
Journal of Clinical Toxicology
Open Access
ISSN: 2161-0495

ISSN: 2161-0495
Review Article - (2021)
Objective: Poisoning from toxic alcohols (methanol, ethylene glycol, diethylene glycol and isopropanol) occurs globally and results in significant morbidity and mortality. Consequently, toxic alcohol poisoning requires prompt diagnosis and management by healthcare workers at the acute presentation. As such, this narrative review aims to provide a practical approach to diagnosis and management of toxic alcohol poisoning.
Methods: PubMed and Scopus databases were searched for studies and clinical practice guidelines on the diagnosis and management of toxic alcohol poisoning.
Results: The osmolar gap, arterial pH, anion gap and serum alcohol concentrations should be evaluated in suspected cases of toxic alcohol poisoning. Gastrointestinal decontamination has limited efficacy due to rapid gastrointestinal absorption of toxic alcohols. Following acute resuscitation and stabilization, treatment modalities include Alcohol Dehydrogenase (ADH) inhibitors, Renal Replacement Therapy (RRT) and correction of metabolic derangements. ADH inhibitors are contraindicated in isopropanol poisoning as they delay its metabolism and clearance. Sodium bicarbonate as well as formic or folinic acid are recommended in methanol poisoning. Thiamine and pyridoxine are recommended in ethylene glycol poisoning.
Conclusion: Clinicians in the acute care setting should exercise a low threshold of suspicion for toxic alcohol poisoning due to the non-specific clinical features at presentation. Timely diagnosis is treatment is critical to minimize mortality and morbidity.
Methanol; Ethylene glycol; Diethylene glycol; Isopropanol; Toxic alcohol poisoning
Early resuscitation and stabilization should be initiated in all patients with toxic alcohol poisoning [10,22,53]. Central nervous depression impairs airway reflexes and obtunds the respiratory drive leading to alveolar hypoventilation with consequent hypercarbia, hypoxia and respiratory failure. Intubation and mechanical ventilation should be considered in the presence of Glasgow Coma Score below 8, actual or impending respiratory failure and severe acidosis [54]. All toxic alcohols depress myocardial contractility and cause vasodilation resulting in hypotension which increases the risk of mortality [55]. Fluids, inotropes and vasopressors should be administered as necessary to maintain hemodynamic stability [56]. Gastrointestinal decontamination through gastric lavage, whole-bowel irrigation, activated charcoal and Ipecac syrup-induced emesis are ineffective due to the rapid absorption of toxic alcohols from the gastrointestinal tract [57,58]. Although rare, acute seizures on presentation can be terminated with benzodiazepines [10,22]. Benzodiazepines, such as lorazepam, demonstrate superior efficacy at terminating established status epilepticus and can be administered more quickly compared to other antiepileptic’s such as phenytoin and phenobarbitone [59]. Barbiturates, such as thiopentone or phenobarbitone, could be considered for adjunctive therapy in refractory seizures as they potentiate the effects of benzodiazepines by increasing the duration of chloride channel opening at GABA receptors [31,60].
The authors received no other forms of assistance in the research, authorship and publication of this article.
The authors declare no potential conflict of interest with respect to the research, authorship and/or potential publication of this article.
The authors received no financial support for the research, authorship and publication of this article.
Toxic alcohol poisoning presents with non-specific clinical features and often mimics ethanol intoxication necessitating perceptive clinical judgement to avoid a missed diagnosis. In the presence of clinical signs and symptoms, the osmolar gap, anion gap, arterial pH and serum alcohol levels help to support the diagnosis and eliminate differentials. Following initial resuscitation and stabilization, ADH inhibitors can be considered in methanol and ethylene glycol poisoning to attenuate the rate of toxic metabolite formation. RRT can also be considered if clinical deterioration persists with HAGMA despite initiating appropriate therapies. Clinicians should be familiar with local and institutional practice guidelines to initiate timely and appropriate treatment. Despite existing progress, further development and refinement of treatments as well as public education are required to reduce mortality and chronic sequelae from exposure to these toxic alcohols.
Methanol and diethylene glycol
Methanol poisoning has a mortality rate of 8.2%-36.0% and ethylene glycol poisoning has a mortality rate of 7.4%-21.5% [80-84]. The severity of the osmolar gap, anion gap and acidosis at presentation are associated with increased mortality. An osmolar gap ≥ 90 mOsm.L-1, anion gap ≥ 41 mmol.L-1 and arterial pH ≤ 7.0 predicts increased mortality in methanol poisoning while an osmolar gap ≥ 79 mOsm.L-1, anion gap ≥ 38 mmol.L-1 and arterial pH ≤ 7.03 predicts increased mortality in ethylene glycol poisoning [81]. Additionally, the presence of acute kidney injury as well as a duration ≥ 6 hours before initiation of antidote treatment increase mortality risk in methanol and ethylene glycol poisoning respectively [83,84]. An arterial pH ≤ 7.20 also predicts an increased likelihood of chronic visual impairment in methanol poisoning [80,82]. A systematic review comparing antidote treatment revealed a mortality rate of 21.8% for ethanol and 17.1% for fomepizole in methanol poisoning and 18.1% for ethanol and 4.1% for fomepizole in ethylene glycol poisoning [85]. Fomepizole might therefore offer survival benefits in ethylene glycol poisoning but further evidence is required to corroborate this finding.
A recent cohort study with 621 participants revealed that individuals with a history of methanol poisoning had an absolute increase in mortality rate of 37.5% over 14 years compared to individuals who did not have a history of methanol poisoning [86]. This finding suggests that the burden of disease from methanol poisoning extends beyond the sentinel event. This could be explained by the persistence of sequelae following the initial poisoning episode. Many individuals who intentionally ingest methanol also have a history of alcohol dependence or psychiatric illness placing them at increased risk of mortality and morbidity from pathology such as cirrhosis and trauma.
Diethylene glycol and isopropanol
Available literature on mortality and morbidity for diethylene glycol and isopropanol poisoning are mainly limited to case reports. Mortality rates in 2 cases of mass diethylene glycol poisoning in paediatric populations reach as high as 70%-80% [87,88]. However, authors acknowledge that delayed diagnosis and treatment might have contributed to higher mortality rates. Overall, mortality and morbidity from diethylene glycol and isopropanol poisoning appears significantly lower than methanol and ethylene glycol [87- 91].
Folic acid and folinic acid as well as pyridoxine (vitamin B6) and thiamine (vitamin B1) can be considered in methanol and ethylene glycol poisoning respectively. Folic and folinic acid circumvent the depletion of endogenous hepatic tetrahydrofolate reserves and mediate the metabolism of formic acid to H2O and CO2 [79]. Folinic acid does not require metabolic reduction and is preferred to folic acid. Similarly, pyridoxine and thiamine mediate the metabolism of glycoxylic acid to non-toxic glycine and α-hydroxy-β-ketoadipate respectively [71]. Although there remains a paucity of evidence demonstrating improved outcomes, these treatments are well tolerated with a high therapeutic index and should be considered as part of a conventional management algorithm [17,22].
Sodium bicarbonate infusions are recommended in methanol poisoning and can be considered if arterial pH<7.30. Formic acid has a pKa of 3.75 and correction of acidosis increases the proportion of ionized formic acid thereby trapping formic acid in the extracellular compartment and minimizing diffusion into the optic nerve [23]. This reduces morbidity from visual deficits secondary to formic acid mediated cellular damage. Sodium bicarbonate infusions should be titrated to achieve an arterial pH of 7.35-7.45 [22].
Renal Replacement Therapy (RRT) is an established method to eliminate toxic alcohols and their metabolites. As general approach, RRT can be considered in (a) new onset visual deficits, (b) refractory acidosis and/or electrolyte derangements, (c) persistently deranged vital signs despite supportive treatment, (d) arterial pH<7.15 and (e) methanol or ethylene glycol serum concentration >50 mg.dL-1 [22,53,72]. All modalities of RRT are acceptable but Continuous Renal Replacement Therapy (CRRT) offers better hemodynamic stability at the expense of reduced efficiency [73]. If RRT is initiated, concurrent antidote treatment is recommended to accelerate toxin clearance and reduce the length of ICU admission and hospitalization [74]. However, RRT eliminates both fomepizole and ethanol and increased doses are required. Ethanol doses are generally increased by 100% but might only require increments of 20% in CRRT due to decreased efficiency [75]. Similarly, fomepizole doses should be increased by 1-1.5 mg.kg.hr-1 during RRT [76]. RRT has been used in isopropanol and diethylene glycol poisoning but thresholds for the initiation of treatment is less clear [77,78].
Fomepizole and ethanol
Fomepizole and ethanol are inhibitors of the active site on ADH and attenuate the hydrolysis of methanol and ethylene glycol to their toxic metabolites (Table 2) [61,62]. Fomepizole is a potent antidote because its affinity for ADH is 1000 times greater than toxic alcohols and it is not metabolized by ADH resulting in a prolonged duration of active site inhibition [63]. Fomepizole is also advantageous as it (a) does not alter mentation, (b) does not require regular monitoring of serum drug concentration and (c) does not necessitate monitoring in the Intensive Care Unit (ICU) [64].
| Treatment | Toxic alcohols | Mechanism of action | Additional considerations |
|---|---|---|---|
| Antidotes -Fomepizole -Ethanol |
Methanol Ethylene glycol Diethylene glycol |
-Competitive ligand for alcohol dehydrogenase -Delays formation of toxic metabolites such as formic acid, glycolic acid, oxalic acid and 2-hydroxyethoxy-acetic acid to reduce the incidence of metabolic acidosis and chronic visual deficits |
Increase dose during renal replacement therapy Fomepizole ->1000x greater affinity for alcohol dehydrogenase -No effect on mentation -Regular serum level monitoring not required -Does not require intensive care admission -Expensive with limited accessibility Ethanol -10x greater affinity for alcohol dehydrogenase -Cheap and globally accessible -Alters mentation -Requires regular monitoring of serum levels (target ≥ 100mg.dL-1) -Requires intensive care admission |
| Renal replacement therapy |
Methanol, Ethylene glycol, Diethylene glycol, Propylene glycol, Isopropanol |
-Augments elimination of toxic alcohol and metabolites | -Clear indications for methanol and ethylene glycol. -Unclear for other toxic alcohols: > Isopropanol: (A) serum concentration ≥ 500mg/dL and (B) hypotension [10] > Diethylene glycol and propylene glycol: guided by clinical trajectory. -Continuous Renal Replacement Therapy (CRRT) offers greater hemodynamic Stability over sustained Low Efficiency Dialysis (SLED) and intermittent dialysis at the expense of lower efficiency |
| sodium bicarbonate | Methanol | -Ion trapping: formic acid remains ionized in extracellular compartment -Reduces incidence of visual deficits secondary to formic acid mediated optic nerve damage |
-Recommended if arterial pH ≤ 7.30 on presentation -Does not treat the underlying cause of metabolic acidosis |
| Redirecting metabolism | Methanol, Ethylene glycol |
-Selectively directs metabolism of formic acid and glycolic acid to non-toxic metabolites: Folic/folinic acid Formic acid à H2O + CO2 Pyridoxine Glycoxylic acid à glycine Thiamine Glycoxylic acid à α-hydroxy-β-ketoadipate |
-No FDA approval but safe with high therapeutic index -Limited evidence supporting efficacy [71]. |
Table 2: Treatments for toxic alcohol poisoning.
Ethanol is a less potent antidote as its affinity for alcohol dehydrogenase is only 10 times greater compared to toxic alcohols (about one hundred-fold less than fomepizole). Ethanol also alters mentation and requires regular 1-2 hourly serum measurements to maintain a concentration greater than 100 mg.dL-1, often necessitating ICU admission for monitoring [65]. Although fomepizole is theoretically advantageous, the superiority of fomepizole over ethanol as an antidote remains debated. Compared to ethanol, fomepizole did not improve survival in a case of methanol mass poisoning in the Czech Republic [66]. Conversely, other experts advocate the use of fomepizole over ethanol in ethylene glycol and methanol poisoning [67]. Globally, ethanol has lower cost and higher accessibility making it a more favourable antidote in resource limited settings [68].
Fomepizole has been used successfully in pediatric populations with diethylene glycol poisoning [69] but evidence of use in other populations is limited. However, advocates of the use of fomepizole in diethylene glycol poisoning remain [61]. Fomepizole and ethanol are not indicated in isopropanol poisoning as they inhibit isopropanol metabolism and paradoxically prolong its clinical effects [70,71].
Toxic alcohols are used in many commercial and industrial products resulting in a risk of acute poisoning. Globally, toxic alcohol poisoning occurs most commonly from ingestion of methanol, ethylene glycol, diethylene glycol and isopropanol (also known as isopropyl alcohol) [1]. Unfortunately, reports of mass poisoning outbreaks from products such as consumable alcohol contaminated with methanol emphasises that the public health impact of toxic alcohol poisoning extends beyond that of isolated individuals [2,3]. Majority of toxic alcohol poisoning is unintentional but intentional exposure is not uncommon amongst individuals with chronic alcohol dependence and psychiatric illness [4]. Indeed, multiple psychological, social and economic stressors arising from the ongoing SARS-CoV-2 pandemic are thought to have increased the global incidence toxic alcohol poisoning in these at-risk groups [5,6]. Toxic alcohol poisoning presents a diagnostic challenge owing to the non-specific signs and symptoms at the initial presentation [7]. Given that delayed treatment increases the risk of cellular dysfunction, end-organ failure and death, prompt diagnosis and the initiation of early management is critical at improving outcomes [8]. This narrative review therefore aims to provide a practical approach to the diagnosis, management and prognosis of acute toxic alcohol poisoning.
A stepwise approach to management is recommended and, owing to geographical and resource limitations, local or institutional guidelines should be adhered to. We propose a management approach involving early resuscitation and stabilization followed by treatments targeting specific alcohols (Figure 2).
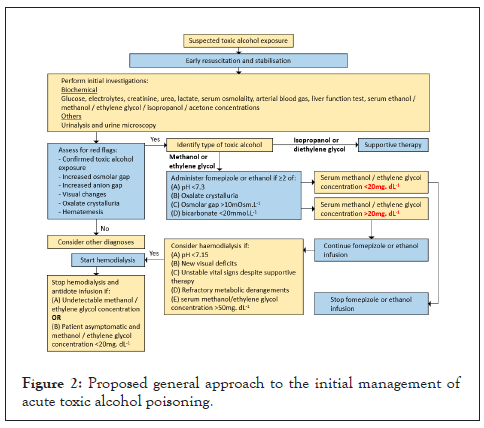
Figure 2: Proposed general approach to the initial management of acute toxic alcohol poisoning.
Serum levels of specific toxic alcohols are most accurately quantified via liquid or gas chromatography [10]. However, this is laborious, expensive, and slow and might not be accessible in all centers. Alternative tests might therefore be considered in resource limited settings. Liquid-based alcohol dehydrogenase assays are useful for multiple toxic alcohols but tend to underestimate serum concentrations [46]. Methanol is detected with the Alco-Screen test and format dehydrogenase assays [47,48]. Alco-Screen is a commercially available test kit utilizing strips containing alcohol oxidase to detect methanol and ethanol in saliva samples. Non-contrasted CT brain scans are useful if putaminal haemorrhage and necrosis is suspected in methanol poisoning [49]. Urinary fluorescein is detected with Wood’s lamp as a surrogate for ethylene glycol poisoning but sensitivity and specificity are low [50]. Furthermore in ethylene glycol poisoning, monohydrate crystalluria is detected via urine microscopy within four hours and dihydrate crystalluria predominates after seven hours [25]. Concomitant hypocalcaemia often accompanies oxalate crystalluria due to sequestration of serum calcium [51]. A positive nitroprusside reaction is a sensitive indicator of the presence of acetone, suggesting isopropanol exposure [52].
Investigations can be divided into general investigations applicable to all cases of toxic alcohol poisoning and investigations targeting specific toxic alcohols [31]. Table 1 summarizes investigations relevant for each toxic alcohol.
General investigations
Arterial blood gas, serum blood glucose, electrolytes and a urine or serum drug screen are useful in the initial work-up to eliminate differential diagnoses including hypoxia, hypercarbia, hypoglycemia, hyponatremia, hypokalemia, hyperkaliemia, and hypomagnesaemia and poisoning from other toxins [32]. In conjunction with the serum lactate trend; these initial investigations are also useful in guiding early efforts at resuscitation and stabilization [33]. Serum creatinine, serum urea and liver function tests should be obtained to screen for acute kidney injury and hepatic injury respectively. As aforementioned, both renal and hepatic dysfunction might result from direct toxicity or ischaemia due to shock. Patients with shock can be further evaluated with a point of care transthoracic echocardiography to ascertain the presence of myocardial hypokinesia, a collapsed inferior vena cava and increased inferior vena cava variability with respiration cycles [34], which are suggestive of decreased inotropy and peripheral vasodilation in toxic alcohol poisoning.
Serum osmolality should also be obtained to calculate the osmolar gap, which is the difference between measured and calculated serum osmolality. The normal osmolar gap is below 10 mOsm.L . Calculated serum osmolality is determined by the following equation:

All toxic alcohols are osmotically active but are not included in conventional formulae used to calculate the serum osmolality (see above) [35,36]. Clinicians should therefore expect an elevated osmolar gap >20 mOsm.L-1 in cases of acute alcohol poisoning [17]. Differential diagnoses such as acute ethanol toxicity, hyponatraemia and lactic acidosis and can also elevate the osmolar gap but these tend to range between 10-20 mOsm.L-1 [37,38].
The osmolar gap has limited negative predictive value and toxic alcohol poisoning cannot be ruled out in the presence of a normal osmolar gap especially if there is strong suspicion from the history and examination. Firstly, the osmolar gap decreases over time as hepatic metabolism progresses and delayed assessment might result in a smaller than expected elevation in the osmolar gap [39]. Secondly, the degree of elevation in the osmolar gap is inversely related to the molecular weight of the toxic alcohol. Diethylene glycol has a relatively high molecular weight of 106.02 g.moL-1 and every increase in serum concentration of 10 mg.dL-1 only increases the osmolar gap by 0.9 mOsm.L-1 [17]. Toxic alcohols with a high molecular weight might therefore result in an unexpectedly small elevation in the osmolar gap. Finally, small amounts of toxic alcohols can produce clinical manifestations without typical increases in the osmolar gap. A serum methanol concentration of 50 mg.dL-1 (sufficient to warrant renal replacement therapy) has been shown to correlate with an osmolar gap of only 16 mOsm.L-1 [40].
Toxic alcohol poisoning typically results in a High Anion Gap Metabolic Acidosis (HAGMA) due to unmeasured anions donated by their acid metabolites [41-44]. Since the acid metabolites are formed at varying rates, the anion gap is often normal in the early stages of poisoning. It is therefore prudent to perform serial arterial blood gases to trend the anion gap over time. In toxic alcohol poisoning, a rising anion gap is expected to coincide with a decreasing osmolar gap owing to the hydrolysis of osmotically active toxic alcohols to their osmotically inactive acid metabolites. Conversely, isopropanol and its metabolite, acetone, do not donate unmeasured anions and therefore do not lead to a HAGMA [45]. As a caveat, concurrent respiratory depression from sedation and hypotension from pump failure and peripheral vasodilation might contribute to a mixed respiratory and metabolic acidosis in severe isopropanol poisoning [23].
Unlike the other toxic alcohols, isopropanol has direct neurotoxic and cardiotoxic effects. Its toxic effects are attenuated following metabolism by ADH and ALDH [10]. Clinical findings closely mimic acute ethanol intoxication and can result in lethargy, sedation, confusion, hyporeflexia, ataxia and coma. Less common neurological findings include seizures, dysarthria, tinnitus and tremors. At serum isopropanol concentrations above 150 mg.dL , systemic arterial vasodilation and decreased myocardial contractility might occur leading to shock, end-organ ischaemia and ultimately death [29]. Isopropanol is an irritant of gastrointestinal mucosa predisposing to massive haemorrhage that further contributes to haemodynamic instability on presentation [30]. Acute renal and hepatic injury characterized by elevated serum creatinine and deranged transaminases respectively might also be observed but it is currently unclear if this is attributable to direct toxic effects or hypoperfusion from shock [10].
2-Hydroxyethoxy-Acetic Acid (HEAA) is postulated to be the primary agent mediating the toxic effects of diethylene glycol. HEAA mediates the accumulation of osmotically active particles in the intracellular compartment leading to transcellular shifts and a disruption of cellular membrane integrity [27]. Subsequent cellular apoptosis and necrosis can result in rapidly progressive organ failure. Diethylene glycol poisoning often manifests as acute kidney injury, pancreatitis, acute hepatitis and peripheral neuropathy [28].
Oxalic acid is the primary toxic metabolite of ethylene glycol. Oxalic acid sequesters calcium to form calcium oxalate crystals that precipitate and deposit in various tissues leading to end-organ dysfunction. Oxalate crystal deposition in pulmonary, myocardial, renal and central nervous tissue leads to Acute Respiratory Distress Syndrome (ARDS), myocarditis with heart failure, acute kidney injury and cranial nerve neuropathy respectively [24,25]. Organ dysfunction is conventionally described as occurring in 3 distinct stages beginning with cardiopulmonary failure followed by renal impairment with oliguria and raised creatinine, finally leading to neurological sequelae [26]. However, overlaps between these stages are common in clinical practice.
Formic acid is the primary toxic metabolite of methanol. It inhibits mitochondrial cytochrome oxidase leading to anaerobic respiration, intracellular adenosine triphosphate depletion and lactic acidosis [20]. Formic acid also promotes lipid peroxidation and free radical formation to accelerate cellular damage [21]. Formic acid is eventually metabolized to H O and CO but this is dependent on endogenous hepatic tetrahydrofolate reserves which are rapidly depleted at toxic doses [22]. Formic acid preferentially targets neurons in the retina, optic nerve and basal ganglia. Consequently, clinical manifestations include visual deficits such as central scotomata, blurred vision and blindness as well as Parkinsonian features secondary to putaminal haemorrhage and necrosis [23].
General clinic pathological features of gastrointestinal stromal tumors
Toxic alcohols are most often utilised as solvents in various commercial and industrial products. Methanol, ethylene glycol and diethylene glycol are found in antifreeze, cleaning solutions and automotive products such as carburettor fluid [9]. Conversely, Isopropanol is an active ingredient in cleaning products, alcohol- based hand sanitizers and cosmetic products such as nail polish removers [10]. As such, there exist minimal barriers to accessing toxic alcohols and the presence of such products in the patient’s vicinity prior to presentation should raise the suspicion of potential exposure. While oral ingestion is the most common route of exposure to toxic alcohols [11-13], absorption through transdermal and inhalational routes are also possible [14,15].
Upon ingestion, toxic alcohols are rapidly absorbed from the gastrointestinal tract and peak serum concentrations are achieved within thirty to sixty minutes [16]. Early symptoms of toxic alcohol poisoning mimic ethanol intoxication owing to the potentiation of GABAA activity. Indeed, sedation, confusion, disorientation, ataxia, abdominal pain and vomiting are common findings [17]. The emergence of defining signs and symptoms as well as clinical deterioration coincides with the formation of toxic metabolites mediated by hepatic hydrolysis from Alcohol Dehydrogenase (ADH) and Acetaldehyde Dehydrogenase (ALDH) (Figure 1).
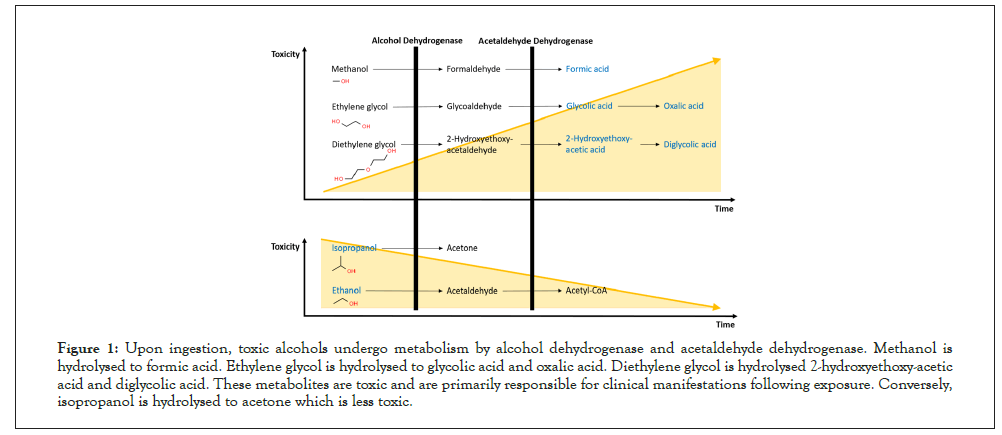
Figure 1: Upon ingestion, toxic alcohols undergo metabolism by alcohol dehydrogenase and acetaldehyde dehydrogenase. Methanol is hydrolysed to formic acid. Ethylene glycol is hydrolysed to glycolic acid and oxalic acid. Diethylene glycol is hydrolysed 2-hydroxyethoxy-acetic acid and diglycolic acid. These metabolites are toxic and are primarily responsible for clinical manifestations following exposure. Conversely, isopropanol is hydrolysed to acetone which is less toxic.
Critically, the duration required for the formation of toxic metabolites is dependent on two key factors. These are (A) the type of toxic alcohol ingested and (B) the presence of co-ingested ethanol. Firstly, toxic alcohols undergo hepatic hydrolysis at differing rates due to varying degrees of affinity for the active site on ADH. For example, methanol has a poor affinity for ADH and up to twenty- four hours may be required before an appreciable rise in serum formic acid concentrations is observed [18]. Secondly, ethanol competes with toxic alcohols for the active site on ADH thus inhibiting ADH mediated hydrolysis and delaying the formation of toxic metabolites [19]. Moreover, ethanol co-ingestion is common in cases of intentional toxic alcohol poisoning. Clinicians should therefore exercise caution by maintaining vigilance and regular reassessments of such patients due to the risk of delayed clinical deterioration. Details specific to the clinical presentation of each toxic alcohol will be discussed below (Table 1).
| Alcohol (molecular weight) |
Major clinical findings | Major investigation findings | ↑ in serum osmolality for each 10 mg/dL increase in serum alcohol level (mOsm.L-1) | Onset time (hrs)-without concurrent ethanol consumption | Onset time (hrs)-with concurrent ethanol consumption |
|---|---|---|---|---|---|
| Methanol (32.04 g.mol-1) |
Central nervous system -sedation -confusion -visual impairment -Parkinsonian features Gastrointestinal system -abdominal pain |
Raised osmolar gap High anion gap metabolic acidosis Raised serum methanol and formic acid Alco-Screen positive Formate Dehydrogenase strip positive |
3.1 | 6-24 | 24-72 |
| Ethylene glycol (62.07 g.mol-1) |
Central nervous system -sedation -confusion -cranial neuropathy Cardiopulmonary system -heart failure -acute respiratory distress syndrome Renal system -acute kidney injury |
Raised osmolar gap High anion gap metabolic acidosis Raised serum ethylene glycol and glycolic acid Calcium oxalate crystalluria Hypocalcaemia Wood’s lamp positive |
1.6 | 4-8 | 8-12 |
| Diethylene glycol (106.02 g.mol-1) |
Gastrointestinal system -pancreatitis -hepatitis renal system -Acute kidney injury |
Raised osmolar gap High anion gap metabolic acidosis |
0.9 | 24-48 | 48-72 |
| Isopropanol (60.1 g.mol-1) |
Central nervous system -sedation -confusion Cardiopulmonary system -respiratory depression -hypotension Gastrointestinal system -haemorrhage |
Raised osmolar gap Raised serum isopropanol and acetone Raised urinary acetone Positive nitroprusside test |
1.7 | 1-4 | Not applicable |
Table 1: Major diagnostic features following toxic alcohol poisoning.
PubMed and Scopus databases were searched. Search terms included “methanol”, “ethylene glycol”, “diethylene glycol”, “isopropanol”, isopropyl alcohol”, “toxic alcohol”, “toxicity” and “poisoning”. Inclusion criteria included English language articles comprising randomized controlled trials, cohort studies, reviews and clinical practice guidelines. Emphasis was placed on identifying randomized controlled trials, observational studies, narrative reviews and systematic reviews which were clinically focused. References cited in selected articles were reviewed to identify additional relevant resources. Articles selected were reviewed and agreed upon by all co-authors.
Citation: Tan K, Ashokka B, Law LSC, Lo EAG, Palaniappan D, Kundra P (2021) Approaching Acute Toxic Alcohol Poisoning: A Narrative Review. J Clin Toxicol. S20:005.
Received: 06-Dec-2021 Accepted: 20-Dec-2021 Published: 27-Dec-2021
Copyright: © 2021 Tan K, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.