Advanced Techniques in Biology & Medicine
Open Access
ISSN: 2379-1764
ISSN: 2379-1764
Mini Review - (2015) Volume 3, Issue 3
Aldose reductase (ALR2) plays a pivotal role in the development of diabetic complications. ALR2 inhibitors (ARIs) have proven efficient to prevent and delay the chronic diseases, and thus attracted significant research interest. This article reviews a series of aromatic heterobicyclics-based ARIs developed over recent years, and discusses about their structure-activity relationships as well as their multifunctionalities such as antioxidant property
Keywords: Aromatic Heterobicyclics, Aldose Reductase, Diabetes
Diabetes mellitus (DM) is associated to the abnormal metabolism of the glucose and is always characterized by long-term complications, such as neuropathy, nephropathy, cataracts, retinopathy [1]. In recent decades, DM is the major threat to human health all over the world and the situation is getting worse rapidly, as over 171 million people worldwide were suffering with this disease in 2000 and this figure is considered to grow more than twice and reach to 366 million by 2030 [2]. In fact, diabetic patients in China have reached 92 million in 2007, and even rocketed to 114 million in 2010 [3,4]. Furthermore, people with any kind of diabetes of sufficient duration, including both type 1 and type 2 diabetes mellitus, are vulnerable to these complications, which are the major distress for the diabetic patients. Increasing evidence demonstrated that aldose reductase (ALR2, EC, 1.1.1.21) plays a pivotal role in development of chronic diabetic complications, as this enzyme accompanied with sorbitol dehydrogenase forms the polyol pathway of glucose metabolism, which supposed to be the main pathogenesis of diabetic complications. In the pathway (Figure 1), the glucose is firstly reduced to sorbitol by ALR2 catalysis with NADPH as coenzyme, and then sorbitol dehydrogenase converts sorbitol into fructose with reduction of NAD+ [5-7]. (Figure 1)
Normally, ALR2 has a low activity and only a small amount of the glucose is metabolized through this pathway. In hyperglycemia, however, ALR2 is activated and one third of the total metabolic glucose turns to the alternative polyol pathway, which leads to the accumulation of sorbitol in tissues possessing insulin-independent uptake of glucose, such as kidney, lens, retina, peripheral nerves [1,5]. Sorbitol is hardly excreted across cell membranes due to strong polarity, and therefore its intracellular accumulation would lead to osmotic imbalance, cell swelling, and changes of membrane permeability, mainly in lens. Also, the abnormal level of NADPH and NAD+ caused by the polyol pathway would induce modification in cellular redox potentials, and unconventionally activated enzymes such as nitric oxide synthase (NOS) and glutathione reductase would give rise to the cellular oxidative stress. As a consequence, the imbalance between increased production of radical oxygen species (ROS) and reduced intracellular antioxidant defense occurs [5]. Furthermore, the increased accumulation of advanced glycation-end products (AGEs) aroused by the enhanced level of glycating agents would cause other pathological alteration in the functions of intracellular proteins and result in further accumulation of ROS [8]. All these stress responses evoked by the activated ALR2 in down-stream of the polyol pathway have also been proposed to form pathogenic mechanisms of the multiple diabetic complications. Therefore, suppression of the ALR2 activity and further oxidative stress, particularly ALR2 inhibitors (ARIs) having antioxidant property, could be an efficient strategy to prevent or delay the progression of diabetic complications.
In the recent few years, the aromatic heterbiocyclic structures including benzothiadiazine, pyridythiadiazine, benzothiazine, and quinoxalinone have been found to be excellent framework for the ARI drug design, which led to a number of novel and potent series 1-7 (Figure 2) [2,8-16] as promising drug candidates for the treatment of diabetic complications. (Figure 2)
Table 1 shows the specific structure and inhibitory activity data of representative samples of each heterocyclic scaffold. Obviously, potent ARIs have been obtained in each of the core structure indicating these scaffolds are in favor of designing ARIs that could intensively interacted with ALR2. Particularly, the samples 1h, 2b, 3b, and 5c (IC50=32, 44, 110, and 6.4 nM, respectively) are the most active compounds to the relevant core. Comparison of the scaffolds to each other indicates that the quinoxalinone may be the core promising for further ARI developments when 5b (IC50=10 nM) in the quinoxalinone series is found to have the ALR2 inhibition greater than its counterparts 1c (IC50=41 nM) and 2b (IC50=44 nM) in benzothiadiazine and pyridythiadiazine series, respectively (Table 1).
| Compd. | Substituent | IC50(nM)afor ALR2 | |
|---|---|---|---|
| R1 | R2 | ||
| 1a[2] | H | 2-F,4-Br | 125 |
| 1b[2] | F | 2-F,4-Br | 90 |
| 1c[2] | Cl | 2-F,4-Br | 41 |
| 1d[2] | Br | 2-F,4-Br | 71 |
| 1f[2] | H | 2,4,5-F3 | 111 |
| 1g[2] | F | 2,4,5-F3 | 66 |
| 1h[2] | Cl | 2,4,5-F3 | 32 |
| 1i[2] | Br | 2,4,5-F3 | 52 |
| 1j[2] | F | 4-CF3 | 931 |
| 1k[2] | Cl | 4-CF3 | 595 |
| 2a[16] | CH3 | 2-F,4-Br | 127 |
| 2b[16] | Cl | 2-F,4-Br | 44 |
| 2c[16] | Br | 2-F,4-Br | 69 |
| 2d[16] | CH3 | 2,4,5-F3 | 84 |
| 2e[16] | Cl | 2,4,5-F3 | 38 |
| 2f[16] | Br | 2,4,5-F3 | 69 |
| 2g[16] | Cl | 4-CF3 | 1010 |
| 2h[16] | Cl | 4-OCH3 | 6540 |
| 3a[15] | H | 2,4,5-F3 | 590 |
| 3b[15] | H | 2-F,4-B3 | 110 |
| 4a[15] | H | 2,4,5-F3 | 340 |
| 5a[13] | H | 2-F,4-Br | 35 |
| 5b[13] | 7-Cl | 2-F,4-Br | 10 |
| 5c[13] | 7-F | 2-F,4-Br | 6.4 |
| 5d[13] | 6-Cl | 2-F,4-Br | 59 |
| 5e[13] | 7-Cl | 4-Br | 23 |
| 6a[14] | H | 2-F,4-Br | 45 |
| 6b[14] | 7-F | 2-F,4-Br | 23 |
| 6c[14] | H | 4-Br | 27 |
| 6d[14] | 7-F | 4-Br | 11 |
| a IC50values represent the concentration required to produce 50% enzyme inhibition | |||
Table 1: The biological activity of ARIs.
For ARIs based on the present scaffolds, the aromatic side chains including benzyl and phenoxyl groups are the other crucial factor besides the typical carboxylate side chain, which has long been the most important group [17]. Benzyl groups as the side chains in the case of quinoxalinone series seem to have more impact on the inhibitory activity against ALR2 than phenoxyl groups as comparing the compounds 5c (IC50=6.4 nM) and 6b (IC50=23 nM) as shown in Table 1, but this effect is depending on substituents both at aromatic rings of the side chain and scaffold. Moreover, longer length of carbon chain spacer of the side chain leads to increase in the potency of quinoxalinone ARIs [8,13].
At the aromatic ring of side chains in all series in this review, groups of 2-F,4-Br, 2,4,5-F3, or 4-Br proved to be preferred substituents whereas the bulky groups of trifluoromethyl and methoxyl had negative substitution effect as comparing 2b (IC50=44 nM) with 2g (IC50=1010 nM) and 2h (IC50=6540 nM). At the aromatic ring of the scaffold, halogens including chloro and bromo are always better substituents than methyl group. However, bulky hydrophobic substituents were found to be favorable for enhancing the ARI activity, and the flexible amide group as one of the substituents could significantly enhance the activity but also selectivity [18].
In the benzothiazine series including compounds 3-4, the orientation of the carboxylic acid head is likely to have a strong influence on the interaction between the ALR2 and inhibitors. Z-isomers of 3 is more active than E-isomers, [11] while R-(−)-enantiomers of 4 (IC50=500 M) than S-(+)-enantiomers (IC50=4150 nM) [10,19] (Table 2).
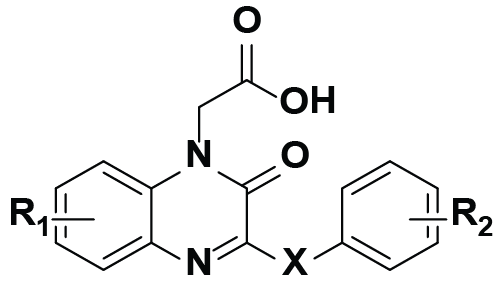 |
||||||||
| Compd. | Substituent | IC50(nM)ALR2a | DPPH sca. % | |||||
| R1 | R2 | X | 100μM | 50μM | ||||
| 6e[9] | 6-F | 4-OH | O | 20 | 23.7 | b | ||
| 6f[9] | 7-Cl | 4-OH | O | 179 | 84.8 | 47.3 | ||
| 6g[9] | 7-Cl | 3,5-(OH)2 | O | 566 | 91.3 | 83.7 | ||
| 7a[8] | H | 4-OH | CH2=CH2 | 182 | 71.8 | 55.8 | ||
| 7b[8] | 7-F | 4-OH | CH2=CH2 | 153 | 67.2 | 45.7 | ||
| 7c[8] | H | 3-OCH3,4-OH | CH2=CH2 | 419 | 95.4 | 68.8 | ||
| epalrestat | 91 | b | b | |||||
| Trolox | b | 98.2 | 90.2 | |||||
| aIC50values represent the concentration required to produce 50%enzyme inhibition. | ||||||||
| b Not determined. | ||||||||
Table 2: Biological activity of multifunctional ARIs based on quinoxalinones.
In order to obtain multifunctional ARIs having antioxidant property, phenolic hydroxyl groups have been introduced to the aromatic ring of the side chain in the quinoxalinone series as shown in Table 2. Hydroxyl substituted phenoxyl side chains integrated good antioxidant activity to ARIs. However, more success resulted from the key strategy by the use of vinyl group as the spacer in combination with the phenolic hydroxyl in the side chain because the products may be more potent in the ALR2 inhibition and antioxidation. These efforts led to achievement of a number of ARIs showing significant antioxidant activity by testing their DPPH radical scavenging rate. Of them, 6g and 7c are excellent both in the ALR2 inhibition and antioxidant activity. Particularly, the antioxidant activity of 7c was also confirmed by testing its inhibition of lipid peroxidation in vivo using animal models [8,9].
In summary, the aromatic heterobicyclics of benzothiadiazine, quinoxalinone, and their analogues have been proved effective scaffolds for the design of ARIs, and in particular quinoxalinone is excellent for the construction of multifunctional ARIs having antioxidant property. However, all these designed inhibitors are in the carboxylate ARI class, which has a shortcoming of poor tissue penetration resulting in poor distribution from blood to the tissues and then in pharmacokinetic drawbacks and even low in vivo efficacies [20]. Therefore, new design of non-carboxylate series of ARIs based on these frameworks may be expected.