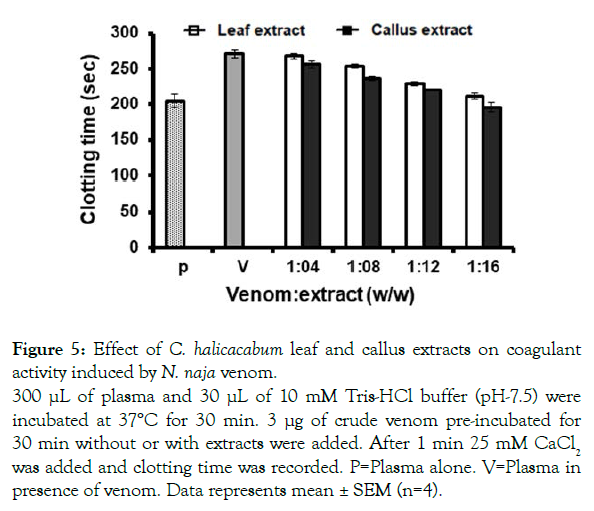
Figure 5: Effect of C. halicacabum leaf and callus extracts on coagulant
activity induced by N. naja venom.
300 μL of plasma and 30 μL of 10 mM Tris-HCl buffer (pH-7.5) were
incubated at 37°C for 30 min. 3 μg of crude venom pre-incubated for
30 min without or with extracts were added. After 1 min 25 mM CaCl2 was added and clotting time was recorded. P=Plasma alone. V=Plasma in
presence of venom. Data represents mean ± SEM (n=4).
Plant extracts constitute rich sources of novel compounds with several pharmacological activities. In many countries, plant extracts have been traditionally used in the treatment of snakebite. The plants with anti-venom activity, protecting humans and animals against systemic envenomation have been demonstrated [23,50]. As the natural habitat for wild plants are becoming endangered and environmental and geopolitical metabolites make it difficult to acquire certain plant-derived chemicals. It may become critical to develop alternative sources of important natural secondary constituents without sacrificing the environment [23]. There has been considerable interest in investigating the potential of plant cell tissue culture as an alternate to traditional agriculture for the industrial production of secondary plant metabolites [33,35,36].
Several reports suggest that plant cell tissue culture is an attractive alternative source to whole plant for the production of high value secondary metabolites without altering the pharmacological activities exerted by natural plant extracts [23,34,51-53]. As C. halicacabum plant has been reported for its anti-venom activity, in this study an attempt was made to systematicinvestigate the neutralization of the lethal and toxic effects of N. naja venom.
N. naja venom is highly lethal and constitutes high content of systemic toxins with little locally acting enzymes. Although ASV is the only therapeutic agent available to treat N. naja envenomed victims, its effectiveness is limited by several side effects and unavailability to many people who live far from hospitals [54]. In areas where no ASV treatment is immediately available or preparation of plants could play an important role by effectively bridging the time until qualified support is available. Although treatment of snakebite with plant extracts does not completely neutralize the toxicity, the lethality is often reduced and by the time victims reach the hospital for treatment [11]. The pharmacological and toxicological properties of snake venom are mainly associated with proteins and peptides, particularly enzymes. C. halicacabum leaf and its callus extracts prolong the death time of experimental animals injected with N. naja venom (Tables 1 and 2). The most lethal fractions of N. naja venom are PLA2 and peptide toxins. Apart from increasing the survival time of the mice and inhibition of PLA2 action in vitro and in vivo, it also inhibited the action of locally acting enzymes like proteases and hyaluronidases. N. naja venom has less content of proteases and hyaluronidases compared to viper venoms. However, their presence plays very important role in spreading of systemic toxins leading to death of the victims [55]. Apart from damaging local tissue in the victim, N. naja venom proteases also interfere in hemostasis and exert anticoagulant effects [56]. Comparatively, callus extract is more potent than leaf extract in antagonizing the action of N. naja venom. C. halicacabum is rich in secondary metabolites such as saponins, tannins, apigenin, proanthocyanidin, stigmosterol and traces of alkaloids [29]. Several studies demonstrate tannins, saponins and alkaloids have ability to neutralize venom action [43].
• C. halicacabum leaf and callus methanol extracts postponed the survival time of mice injected with lethal dose of N. naja venom.
• Both C. halicacabum leaf and callus methanol extracts significantly inhibited toxic PLA2, protease, hyaluronidase activities and anticoagulant property of N. naja venom.
• Both C. halicacabum leaf and callus methanol extracts neutralized edema inducing activity of N. naja venom in mice.
• C. halicacabum leaf and callus methanol extracts significantly neutralized N. naja venom-induced myotoxicity.
• Comparatively, callus extract exhibited more potent antiophidian activity than leaf extract.
Since C. helicacabum is rich in these metabolites, they might have a role in the neutralization of N. naja venom. Comparatively callus extract showed a potent anti-venom activity than leaf extract, suggesting plant cell culture potentiates the production of these secondary metabolites having anti-snake venom activity. Isolation of these metabolites using callus extract will be of an immense therapeutic application in neutralizing snake venom and also as anti-inflammatory drugs.
Authors thank University of Mysore for providing facilities to conduct experiments. We thank central animal facility, Department of Studies in Zoology, University of Mysore for providing animals for in vivo experiments.
The authors have not declared any conflict of interests.
Citation: Girish HV, Nataraju A, Rajaiah R, Vishwanath BS, Sudarshana MS, Reshi NA (2021) Appraisal of Antiophidian Properties of Cardiospermum halicacabum L. Leaf and Callus Extracts against Naja Naja Venom. Med Aromat Plants (Los Angeles) 10: 399.
Received: 16-Apr-2021 Accepted: 09-Aug-2021 Published: 16-Aug-2021 , DOI: 10.35248/2167-0412.21.10.399
Copyright: © 2021 Girish HV, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.