Mini Review - (2022)Volume 6, Issue 3
Arecoline is a controversial composition with pharmacological activity and toxicities, which is speculated to the major cause of oral carcinogensis reported by many studies. However, there is no data or evidence in humans regarding the carcinogenicity of arecoline due to indirect exposure to it. In 2020, arecoline was classified as “possibly carcinogenic to humans” (Group 2B) on the basis of “strong” mechanistic evidence by International Agency for Research on Cancer (IARC). In fact, the inference of arecoline leading to carcinogenic processes in oral carcinogensis has not been proven by experiments in vivo. Hence, this review was aimed at evaluating the existing articles regarding the arecoline induced cancer in the animal experiments. The PubMed database of the National Library of Medicine was used to search for publications that investigated the association with the arecoline caused carcinogensis up to August 2022. The search terminology was the keywords “Cancer with arecoline or its metabolite, arecaidine”.The search was conducted under the clear inclusion and exclusion criteria. This review shows that there is insufficient evidence about the carcinogenic effects of arecoline in the animal experiments. The multifactors in betel quid chewing contribute to the oral cancer. The arecoline deemed as the major factor induced oral cancer in previous statement is not proven by experiments in vivo. Our pilot study provides the information associated with arecoline and carcinogensis in animal experiments and proves that there is no solid evidence indicating arecoline lead to cancer in vivo.
Arecoline; Areca nut; Betel quid; Oral carcinogenesis
Betel-quid (BQ) chewing is considered as the fourth most widely used addictive substance in the world following tobacco, alcohol and coffee [1]. BQ chewing is wildly prevalent in the areas of Southeast Asia, East African seaboard, and Western Pacific [2]. Achieving euphoria, combating fatigue, increasing salivation, attaining satiation, and even seeking relief of toothaches are the main reasons for chewing BQ [3]. BQ is usually composed of areca nut, betel leaf, slaked lime, with or without tobacco and other additives like spices, sweeteners, essences, catechu [4]. Areca Nut (AN) is proverbially basic ingredient of the different types of chewing products. The major compositions of the AN is alkaloids, crude fibre, polyphenols, proteins, lipids, mineral matter and carbohydrates [5]. Importantly, arecoline is an alkaloid isolated from AN, which is considered as the major effective psychoactive component of AN [6]. Arecoline has many pharmacological activities with nervous, cardiovascular, endocrine, and digestive systems.
The safety of BQ chewing has been drawing broad attention for a long time. Since International Agency for Research on Cancer (IARC) found sufficient evidence that the habit of chewing betel quid, with or without tobacco, causes cancer in human [4], a plenty of studies have been carried out to explore the mechanism of oral carcinogensis induced by BQ chewing [7]. Extensive studies have suggested that a number of factors play a role in the pathogenesis of oral cancer induced by BQ chewing [8,9]. Typically, a combination of mechanical irritation and chemical irritation together leads to the Oral Submucous Fibrosis (OSF) or oral cancer. One hand, the chronic mechanical irritation derived from coarse fibers in the AN of the oral mucosa has been proposed as a risk factor for oral cancer [10].On the other hand, chemical irritation originated from compositions of BQ is also the most potent factor in OSF or oral cancer. Areca alkaloids are deemed to be the causative ingredients in the pathogenesis of OSF by increasing collagen production; the flavonoids and polyphenols also have been found to show negative effect on collagen metabolism; in addition, the irritant additives such as slaked lime, pepper, ginger or tobacco in BQ, which also contribute to the incidence of OSF or oral cancer. In fact, there is no solid evidence in clinic to prove theses proposed carcinogenic processes. Among the multitudinous carcinogenic factors, arecoline has been attracted more attentions because it is a mainly pharmacologically active ingredient in AN. Unfortunately, there was no information on population direct exposure to arecoline, which was generally indirect via the use of areca nut and areca nut-derived products. Therefore, this review mainly focuses on the arecoline induced cancer cases in animal experiments in the past years to illustrate the effect of arecoline on the carcinogensis in vivo.
The data for this review was derived from PubMed database of the National Library of Medicine by searching paper published from 1969 to 2022, using the “Cancer with arecoline or its metabolite, arecaidine” as keywords. Details of the selection process are shown in Figure 1.
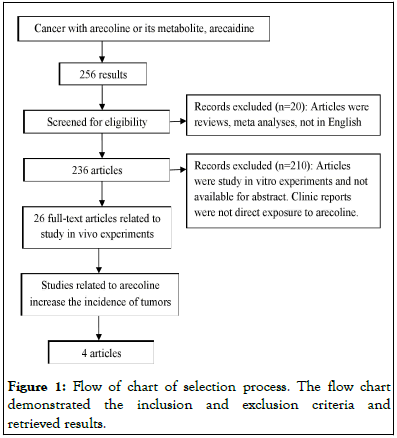
Figure 1: Flow of chart of selection process. The flow chart demonstrated the inclusion and exclusion criteria and retrieved results.
The articles were excluded in languages other than English, studies associated with meta-analyses and reviews, reports without full text, studies in vitro, as well as studies in vivo and clinic trials indirect exposure to arecoline. Articles mainly focus on the studies associated with arecoline increase the incidence of tumours alone or in combination with other chemical when direct exposure to animals or human. Finally, a total of 256 articles in PubMed were found to satisfy the search words. After manual screening the exclusion criteria, only 4 articles were deemed to be eligible.
Three papers focused on the study of co-carcinogenicity in combination with the 4-nitroquinoline 1-oxide (4-NQO) and arecoline, and one paper investigated full carcinogenicity in arecoline alone. In fact, only two of them mimicked oral tumorgenesis in animal experiments as shown in Table 1. Chang, et al. [11] established a C57BL/6JNarl male mouse model mimicking oral tumorigenesisby co-treating with arecoline and 4-NQO (in the drinking-water). After 28 exposure weeks, the results indicated that tongue tumor incidence rate was 100% in mice exposed to concomitant 4-NQO (200 μg/mL) and arecoline (500 μg/mL) treatment, 57% in mice exposed to 4-NQO alone, and 0% in mice exposed to arecoline alone. Immunohistochemical analysis in study have demonstrated that the murine oral cancer tumor progression was due to the upregulation of αB-crystallin and Hsp27. Following the above study, other two cocarcinogenicity studies mainly investigated the mechanism of carcinogenesis Chen, et al. [12] established the NHRI-HN1 cell line from a male mouse tongue tumor induced by 4-NQO and arecoline to illustrate NHRI-HN1 cells have tumorigenic characteristics of Epithelial Mesenchymal Transition (EMT), extracellular signal-regulated kinase activation and cancer stemness. Huang, et al. [13] have found various subtype cell lines contributed to oral carcinogenesis induced by 4NQO and areocline administration in a mouse model. In full carcinogenicity study [14], Swiss mice treated with arecoline, either alone or in combination with KNO3 or KNO3+ lime by oral administration (gavage), which kept on a vitamin B complexdeficient or normal diet. The results have showed that mice receiving a normal diet induced tumors in 43% of males but failed to produce tumors of females in expose to arecoline alone.
| Species | Duration | Method | Groups | Lesion incidence |
|---|---|---|---|---|
| C57BL/6J [11] | 28 week | Oral administration (drinking-water) | Nine groups | Tongue or Oesophagus including hyperplasia, dysplasia, papilloma, or invasive squamous cell carcinoma |
| Mouse | 3 mice were sacrificed after the 8-week; the remaining mice were sacrificed after 28-week | G1.Control group (n=10) | G1.T-0/7, O-0/7 | |
| Male | G2.4-NQO 100 µg/mL (n=10) | G2.T-2/7 (29%), O-1/7 (14%) | ||
| 6 week | G3 4-NQO 200 µg/mL (n=10) | G3.T-4/7 (57%), O-1/7 (14%) | ||
| G4. ARC 250 µg/mL (n=10) | G4.T-0/7(0%), 0/7(0%) | |||
| G5. ARC 500 µg/mL (n=10) | G5.T-0/7 (0%), 0/7 (0%) | |||
| G6. ARC /4-NQO 250/100 µg/mL (n=11) | G6.T-4/8 (50%), 4/8 (50%) | |||
| G7. ARC/4-NQO 500/100 µg/mL (n=11) | G7.T-3/8 (38%), 1/8 (13%) | |||
| G8. ARC/4-NQO 250/200 µg/mL (n=11) | G8.T-4/8 (50%), 1/8 (13%) | |||
| G9 ARC/4-NQO 500/200 µg/mL (n=11) | G9.T-8/8 (100%), 0/8 (0%) | |||
| Swiss [14] | 25 month | Oral administration (gavage) | Nine groups | Tumors in liver lung and stomach |
| Mouse Female/Male | Treated groups received 1 mg ARC/day/mouse either alone or in combination with other substances | G1.untreated (n=40) | G1.M-1/20(5%), F-0/20(0%) | |
| 6 week | G2. ARC (n=53) | G2.M-15/35 (43%), F-0/18(0%) | ||
| G3 ARC +KNO3 (n=33) | G3.M-3/19 (16%), F-0/14(0%) | |||
| G4. ARC +KNO3+ lime (n=28) | G4.M-1/16(6%), F-0/12(0%) | |||
| G5. KNO3+ lime (n=25) | G5.M-2/17 (10%), F-0/8(0%) | |||
| G6. untreated/B-complex-deficient (n=37) | G6.M-2/21 (10%), F-1/16(6%) | |||
| G7. ARC /B-complex-deficient (n=28) | G7.M-7/21 (6%), F-6/12(6%) | |||
| G8. ARC +KNO3/B-complex-deficient (n=34) | G8.M-1/16 (6%), F-2/16(12%) | |||
| G9. ARC +KNO3+ lime/B-complex-deficient (n=36) | G9.M-7/18 (39%), F-8/18(44%) | |||
| Note: 4-NQO: 4-nitroquinoline 1-oxide; ARC: arecoline; M: male; F: female; G: group; T: Tongueo; O: Oesophagus. | ||||
The retrieved results indicated that there was no solid evidence to prove the arecoline alone direct induced the OSF or oral cancer in animal experiments. Arecoline only increased the incidence of tumours in combination with the 4-NQO and carcinogenesis of which were illustrated on the cellular and genetic level in three studies [11-13].The other study was only increased the incidence of tumors in male mice treated with arecoline, while not influence on the female mice. There is limited evidence in experimental animals for the carcinogenicity of arecoline. The evidence regarding cancer in humans is inadequate, as no studies were available [15].There is also reason why the arecoline was classified as “possibly carcinogenic to humans” (Group 2B) by IARC in 2020.
In summary, our review provides the data results of shed light on the association with arecoline and carcinogensis in animal experiments. Analysis of the existing articles indicates that the inference of arecoline is possibly major factor induced oral carcinogensisin humans, which is only according to mechanistic evidence in vitro experiments and lack of convincing evidence in vivo experiments. To data, there is inadequate evidence in humans regarding the carcinogenicity of arecoline. In future, the more studies of arecoline regarding toxicity in vivo are needed to carry out to illustrate the carcinogensis in humans.
We would like to thank the Laboratory of Life and Health Sciences, Shenzhen First Union Technology Co., Ltd; Shenzhen First Union Technology Co., Ltd- Shenzhen Institute of Advanced Technology, Chinese Academy of Sciences Joint Laboratory; Shenzhen Icybetel Biotechnology Co., Ltd; and Hainan Elka Biotechnology Co., Ltd for all support.
None.
Citation: Lu J, Chu M (2022) Arecoline is the Causative Factor for Oral Carcinogensis? A Review of Arecoline Associated Cancer in Animal Experiments. J Pharma Reports. 06: 132.
Received: 14-Sep-2022, Manuscript No. JPR-22-19188; Editor assigned: 19-Sep-2022, Pre QC No. JPR-22-19188 (PQ) ; Reviewed: 04-Oct-2022, QC No. JPR-22-19188; Revised: 10-Oct-2022, Manuscript No. JPR-22-19188 (R); Published: 17-Oct-2022 , DOI: 10.35248/JPR.22.6.132
Copyright: © 2022 Lu J, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.